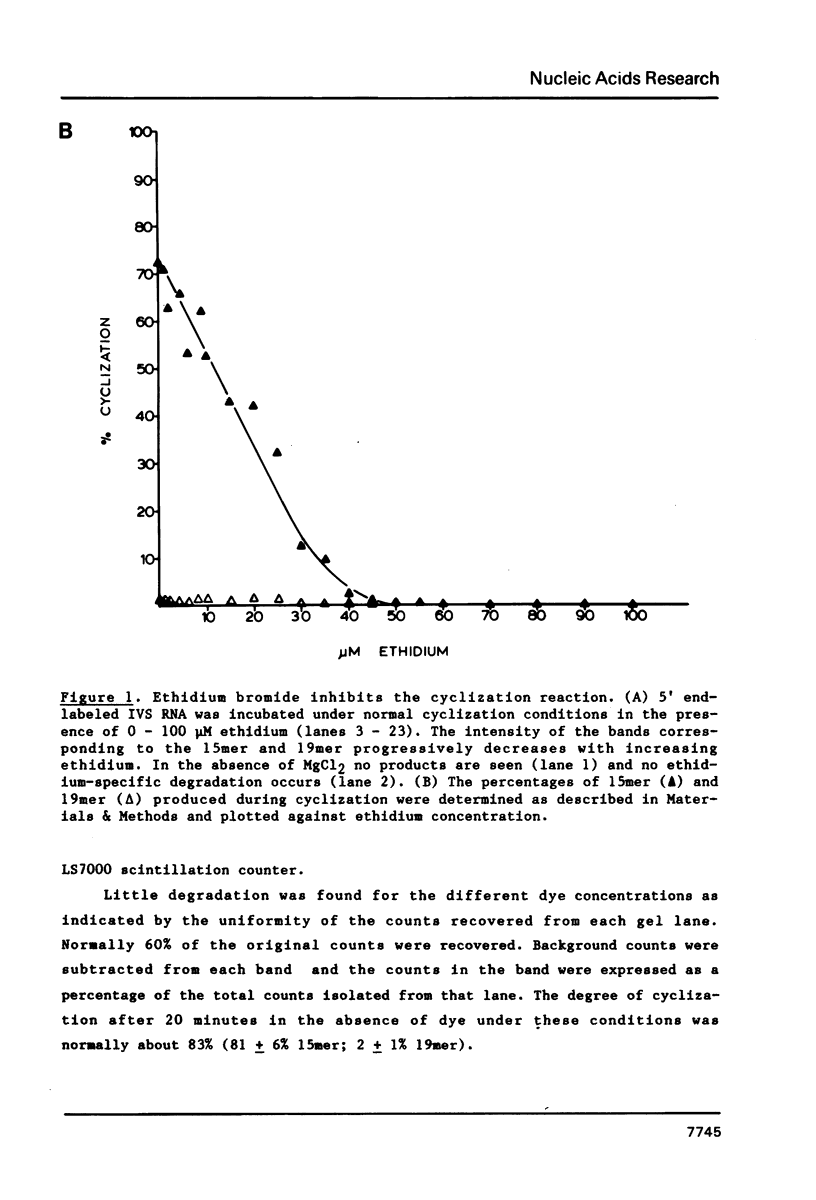
Abstract
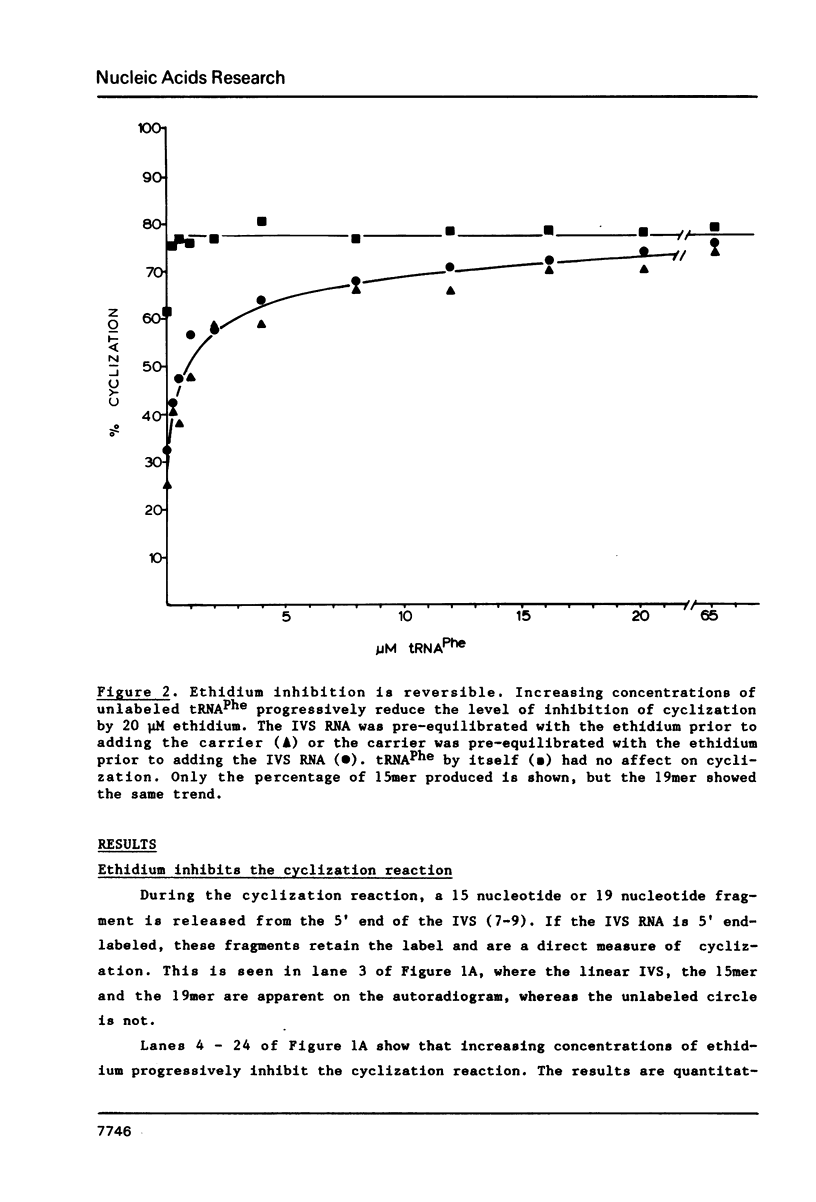
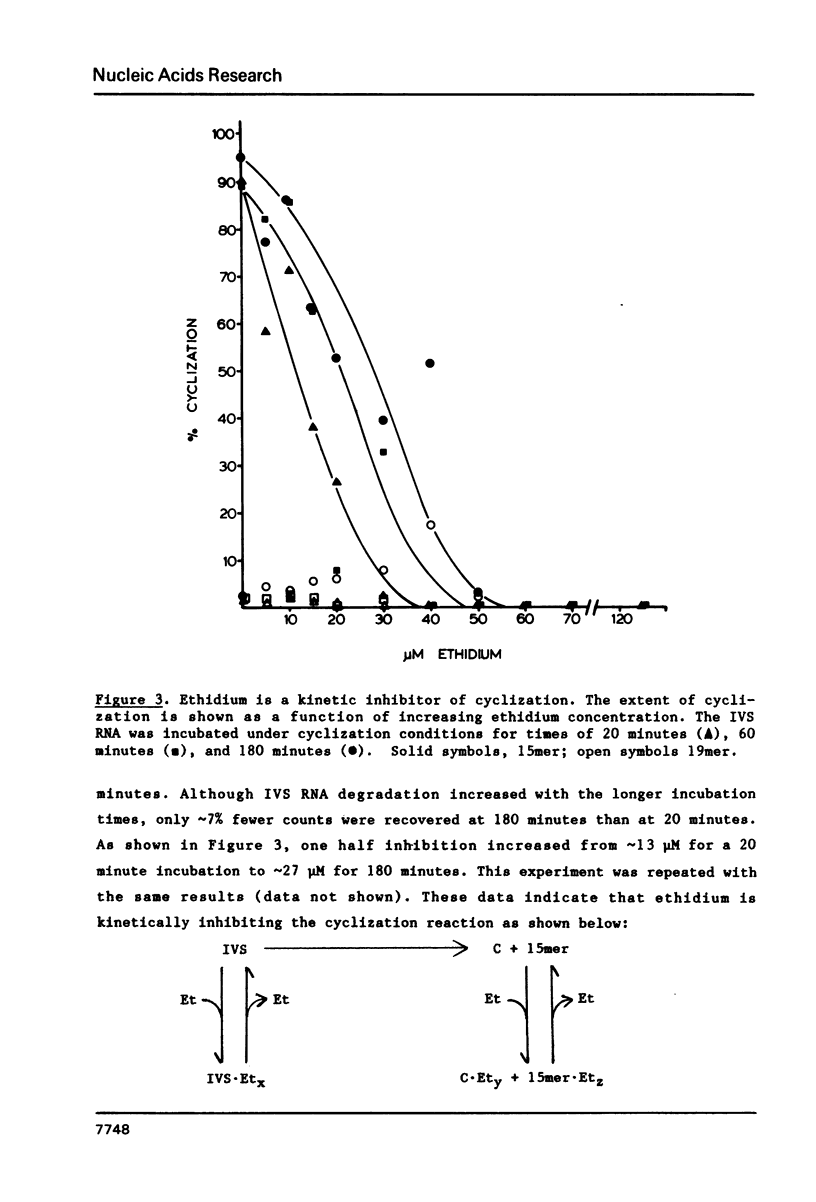
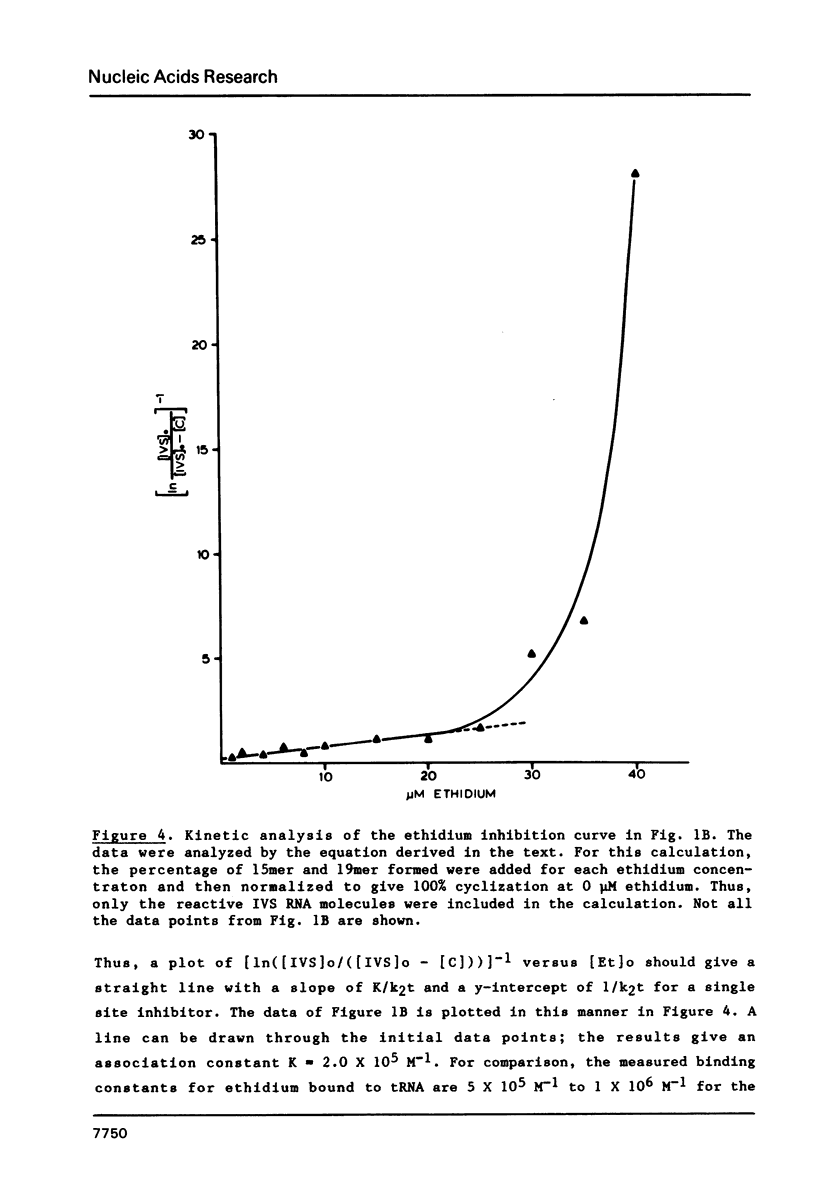
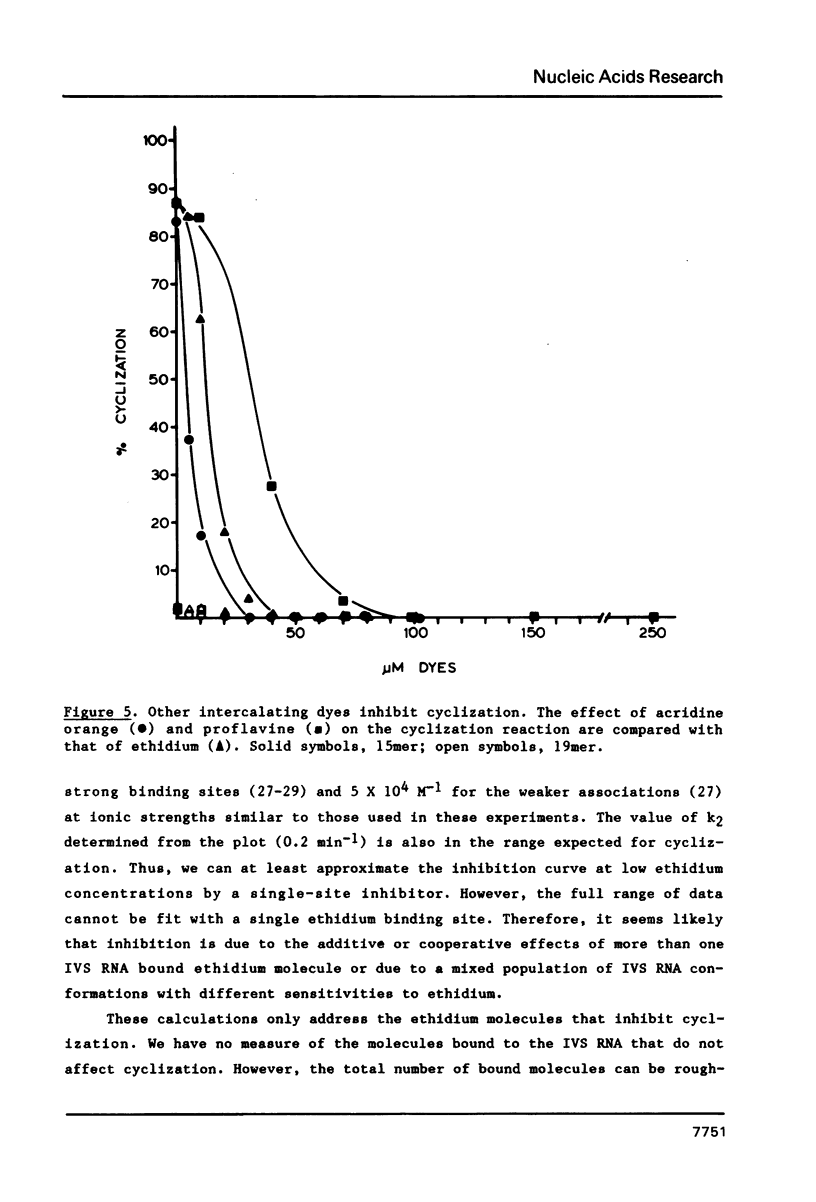
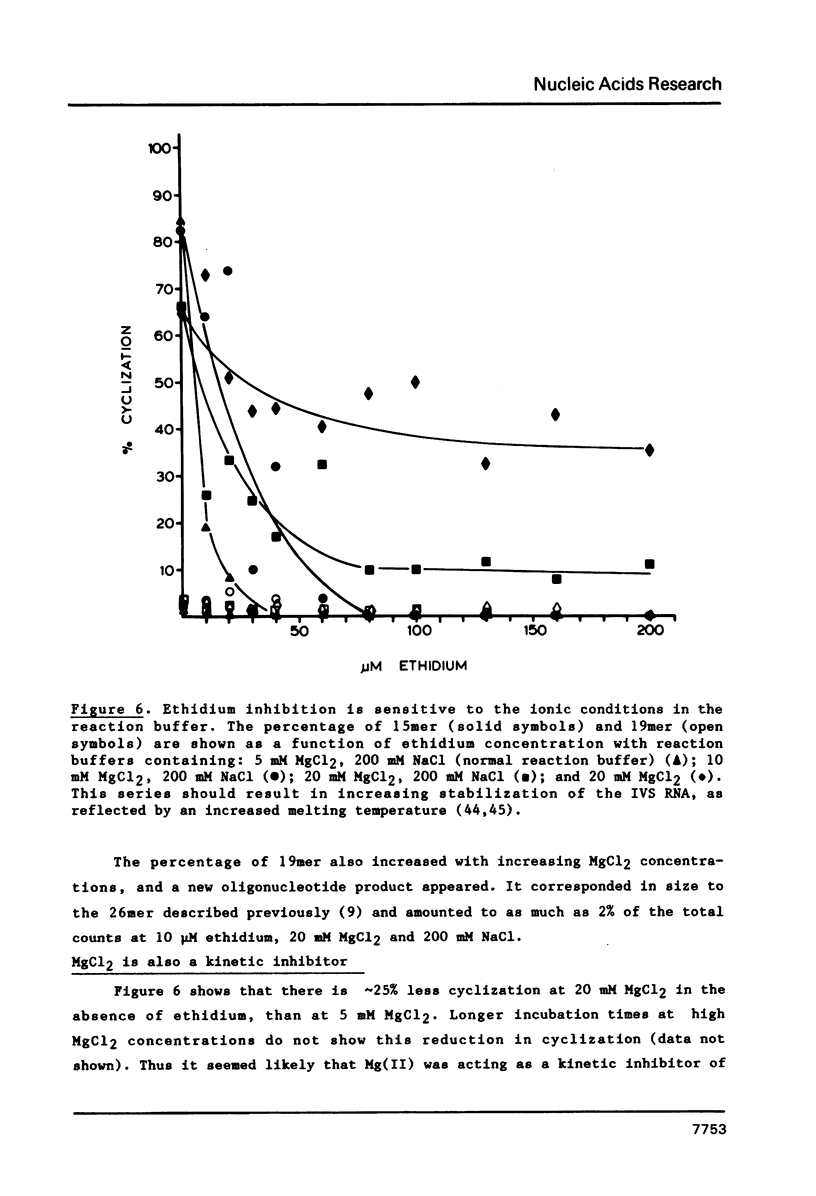
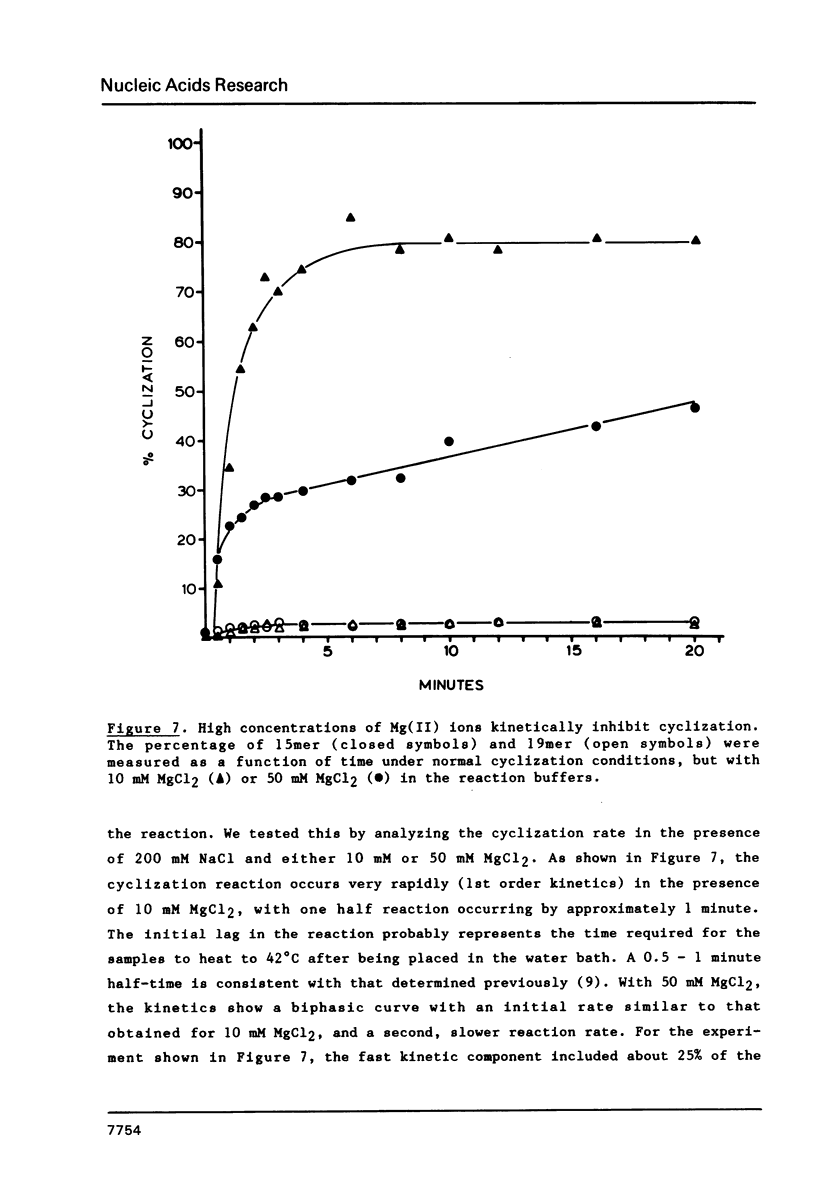
The intervening sequence (IVS) excised from the pre-rRNA of Tetrahymena undergoes a self-catalyzed cleavage-ligation reaction to form a covalently closed circular RNA. This cyclization reaction is kinetically inhibited by ethidium bromide (50% inhibition at 22 +/- 14 microM, greater than 99% inhibition at 53 +/- 16 microM for a 20 minute reaction). The dye does not alter the sites of the cyclization reaction, but it does increase the relative amount of reaction at a minor site 19 nucleotides from the 5' end of the IVS. The reversibility of the inhibition and the relative inhibitory strength of acridine orange, ethidium and proflavine suggest that inhibition is due to intercalation of the dye in functionally important secondary or tertiary structures of the IVS. The concentration of dye required to inhibit cyclization is much higher than expected from the known binding constants of such dyes to tRNA. At high Mg2+ to Na+ ratios, conditions which should stabilize RNA structure, a subpopulation of the IVS RNA molecules is resistant to ethidium inhibition, even at 200 microM ethidium. These data are interpreted as reflecting two conformational isomers of the IVS that differ in their reactivity and in their sensitivity to dye binding.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman H. M., Young P. R. The interaction of intercalating drugs with nucleic acids. Annu Rev Biophys Bioeng. 1981;10:87–114. doi: 10.1146/annurev.bb.10.060181.000511. [DOI] [PubMed] [Google Scholar]
- Blake A., Peacocke A. R. The interaction of aminocridines with nucleic acids. Biopolymers. 1968;6(9):1225–1253. doi: 10.1002/bip.1968.360060902. [DOI] [PubMed] [Google Scholar]
- Bresloff J. L., Crothers D. M. Equilibrium studies of ethidium--polynucleotide interactions. Biochemistry. 1981 Jun 9;20(12):3547–3553. doi: 10.1021/bi00515a038. [DOI] [PubMed] [Google Scholar]
- Carin M., Jensen B. F., Jentsch K. D., Leer J. C., Nielsen O. F., Westergaard O. In vitro splicing of the ribosomal RNA precursor in isolated nucleoli from Tetrahymena. Nucleic Acids Res. 1980 Dec 11;8(23):5551–5566. doi: 10.1093/nar/8.23.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R. RNA splicing: three themes with variations. Cell. 1983 Oct;34(3):713–716. doi: 10.1016/0092-8674(83)90527-5. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Rio D. C. Localization of transcribed regions on extrachromosomal ribosomal RNA genes of Tetrahymena thermophila by R-loop mapping. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5051–5055. doi: 10.1073/pnas.76.10.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Tanner N. K., Tinoco I., Jr, Weir B. R., Zuker M., Perlman P. S. Secondary structure of the Tetrahymena ribosomal RNA intervening sequence: structural homology with fungal mitochondrial intervening sequences. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3903–3907. doi: 10.1073/pnas.80.13.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Zaug A. J., Grabowski P. J. In vitro splicing of the ribosomal RNA precursor of Tetrahymena: involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell. 1981 Dec;27(3 Pt 2):487–496. doi: 10.1016/0092-8674(81)90390-1. [DOI] [PubMed] [Google Scholar]
- Davies R. W., Waring R. B., Ray J. A., Brown T. A., Scazzocchio C. Making ends meet: a model for RNA splicing in fungal mitochondria. Nature. 1982 Dec 23;300(5894):719–724. doi: 10.1038/300719a0. [DOI] [PubMed] [Google Scholar]
- Din N., Engberg J., Kaffenberger W., Eckert W. A. The intervening sequence in the 26S rRNA coding region of T. thermophila is transcribed within the largest stable precursor for rRNA. Cell. 1979 Oct;18(2):525–532. doi: 10.1016/0092-8674(79)90069-2. [DOI] [PubMed] [Google Scholar]
- Garriga G., Lambowitz A. M. RNA splicing in Neurospora mitochondria. The large rRNA intron contains a noncoded, 5'-terminal guanosine residue. J Biol Chem. 1983 Dec 25;258(24):14745–14748. [PubMed] [Google Scholar]
- Garriga G., Lambowitz A. M. RNA splicing in neurospora mitochondria: self-splicing of a mitochondrial intron in vitro. Cell. 1984 Dec;39(3 Pt 2):631–641. doi: 10.1016/0092-8674(84)90470-7. [DOI] [PubMed] [Google Scholar]
- Inoue T., Cech T. R. Secondary structure of the circular form of the Tetrahymena rRNA intervening sequence: a technique for RNA structure analysis using chemical probes and reverse transcriptase. Proc Natl Acad Sci U S A. 1985 Feb;82(3):648–652. doi: 10.1073/pnas.82.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger K., Grabowski P. J., Zaug A. J., Sands J., Gottschling D. E., Cech T. R. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982 Nov;31(1):147–157. doi: 10.1016/0092-8674(82)90414-7. [DOI] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Tinoco I., Jr Mutagen--oligonucleotide complexes with a bulged base as models for frameshift mutation. Nature. 1978 Aug 10;274(5671):609–610. doi: 10.1038/274609a0. [DOI] [PubMed] [Google Scholar]
- Lehrman E. A., Crothers D. M. An ethidium-induced double helix of poly(dA)-poly(rU). Nucleic Acids Res. 1977;4(5):1381–1392. doi: 10.1093/nar/4.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman M., Rubin J., Sundaralingam M. Nonintercalative binding of ethidium bromide to nucleic acids: crystal structure of an ethidium--tRNA molecular complex. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4821–4825. doi: 10.1073/pnas.74.11.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Michel F., Dujon B. Conservation of RNA secondary structures in two intron families including mitochondrial-, chloroplast- and nuclear-encoded members. EMBO J. 1983;2(1):33–38. doi: 10.1002/j.1460-2075.1983.tb01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F., Jacquier A., Dujon B. Comparison of fungal mitochondrial introns reveals extensive homologies in RNA secondary structure. Biochimie. 1982 Oct;64(10):867–881. doi: 10.1016/s0300-9084(82)80349-0. [DOI] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Interactions of heteroaromatic compounds with nucleic acids. 1. The influence of heteroatoms and polarizability on the base specificity of intercalating ligands. Eur J Biochem. 1975 May;54(1):267–277. doi: 10.1111/j.1432-1033.1975.tb04137.x. [DOI] [PubMed] [Google Scholar]
- Nelson J. W., Tinoco I., Jr Intercalation of ethidium ion into DNA and RNA oligonucleotides. Biopolymers. 1984 Feb;23(2):213–233. doi: 10.1002/bip.360230205. [DOI] [PubMed] [Google Scholar]
- Nielsen O. F., Carin M., Westergaard O. Studies on transcription termination and splicing of the rRNA precursor in vivo in the presence of proflavine. Nucleic Acids Res. 1984 Jan 25;12(2):873–886. doi: 10.1093/nar/12.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M., Baehr W., Holbrook J. J. Ethidium bromide as a cooperative effector of a DNA structure. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3805–3809. doi: 10.1073/pnas.69.12.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. V., Kieft G. L., Kent J. R., Sievers E. L., Cech T. R. Sequence requirements for self-splicing of the Tetrahymena thermophila pre-ribosomal RNA. Nucleic Acids Res. 1985 Mar 25;13(6):1871–1889. doi: 10.1093/nar/13.6.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record M. T., Jr, Anderson C. F., Lohman T. M. Thermodynamic analysis of ion effects on the binding and conformational equilibria of proteins and nucleic acids: the roles of ion association or release, screening, and ion effects on water activity. Q Rev Biophys. 1978 May;11(2):103–178. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]
- Sakai T. T., Torget R., I J., Freda C. E., Cohen S. S. The binding of polyamines and of ethidium bromide to tRNA. Nucleic Acids Res. 1975 Jul;2(7):1005–1022. doi: 10.1093/nar/2.7.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell H. M., Sakore T. D., Jain S. C., Banerjee A., Bhandary K. K., Reddy B. S., Lozansky E. D. beta-kinked DNA--a structure that gives rise to drug intercalation and DNA breathing--and its wider significance in determining the premelting and melting behavior of DNA. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):293–314. doi: 10.1101/sqb.1983.047.01.035. [DOI] [PubMed] [Google Scholar]
- Sullivan F. X., Cech T. R. Reversibility of cyclization of the Tetrahymena rRNA intervening sequence: implication for the mechanism of splice site choice. Cell. 1985 Sep;42(2):639–648. doi: 10.1016/0092-8674(85)90121-7. [DOI] [PubMed] [Google Scholar]
- Tabak H. F., Van der Horst G., Osinga K. A., Arnberg A. C. Splicing of large ribosomal precursor RNA and processing of intron RNA in yeast mitochondria. Cell. 1984 Dec;39(3 Pt 2):623–629. doi: 10.1016/0092-8674(84)90469-0. [DOI] [PubMed] [Google Scholar]
- Urbanke C., Römer R., Maass G. The binding of ethidium bromide to different conformations of tRNA. Unfolding of tertiary structure. Eur J Biochem. 1973 Mar 15;33(3):511–516. doi: 10.1111/j.1432-1033.1973.tb02710.x. [DOI] [PubMed] [Google Scholar]
- Von Hippel P. H., McGhee J. D. DNA-protein interactions. Annu Rev Biochem. 1972;41(10):231–300. doi: 10.1146/annurev.bi.41.070172.001311. [DOI] [PubMed] [Google Scholar]
- Waring M. J. Complex formation between ethidium bromide and nucleic acids. J Mol Biol. 1965 Aug;13(1):269–282. doi: 10.1016/s0022-2836(65)80096-1. [DOI] [PubMed] [Google Scholar]
- Waring M. J. DNA modification and cancer. Annu Rev Biochem. 1981;50:159–192. doi: 10.1146/annurev.bi.50.070181.001111. [DOI] [PubMed] [Google Scholar]
- Waring M. J. Stablilzation of two-standard ribohomopolymer helices and destabilzation of a three-stranded helix by ethidium bromide. Biochem J. 1974 Nov;143(2):483–486. doi: 10.1042/bj1430483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring R. B., Scazzocchio C., Brown T. A., Davies R. W. Close relationship between certain nuclear and mitochondrial introns. Implications for the mechanism of RNA splicing. J Mol Biol. 1983 Jul 5;167(3):595–605. doi: 10.1016/s0022-2836(83)80100-4. [DOI] [PubMed] [Google Scholar]
- Wells B. D., Cantor C. R. A strong ethidium binding site in the acceptor stem of most or all transfer RNAs. Nucleic Acids Res. 1977;4(5):1667–1680. doi: 10.1093/nar/4.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaug A. J., Grabowski P. J., Cech T. R. Autocatalytic cyclization of an excised intervening sequence RNA is a cleavage-ligation reaction. Nature. 1983 Feb 17;301(5901):578–583. doi: 10.1038/301578a0. [DOI] [PubMed] [Google Scholar]
- Zaug A. J., Kent J. R., Cech T. R. A labile phosphodiester bond at the ligation junction in a circular intervening sequence RNA. Science. 1984 May 11;224(4649):574–578. doi: 10.1126/science.6200938. [DOI] [PubMed] [Google Scholar]
- van der Horst G., Tabak H. F. Self-splicing of yeast mitochondrial ribosomal and messenger RNA precursors. Cell. 1985 Apr;40(4):759–766. doi: 10.1016/0092-8674(85)90335-6. [DOI] [PubMed] [Google Scholar]