Abstract
OBJECTIVE
Participants in ADVANCE were drawn from many countries. We examined whether the effects of intensive glycemic control on major outcomes in ADVANCE differ between participants from Asia, established market economies (EMEs), and eastern Europe.
RESEARCH DESIGN AND METHODS
ADVANCE was a clinical trial of 11,140 patients with type 2 diabetes, lasting a median of 5 years. Demographic and clinical characteristics were compared across regions using generalized linear and mixed models. Effects on outcomes of the gliclazide modified release–based intensive glucose control regimen, targeting an HbAlc of ≤6.5%, were compared across regions using Cox proportional hazards models.
RESULTS
When differences in baseline variables were allowed for, the risks of primary outcomes (major macrovascular or microvascular disease) were highest in Asia (joint hazard ratio 1.33 [95% CI 1.17–1.50]), whereas macrovascular disease was more common (1.19 [1.00–1.42]) and microvascular disease less common (0.77 [0.62–0.94]) in eastern Europe than in EMEs. Risks of death and cardiovascular death were highest in eastern Europe, and the mean difference in glycosylated hemoglobin between the intensive and standard groups was lowest in EMEs. Despite these and other differences, the effects of intensive glycemic control were not significantly different (P ≥ 0.23) between regions for any outcome, including mortality, vascular end points, and severe hypoglycemic episodes.
CONCLUSIONS
Irrespective of absolute risk, the effects of intensive glycemic control with the gliclazide MR-based regimen used in ADVANCE were similar across Asia, EMEs, and eastern Europe. This regimen can safely be recommended for patients with type 2 diabetes in all of these regions.
Estimates and projections of the global burden of diabetes clearly show that this burden is widely dispersed and that diabetes is by no means merely a problem of established market economies (EMEs) (1). Indeed, none of the top ten countries by diabetes prevalence in the latest International Diabetes Federation statistics are EMEs (2). Furthermore, in all parts of the world the prevalence of diabetes is expected to rise over the next 20 years (1,2). This argues for a global approach to prevention of the undesirable consequences of diabetes, the most important of which are macrovascular and microvascular disease.
Guidelines for treatment of patients with diabetes across the world stress the importance of glycemic control, although the target levels and first-line drugs of choice vary (3). Whether these variations reflect real differences in the effects of glycemic control across regions is unknown. The ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release (MR) Controlled Evaluation) trial (4) had the broadest worldwide coverage of patients of any trial of diabetes yet conducted, although relatively few participants (4%) came from North America. In general, ADVANCE found favorable, but nonsignificant, effects of an intensive glycemic control regimen, compared with local standard control regimens, on cardiovascular events and all-cause mortality and beneficial effects on microvascular complications. The objective of this study was to determine whether these generally favorable effects of glycemic control were consistent across the ADVANCE trial populations recruited from Asia, EMEs, and eastern Europe.
RESEARCH DESIGN AND METHODS
ADVANCE was a double-blind factorial randomized controlled trial conducted by 215 collaborating centers in 20 countries, involving 11,140 patients (lists of participating centers and personnel can be found in the Supplementary Appendix). Detailed study methods have previously been published (4,5). In brief, subjects had type 2 diabetes diagnosed at ≥30 years of age, were ≥55 years of age at entry to the study, and had either existing cardiovascular disease or at least one risk factor for that disease. Patients were ineligible if they had a definite indication for, or contraindication to, either of the active study treatments or had a definite indication for long-term insulin therapy. Subjects were randomized to 1) the fixed combination of the ACE inhibitor, perindopril, and the diuretic, indapamide (2 mg/0.625 mg, doubled to 4 mg/1.25 mg after 3 months) or matching placebos and 2) intensive (gliclazide MR based, to an HbA1c target of ≤6.5%) or standard (usual care) glucose control. Patients were followed up for a median of 5.0 years.
The two primary study outcomes were as follows: macrovascular events (cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) and microvascular events (new or worsening nephropathy or retinopathy), analyzed separately and jointly. Prespecified secondary outcomes were total deaths, cardiovascular disease deaths, major coronary events (death due to coronary heart disease, including sudden death, and nonfatal myocardial infarction), total coronary events (major coronary events, silent myocardial infarction, coronary revascularization, or hospital admission for unstable angina), major cerebrovascular events (death due to cerebrovascular disease or nonfatal stroke), total cerebrovascular events (major cerebrovascular events, transient ischemic attack, or subarachnoid hemorrhage), new or worsening nephropathy (development of new macroalbuminuria, doubling of serum creatinine to a level of at least 200 μmol/L, need for renal replacement therapy, or death due to renal disease), development of new microalbuminuria, total renal events (new or worsening nephropathy or new microalbuminuria), new or worsening retinopathy, visual deterioration, and total retinopathy (development of proliferative retinopathy, macular edema, or diabetes-related blindness or retinal photocoagulation therapy). An independent Endpoint Adjudication Committee, blinded to treatment allocation, reviewed source documentation for all individuals who had a suspected primary end point or who died during follow-up.
Statistical methods
The 20 countries involved in ADVANCE were divided according to standard definitions (1) into three groups, listed with number of patients per country in parentheses: Asia (n = 4,136), comprising China (n = 3,293), India (n = 471), Malaysia (n = 236), and the Philippines (n =136); EMEs (n = 4,862), comprising Australia (n = 978), Canada (n = 436), France (n = 196), Germany (n = 327), Ireland (n = 442), Italy (n = 21), the Netherlands (n = 507), New Zealand (n = 630), and the U.K. (n = 1,325); and eastern Europe (n = 2,142), comprising the Czech Republic (n = 209), Estonia (n = 155), Hungary (n = 434), Lithuania (n = 118), Poland (n = 604), Russia (n = 164), and Slovakia (n = 458).
Baseline demographic and clinical data, including methods of glycemic control, were compared between the three regions, unadjusted and after adjustment for age, sex, and duration of diabetes, using generalized linear models. Skewed continuous variables were transformed to approximate normality using logarithmic transformations. Event rates during the trial were compared between the regions using Cox proportional hazards regression, adjusting for a range of potential confounding variables, taking EMEs as the reference group.
Mean differences in HbA1c between the intensive and the standard glycemic control regimens during the trial were estimated for each region using linear mixed models. The efficacy of more versus less glycemic control was estimated for each region and reported as the relative risk reduction for each of the primary and secondary outcomes. This was calculated from the hazard ratio obtained from an unadjusted Cox proportional hazards regression model applied to the region-specific data for each outcome. The number and percentage of people suffering a severe hypoglycemic episode were also computed and analyzed using a logistic regression model. In each analysis, the regions were compared by adding a region by treatment interaction term to the relevant statistical model. All tests were considered significant if P < 0.05.
Although this study is concerned with comparisons of regions of domicile, issues of ethnicity are clearly related. Hence, in secondary analyses, baseline differences and treatment effects were also analyzed by self-reported ethnicity.
RESULTS
Comparisons between regions at baseline
At baseline, there were significant (P ≤ 0.02) differences in all the demographic and clinical variables considered a priori except history of microvascular disease (Table 1). On average, people in EMEs were the oldest, most often male, and had the shortest lead time since diabetes was diagnosed prior to recruitment. Sulphonylureas were the most common type of glucose-lowering therapy in all regions but were most commonly used in Asia (Table 2). Metformin was least likely to be taken in eastern Europe. Statins were far more commonly used in EMEs than in eastern Europe, which itself had a far higher prevalence of statin use than Asia. More patients in eastern Europe were taking blood pressure medications than in the other two regions. With the exception of nonaspirin antiplatelet agents and nonstatin lipid modifiers, for which numbers were small, in unadjusted analyses medication use was always significantly different between regions (P ≤ 0.05). Adjustment for age, sex, and duration of diabetes made little difference, except that insulin use then became marginally nonsignificant (P = 0.06) (Table 2). At the end of trial follow-up, medication use remained different across regions (Supplementary Table 1).
Table 1.
Baseline characteristics in the ADVANCE population by region
| Asia | EME | Eastern Europe | P | |
|---|---|---|---|---|
| n | 4,136 | 4,862 | 2,142 | |
| Age (years) | 64.5 ± 5.7 | 67.1 ± 6.4 | 65.3 ± 6.9 | <0.0001 |
| Women | 1,926 (46.6) | 1,614 (33.2) | 1,193 (55.7) | <0.0001 |
| Age diabetes first diagnosed (years) | 56.1 ± 8.3 | 59.6 ± 8.8 | 57.1 ± 8.6 | <0.0001 |
| Duration of diabetes (years) | 8 (3–12) | 6 (3–11) | 7 (3–12) | <0.0001 |
| History of macrovascular disease | 1,261 (30.5) | 1,558 (32.0) | 771 (36.0) | <0.0001 |
| Myocardial infarction | 264 (6.4) | 734 (15.1) | 336 (15.7) | <0.0001 |
| Stroke | 576 (13.9) | 275 (5.7) | 172 (8.0) | <0.0001 |
| Other | 3,335 (80.6) | 3,910 (80.4) | 1,666 (77.8) | 0.02 |
| History of microvascular disease | 496 (12.0) | 461 (9.5) | 198 (9.2) | 0.44 |
| Macroalbuminuria | 178 (4.3) | 151 (3.1) | 75 (3.5) | 0.80 |
| Microalbuminuria | 1,272 (31.5) | 1,079 (23.6) | 506 (25.0) | 0.19 |
| Eye disease | 340 (8.2) | 327 (6.7) | 128 (6.0) | 0.08 |
| Current smoking | 561 (13.6) | 627 (12.9) | 362 (16.9) | <0.0001 |
| BMI (kg/m2) | 25.3 ± 3.4 | 30.0 ± 5.3 | 30.6 ± 5.0 | <0.0001 |
| Waist circumference (cm) | 90.1 ± 9.4 | 103.6 ± 12.7 | 103.4 ± 11.8 | <0.0001 |
| Fasting blood glucose (mmol/L) | 8.55 ± 3.00 | 8.32 ± 2.50 | 8.76 ± 2.88 | <0.0001 |
| Nonstandardized HbA1c (%) | 7.75 ± 1.76 | 7.28 ± 1.22 | 7.60 ± 1.73 | <0.0001 |
| Standardized HbA1c (%)* | 7.72 ± 1.87 | 7.26 ± 1.30 | 7.52 ± 1.78 | <0.0001 |
| Systolic blood pressure (mmHg) | 141.1 ± 21.7 | 146.1 ± 20.7 | 150.0 ± 21.7 | <0.0001 |
| Diastolic blood pressure (mmHg) | 78.8 ± 10.9 | 80.5 ± 10.4 | 84.5 ± 11.1 | <0.0001 |
| Serum LDL cholesterol (mmol/L) | 3.18 ± 1.01 | 2.90 ± 1.00 | 3.50 ± 1.05 | <0.0001 |
| Serum HDL cholesterol (mmol/L) | 1.29 ± 0.37 | 1.22 ± 0.34 | 1.26 ± 0.33 | <0.0001 |
| Serum triglycerides (mmol/L) | 1.60 (1.10–2.30) | 1.62 (1.20–2.30) | 1.77 (1.27–2.50) | <0.0001 |
| Serum creatinine (μmol/L) | 80 (66–96) | 86 (74–100) | 85 (74–98) | <0.0001 |
| Urinary albumin-to-creatinine ratio | 18.0 (8.8–49.0) | 13.3 (6.2–32.7) | 12.6 (5.3–34.9) | <0.0001 |
Data are means ± SD, n (%), or quartile 2 (quartile 1–quartile 3) unless otherwise indicated. Triglycerides, HbA1c, creatinine, and albumin-to-creatinine ratio were tested after transforming to approximate normality. The P value is for a test of equality between the regions after adjustment for age, sex, and duration of diabetes.
*Standardized by analyses on common standard samples (5). (All other results quoted in this article are unstandardized.)
Table 2.
Drugs being taken at recruitment by region
| Asia | EME | Eastern Europe | P* | |
|---|---|---|---|---|
| n | 4,136 | 4,862 | 2,142 | |
| ≥1 oral glucose–lowering drugs | 3,926 (94.9) | 4,280 (88.0) | 1,922 (89.8) | <0.0001 |
| Gliclazide | 159 (3.8) | 290 (6.0) | 416 (19.4) | <0.0001 |
| Sulphonylureas | 3,048 (73.7) | 2,828 (58.2) | 1,215 (56.7) | <0.0001 |
| Metformin | 2,639 (63.8) | 3,106 (63.9) | 1,007 (47.0) | <0.0001 |
| Thiazolidinedione | 98 (2.4) | 300 (6.2) | 9 (0.4) | <0.0001 |
| Arcabose | 595 (14.4) | 201 (4.1) | 164 (7.7) | <0.0001 |
| Glinide | 40 (1.0) | 120 (2.5) | 27 (1.3) | <0.0001 |
| Insulin therapy | 66 (1.6) | 50 (1.0) | 43 (2.0) | 0.06 |
| No glucose-lowering drugs | 191 (4.6) | 579 (11.9) | 218 (10.2) | <0.0001 |
| Aspirin | 1,734 (41.9) | 2,220 (45.7) | 941 (43.9) | 0.03 |
| Other antiplatelet agents | 168 (4.1) | 236 (4.9) | 102 (4.8) | 0.40 |
| Statins | 458 (11.1) | 2,170 (44.6) | 518 (24.2) | <0.0001 |
| Other lipid-modifying drugs | 370 (8.9) | 376 (7.7) | 190 (8.9) | 0.07 |
| Any blood pressure–lowering drugs | 2,812 (68.0) | 3,617 (74.4) | 1,936 (90.4) | <0.0001 |
Data are n (%) unless otherwise indicated.
*Adjusted for age, sex, and duration of diabetes.
Comparisons between regions over follow-up
After differences in demographics and baseline clinical variables, including blood pressure and medication use, were allowed for, there were highly significant (P ≤ 0.001) differences in event rates during follow-up for all outcomes when all three regions were compared against each other (Table 3). The risks of macrovascular and microvascular disease, analyzed jointly and separately, were highest in Asia. In eastern Europe, macrovascular disease was significantly (P ≤ 0.05) more common but microvascular disease significantly less common than in EMEs. The risk of death during the study, including cardiovascular death, was highest in eastern Europe. Coronary events were significantly more likely to occur in EMEs than in Asia or eastern Europe but major cerebrovascular events were significantly less likely to occur in EMEs. Total renal events and new microalbuminuria were significantly less likely in EMEs than in Asia or eastern Europe. The risk of eye events differed significantly between EMEs and each of the other regions, being highest in Asia and lowest in eastern Europe. Similar differences were found when unadjusted analyses were made.
Table 3.
Hazard ratios for end points comparing regions (EMEs: base)
| Hazard ratio (95% CI) | P* | |
|---|---|---|
| Combined macro- plus microvascular disease | ||
| Asia | 1.33 (1.17–1.50) | <0.001 |
| Eastern Europe | 1.01 (0.88–1.16) | 0.87 |
| Macrovascular disease | ||
| Asia | 1.31 (1.11–1.56) | 0.002 |
| Eastern Europe | 1.19 (1.00–1.42) | 0.05 |
| Microvascular disease | ||
| Asia | 1.36 (1.15–1.60) | <0.001 |
| Eastern Europe | 0.77 (0.62–0.94) | 0.01 |
| All deaths | ||
| Asia | 1.08 (0.90–1.30) | 0.38 |
| Eastern Europe | 1.43 (1.20–1.71) | <0.001 |
| Cardiovascular death | ||
| Asia | 1.19 (0.92–1.54) | 0.18 |
| Eastern Europe | 1.64 (1.29–2.09) | <0.001 |
| Total coronary events | ||
| Asia | 0.72 (0.60–0.85) | <0.001 |
| Eastern Europe | 0.79 (0.67–0.95) | 0.01 |
| Major coronary events | ||
| Asia | 0.84 (0.66–1.05) | 0.13 |
| Eastern Europe | 1.11 (0.88–1.38) | 0.38 |
| Total cerebrovascular events | ||
| Asia | 2.02 (1.62–2.51) | <0.001 |
| Eastern Europe | 1.23 (0.96–1.57) | 0.10 |
| Major cerebrovascular events | ||
| Asia | 2.40 (1.84–3.14) | <0.001 |
| Eastern Europe | 1.52 (1.14–2.03) | 0.005 |
| Total renal events | ||
| Asia | 1.73 (1.57–1.91) | <0.001 |
| Eastern Europe | 1.29 (1.16–1.43) | <0.001 |
| New or worsening nephropathy | ||
| Asia | 1.42 (1.11–1.82) | 0.005 |
| Eastern Europe | 0.80 (0.60–1.08) | 0.15 |
| New microalbuminuria | ||
| Asia | 1.97 (1.77–2.19) | <0.001 |
| Eastern Europe | 1.36 (1.21–1.53) | <0.001 |
| Total eye events | ||
| Asia | 1.21 (1.13–1.30) | <0.001 |
| Eastern Europe | 0.72 (0.66–0.79) | <0.001 |
| New or worsening retinopathy | ||
| Asia | 1.35 (1.09–1.67) | 0.006 |
| Eastern Europe | 0.74 (0.56–0.97) | 0.03 |
| Visual deterioration | ||
| Asia | 1.22 (1.14–1.31) | <0.001 |
| Eastern Europe | 0.72 (0.66–0.79) | <0.001 |
Hazard ratios adjusted for age, sex, duration of diabetes, histories of macrovascular and microvascular disease, current smoking, waist circumference, systolic and diastolic blood pressure, total and HDL cholesterol, triglycerides, HbA1c, creatinine, albumin-to-creatinine ratio, any oral hypoglycemic drug, aspirin plus other antiplatelet agents, statins plus other lipid-modifying drugs, any blood pressure–lowering drug, and randomized treatment allocations (n = 11,140).
*Comparison with EMEs. (All three-way comparisons have P < 0.001.)
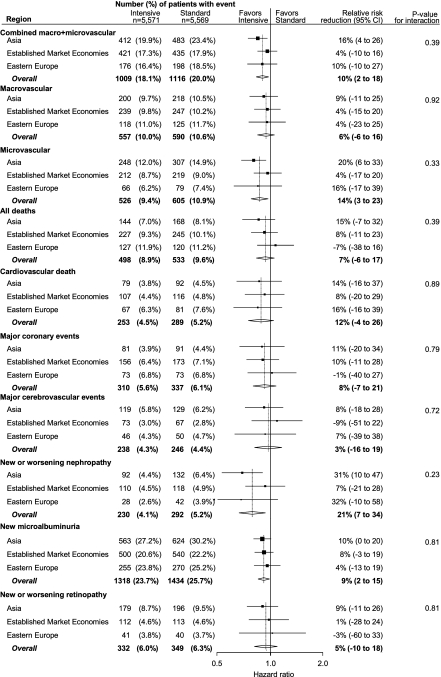
The mean (95% CI) differences in HbA1c during the trial, intensive minus standard control, were −0.78% (−0.83 to −0.74) for Asia, −0.55% (−0.59 to −0.50) for EMEs, and −0.71% (−0.78 to −0.64) for eastern Europe (P < 0.0001). Adjustment for age, sex, and duration of diabetes did not change any of these estimates or CIs by >0.01. The effects of intensive glycemic control tended to be greater in Asia than elsewhere for both macro- and microvascular events (Fig. 1), although any differences are explainable by chance (P ≥ 0.23). There was also no evidence of any differences in the relative number of people (intensive vs. standard) suffering a severe hypoglycemic event between the regions (P = 0.66). In Asia, 30 (1.5%) in the standard group and 64 (3.1%) in the intensive group had a severe hypoglycemic event; corresponding numbers in EMEs were 42 (1.7%) and 74 (3.0%) and in eastern Europe were 9 (0.8%) and 16 (1.2%).
Figure 1.
Relative risk reduction, intensive vs. standard glycemic control, for primary outcomes and main secondary outcomes in ADVANCE. The bars show 95% CIs, and the diamonds show the overall estimate, also illustrated by the vertical line. The width of the diamonds shows the 95% CI for the overall result.
Ethnicity
Of participants in Asia, 99.7% reported themselves to be of Asian ethnicity while 99.8% of those in eastern Europe and 93.4% of those in EMEs reported themselves to be of “Caucasian/European” ethnicity (Supplementary Table 2). Consequently, results were virtually the same for Asians as for residents of Asia and results for Caucasian/Europeans were essentially a weighted average of those for eastern Europe and EMEs in the regional analyses (Supplementary Tables 3 and 4).
CONCLUSIONS
ADVANCE was a global study of patients with diabetes at moderate to high risk of future vascular disease. Subjects in ADVANCE differed significantly between geographical regions in age, sex, and other baseline characteristics, as well as in the medications taken. There were also important differences between the regions in the rates at which major clinical outcomes occurred during the trial. The risk of coronary events was higher, but the risk of cerebrovascular events lower, in EMEs than in the other regions. Adverse renal and ocular microvascular events were more likely in Asia than in EMEs. Eastern European patients were more likely than their EME counterparts to experience new albuminuria and significantly less likely to report an eye event. Eastern Europeans had by far the highest rates of death. These event rates differed even after accounting for differences in baseline clinical characteristics and medication use across the regions.
Intensive treatment, as expected, controlled HbA1c better than standard care in all regions, but there was a small but significant difference between the regions, such that intensive care had less effect in EMEs than elsewhere. To some extent, this was due to the differential levels of HbA1c across regions at baseline. The relatively smaller reduction in glycated hemoglobin probably contributed to the lower risk reduction seen for the effect of randomized treatment on the composite primary outcome in EMEs (4% relative risk reduction) versus both eastern Europe (16%) and Asia (10%). Despite this, there were no significant differences between regions in the effect of intensive treatment for any of the hard outcomes analyzed.
As far as we are aware, this is the first comparison of international regional effects within a randomized clinical trial of glycemic control. The strengths of our study are the large numbers, wide geographical coverage, and standardized procedures. Even so, we do not have the numbers to produce reliable estimates for individual countries or any subjects from the Americas south of Canada or from Africa.
In conclusion, we have shown that the benefits of intensive glycemic control on vascular disease and the lack of harm in relation to mortality, which were manifest with the gliclazide MR-based regimen in ADVANCE overall, are shared between the three major regions from which subjects were recruited. This is so despite differences in health care systems, clinical practice, and use of medications (including statins and blood pressure–lowering medication). The methods of glycemic control used in ADVANCE can thus be safely recommended for Caucasian and Asian patients with type 2 diabetes in all three regions studied who are at moderate to high risk of cardiovascular disease.
Supplementary Material
Acknowledgments
ADVANCE was funded by the National Health and Medical Research Council of Australia.
ADVANCE was also funded by Servier. J.C. holds research grants from Servier as a principal investigator for ADVANCE. M.W., A.P., S.Z., L.L., C.P., A.J., S.H., B.N., and J.C. have received lecturing fees from Servier. No other potential conflicts of interest relevant to this article were reported.
M.W. researched data and wrote the manuscript. A.P., L.L., C.P., N.P., A.J., N.T., P.J., S.H., and B.N. researched data and contributed to discussion. S.Z. and J.C. researched data and edited the manuscript.
Footnotes
Clinical trial reg. no. NCT00145925, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0755/-/DC1.
The sponsors had no role in the design of the study, data collection, data analysis, data interpretation, or writing of the manuscript. The Management Committee, whose membership did not include any sponsor representatives, had final responsibility for the decision to submit the manuscript for publication.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. Diabetes atlas 2010 [article online], 2011. Available from http://www.diabetesatlas.org/ Accessed 29 September 2011
- 3.Burgers JS, Bailey JV, Klazinga NS, Van Der Bij AK, Grol R, Feder G; AGREE COLLABORATION Inside guidelines: comparative analysis of recommendations and evidence in diabetes guidelines from 13 countries. Diabetes Care 2002;25:1933–1939 [DOI] [PubMed] [Google Scholar]
- 4.Patel A, MacMahon S, Chalmers J, et al. ; ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 5.ADVANCE Management Committee Rationale and design of the ADVANCE study: a randomised trial of blood pressure lowering and intensive glucose control in high-risk individuals with type 2 diabetes mellitus. Action in Diabetes and Vascular Disease: PreterAx and DiamicroN Modified-Release Controlled Evaluation. J Hypertens Suppl 2001;19:S21–S28 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.