Abstract
OBJECTIVE
To study the effects of lifestyle intervention on gestational weight gain (GWG) and obstetric outcomes.
RESEARCH DESIGN AND METHODS
The LiP (Lifestyle in Pregnancy) study was a randomized controlled trial in 360 obese women allocated in early pregnancy to lifestyle intervention or control. The intervention program included dietary guidance, free membership in fitness centers, physical training, and personal coaching.
RESULTS
A total of 360 obese pregnant women were included, and 304 (84%) were followed up until delivery. The intervention group had a significantly lower median (range) GWG compared with the control group of 7.0 (4.7–10.6) vs. 8.6 kg (5.7–11.5; P = 0.01). The Institute of Medicine (IOM) recommendations on GWG were exceeded in 35.4% of women in the intervention group compared with 46.6% in the control group (P = 0.058). Overall, the obstetric outcomes between the two groups were not significantly different.
CONCLUSIONS
Lifestyle intervention in pregnancy resulted in limited GWG in obese pregnant women. Overall obstetric outcomes were similar in the two groups. Lifestyle intervention resulted in a higher adherence to the IOM weight gain recommendations; however, a significant number of women still exceeded the upper threshold.
Maternal obesity has become highly prevalent worldwide and is associated with adverse outcomes for mothers and infants (1). As one of the most common risk factors, maternal obesity remains a significant obstetric challenge, and 12.2% of Danish pregnant women are obese, with a BMI of ≥30 kg/m2 (2). Several studies have shown that maternal obesity is related to a number of adverse outcomes, including miscarriage, gestational diabetes mellitus (GDM), preeclampsia, stillbirth, macrosomia, and cesarean section (3–5). Maternal obesity leads to obesity in infancy and among young adults, and the long-term consequences can be seen in observational studies.
Prepregnancy BMI and gestational weight gain (GWG) are strong predictors for high birth weight and obesity in infancy and adulthood (6,7). High GWG in the first part of pregnancy is especially associated with obesity in later life (8). In addition, interpregnancy weight gain increases the risk of GDM in future pregnancies (9). The increased risk of GDM has short- and long-term consequences for the mother and her offspring and increases the development of diabetes in future generations (10,11). The management of obesity in pregnancy includes the recommendation of appropriate GWG. In 2009, the U.S. Institute of Medicine (IOM) recommended a GWG of 5–9 kg in obese women (BMI ≥30 kg/m2) (12). Some observational studies have suggested that a GWG of <5 kg may reduce the number of complications (13,14) without increasing the risk of adverse outcomes.
The evidence for clinical effects of lifestyle intervention in pregnancy is conflicting. Only a small number of studies have addressed the issues of limiting GWG exclusively in obese women (15–17). Other interventional studies included normal weight and overweight/obese women, without success in limiting GWG among the obese (18–20). None of these interventions significantly improved obstetric outcomes, but the studies were not powered to address these. The exception was the Australian study by Quinlivan et al. (20), who succeeded in limiting GWG and found a lower incidence of GDM in a randomized trial of overweight/obese women.
Accordingly, our aim with this randomized trial was to study the effects of lifestyle intervention on GWG and obstetric and neonatal outcomes in a large group of obese pregnant women.
RESEARCH DESIGN AND METHODS
The LiP (Lifestyle in Pregnancy) study was a randomized controlled trial running from October 2007 to October 2010 in two university hospitals in Denmark: Odense and Aarhus University Hospital, serving a population with 8,500 combined deliveries annually. The project was approved by the local ethics committee of the Region of Southern Denmark (S-20070058) and registered at clinicaltrials.gov as NCT00530439.
Women aged 18–40 years were recruited and included at 10–14 weeks’ gestation after referral to the Department of Gynecology and Obstetrics. The inclusion criteria required a BMI of 30–45 kg/m2 as calculated from the prepregnancy weight or first measured weight in pregnancy.
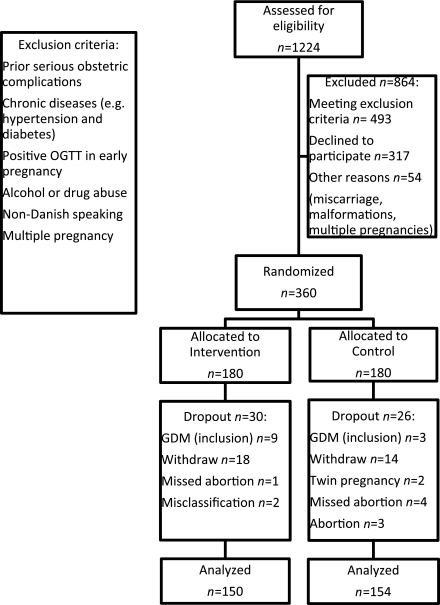
In the inclusion period, 1,224 pregnant women with BMI 30–45 kg/m2 were referred to the obstetric departments and assessed for eligibility, and 547 were excluded due to our exclusion criteria (Fig. 1). The main reasons for exclusion were chronic medical disorders (n = 137) and prior serious obstetric complications (n = 101). Another 317 declined participation, resulting in 360 women who were admitted to the study. Among the 360 women included, 56 dropped out for reasons shown in Fig. 1. The study group therefore comprised 304 women who completed the study: 150 in the intervention group and 154 in the control group. GWG and other recordings made at 35 weeks’ gestation were missing in 12 of the 304 women because they failed to attend the last appointment in pregnancy.
Figure 1.
Flowchart shows selection and participation in the LiP study. OGTT, oral glucose tolerance test.
After receiving written and oral information and giving written consent, the 360 participants were randomized 1:1 by computer-generated numbers in closed envelopes to receive the intervention or to the control group. Stratified randomization was applied to smokers to ensure equal distribution between the groups. Both groups were monitored throughout the study by a project physician or midwife. A baseline questionnaire provided information about previous pregnancies, smoking, demographic information, dietary habits, and physical activities.
Weight was measured at all antenatal visits using the same medical scales (model 704, Seca, Hamburg, Germany), with the women wearing light indoor clothes and no shoes. All women had the same follow-up program in pregnancy, including repeated monitoring of blood pressure and two additional ultrasound examinations in the third trimester. Blood pressure was measured with an electronic instrument (model Boso-medicus control, CMA Medico, Skaevinge, Denmark) under standard conditions and using a large cuff when needed.
A short fitness test (the Danish Steptest) was performed at inclusion and at 34–36 weeks of gestation. The features of this test are that it is simple to conduct and requires minimal equipment: a bench/platform and an online computer program. A fitness score is calculated from minutes to exhaustion, body weight, and height of the bench (21). This result is categorized according to age as very low to very high. All women were tested for GDM with a 75-g, 2-h oral glucose tolerance test on three occasions during pregnancy, at 12–14, 28–30, and 34–36 weeks’ gestation.
The intervention consisted of two major components: dietary counseling and physical activity. Dietary counseling was performed by trained dietitians on four separate occasions, at 15, 20, 28, and 35 weeks’ gestation. The aim was to limit GWG to 5 kg. The counseling included dietary advice based on the official Danish recommendations. Energy requirements for each participant were individually estimated according to weight and level of activity. Women in the intervention group were encouraged to be moderately physically active 30–60 min daily and were equipped with a pedometer to motivate and improve daily activity. Women in this group also had free full-time membership in a fitness center for 6 months, where they had closed training classes with physiotherapists for 1 h each week. Training consisted of aerobic (low-step), training with light weights and elastic bands, and balance exercises. After physical training, the women were grouped 4–6 times in pregnancy with the physiotherapist using coaching-inspired methods for improving participants’ integration of physical activities in pregnancy and daily life.
The women in the control group received the same initial information about the purpose and content of the study, including access to a website with advice about dietary habits and physical activities in pregnancy, but no additional intervention.
Outcomes
We assessed a number of primary obstetric and neonatal outcomes: GWG, preeclampsia, pregnancy-induced hypertension (PIH), GDM, cesarean section, macrosomia/large for gestational age (LGA), and admission to neonatal intensive care unit (NICU). GWG was defined as weight at the 35-week visit minus measured weight at inclusion. Preeclampsia was defined as proteinuria and persistently elevated blood pressure (>140/90 mmHg) on more than one occasion. PIH was diagnosed if the blood pressure met the previously mentioned criteria but without the presence of proteinuria. GDM was diagnosed if the 2-h oral glucose tolerance test capillary blood glucose result was ≥9 mmol/L. A record of cesarean section included planned and emergency cesareans. Macrosomia was defined as birth weight of ≥4,000 g, and LGA was defined as ≥90th percentile for a Danish standard population. LGA was calculated from the Marsál formula, which includes fetal sex, birth weight, and GA (22).
Statistical analysis
All analyses were performed with STATA 10.0 software (StataCorp, College Station, TX). The underlying basis for power calculations were from the Danish observational study on GWG among 481 obese women (13), where there was a significant difference in the clinical outcomes among four GWG categories. Because the frequencies of each adverse outcome were small, we chose a combined end point for our intervention study. Each of the five outcomes—GDM, preeclampsia/hypertension, emergency cesarean section, LGA, and NICU admission—was given 1 point, and thus, each participant could obtain a combined score of between 0 and 5 points. To detect a significant difference in this score (Wilcoxon rank-sum test, 85% power), 180 women were required in each arm (P < 0.05). Power calculations were based on the assumption that women in the intervention group behaved like women from the observational study who gained <5 kg and that the control group behaved like the whole group of 481 obese women. Differences between the groups were analyzed with the χ2 test for categoric variables. The Student t test was used for continuous variables, provided there was a normal distribution; otherwise, the Mann-Whitney U test was used. A significance level of 0.05 (two-sided) was chosen. Logistic regression models were used to estimate smoking as a confounder for GWG and birth weight.
RESULTS
The intervention and control groups did not differ significantly with respect to maternal baseline characteristics (Table 1). The drop-out group was characterized by being older and having a higher percentage with BMI ≥40 kg/m2 and a higher percentage of smokers compared with the completing groups. These differences were not statistically significant because of the small numbers. Information on weight gain and other factors measured at GA 35 was missing in 12 of the 304 women because they failed to attend the last follow-up in pregnancy, 9 because of preterm delivery, and they were equally distributed within the randomization groups.
Table 1.
Maternal baseline characteristics in 360 obese pregnant women according to randomization and dropout
| Intervention | Control | Dropout | |
|---|---|---|---|
| Characteristic | n = 150 | n = 154 | n = 56 |
| Maternal | |||
| Age (years) | 29 (27–32) | 29 (26–31) | 30 (27–33) |
| BMI (kg/m2) | 33.4 (31.7–36.5) | 33.3 (31.7–36.9) | 34.4 (30.8–35.9) |
| Obesity class | |||
| I (BMI 30–34.9 kg/m2) | 95 (63.3) | 102 (66.2) | 34 (60.7) |
| II (BMI 35–39.9 kg/m2) | 42 (28) | 45 (29.2) | 14 (25) |
| III (BMI 40–45 kg/m2) | 13 (8.7) | 7 (4.6) | 8 (14.3) |
| Blood pressure (mmHg) | |||
| Systolic | 124 (118–132) | 122 (117–130) | 123 (114–130) |
| Diastolic | 80 (76–84) | 81 (76–86) | 80 (76–85) |
| Smokers | 11 (7.3) | 18 (11.7) | 10 (17.7) |
| Primiparous | 79 (52.7) | 84 (54.6) | 22 (39.3) |
| Caucasians | 150 (100) | 154 (100) | 54 (96.4) |
| School ≥12 years | 111 (74) | 100 (64.9) | 30 (53.6) |
| Further education ≥3 years | 75 (50) | 67 (43.5) | 23 (41.1) |
| Gainfully employed | 103 (68.7) | 106 (68.8) | 38 (67.9) |
| Vo2max (mL/kg/min)* | 25 (22–28) | 24 (21–28) | 23 (19–27) |
Data are given as median (interquartile range) or number (%). Differences between groups were analyzed with the χ2 test for categoric variables. The Student t test was used for continuous variables with normal distribution; otherwise, the Mann-Whitney U test was used. At a significance level of 0.05 (two-sided), there were no statistically significant differences in any variables between the intervention and control groups. Owing to small numbers, the differences between drop-out and completing groups were not statistically significant.
*As an indicator of physical fitness.
Women invited but not participating in the study (n = 317) were characterized by same mean age, parity, and distribution within the three obesity classes. A higher percentage was smokers (16.4% vs. 9.5%, P = 0.01). The intervention group had a lower median (range) GWG compared with the control group: 7.0 (4.7–10.6) vs. 8.6 kg (5.7–11.5; P = 0.01). In the intervention group, 28% gained ≤5 kg compared with 20% in the control group (P = 0.102; Table 2). With respect to weight gain according to the IOM guidelines (≤9 kg), 65% were within this range in the intervention group compared with 53% in the control group (P = 0.058).
Table 2.
GWG, obstetric, and neonatal outcomes
| Intervention | Control | ||
|---|---|---|---|
| Variable | n = 150 | n = 154 | P |
| GA 35 | |||
| GWG (kg) | 7.0 (4.7–10.6) | 8.6 (5.7–11.5) | 0.014 |
| ≤5 kg | 41 (28.5) | 30 (20.3) | 0.102 |
| ≤9 kg | 93 (64.6) | 79 (53.4) | 0.058 |
| Blood pressure (mmHg) | |||
| Systolic | 122 (117–130) | 124 (116–129) | 0.693 |
| Diastolic | 82 (77–88) | 83 (78–89) | 0.263 |
| Vo2max (mL/kg/min)* | 23 (19–27) | 22 (19–24.5) | 0.049 |
| Obstetric outcomes | |||
| Cesarean section | |||
| All | 40 (26.7) | 39 (25.3) | 0.790 |
| Emergency | 22 (14.7) | 28 (18.2) | 0.408 |
| Planned | 18 (12) | 11 (7.1) | 0.149 |
| GDM | 9 (6.0) | 8 (5.2) | 0.760 |
| Preeclampsia/PIH | 23 (15.4) | 28 (18.2) | 0.506 |
| Neonatal outcomes | |||
| Birth weight (g) | 3,742 (3,464–4,070) | 3,593 (3,335–3,930) | 0.039 |
| GA (days) | 283 (273–290) | 283 (274–289) | 0.952 |
| LGA | 23 (15.4) | 18 (11.7) | 0.340 |
| Birth weight >4,000 g | 40 (32) | 39 (25.3) | 0.070 |
| Admission to NICU | 21 (14.0) | 22 (14.3) | 0.943 |
Data are given as median (interquartile range) or n (%). For the GWG variables, the total number is <304 due to missing values: n = 144 in the intervention group and n = 148 in the control group. For the physical fitness score, the number is n = 90 in the intervention group and n = 76 in the control group. Differences between groups were analyzed with the χ2 test for categoric variables. The Student t test was used for continuous variables with normal distribution; otherwise, the Mann-Whitney U test was used.
*As an indicator of physical fitness.
Overall, there was no significant difference in obstetric and neonatal outcomes in intervention versus control groups (Table 2). When the five end points of emergency caesarean section, preeclampsia, and/or PIH, GDM, LGA, and admission to NICU were combined, there was still no significant difference in the combined scores: 0.65 for the intervention group compared with 0.67 for the control group (P = 0.39). Birth weight was significantly higher in the intervention group (median 3,742 vs. 3,596 g, P = 0.039). Because there were more smokers in the control group, we did logistic regression analysis to adjust for smoking as a confounding factor on birth weight and GWG. Smoking was negatively associated with birth weight and GWG, but this was not statistically significant.
One woman had an unexplained stillbirth after induction of labor in GA 42. Two additional women had a preterm delivery with stillborn infants in second trimester of pregnancy, one from each randomization group.
With respect to compliance with intervention, 92% of the women completed all four dietetic counseling sessions and 98% completed at least three sessions. When asked at 35 weeks’ gestation whether participation in the LiP study had resulted in more healthy eating habits, 85% of women in the intervention group responded affirmatively. In addition, 21% of women in the control group thought that their dietary habits in pregnancy were positively influenced by their participation. The mean attendance for the 20 aerobic classes was 10.4 h, and 56% of women in the intervention group attended the aerobic classes for at least half of the lessons. Among women in the intervention group, 77.5% undertook leisure time sporting activities in addition to the aerobic classes. In addition, 65% of women in the control group engaged in some type of leisure time sporting activities during pregnancy (P = 0.016).
CONCLUSIONS
In this controlled trial in which obese pregnant women were randomized to receive lifestyle intervention or control, we have demonstrated that 1) lifestyle intervention resulted in a significantly lower GWG; and 2) adherence to weight gain according to IOM recommendations was higher in the intervention group compared with the control group. Although GWG was lower in the intervention group, obstetric outcomes were similar in the two groups. To our knowledge the LiP study is the largest randomized study of intensive lifestyle intervention in obese pregnant women. Power calculations were based on the expectation of a larger difference in GWG between groups than we actually found. This should be considered in the conclusions regarding clinical effects of intervention.
Health at study entry
The primary reason for exclusion was medical disorders, which is disturbing in obese women aged younger than 40 years. Also, a large group of women were excluded due to “previous serious obstetric complications.” These exclusion criteria were chosen to ensure study safety and to facilitate compliance with the intervention. This demonstrates that our cohort is a selected group of obese women. We would expect a higher degree of adverse outcomes if participants had not fulfilled these criteria. It is well described that the highest prevalence of obesity is evident in lower socioeconomic groups and that the increase in obesity is mainly seen among women with a low educational level (23). This was not a predominant feature of our study population (Table 1). More than 68% in both groups were in employment. A high percentage was well educated: 74% in the intervention group and 65% in the control group had at least 12 years of school, and 50% of women in the intervention group and 43.5% in the control group had at least 3 years of further education. The 12.2% incidence of obesity in our study population was markedly lower than rates of obesity in the U.K. and the U.S. and may reflect participants with a relatively high socioeconomic status.
Fitness levels at study entry
At inclusion, results for the fitness test (Steptest) were similar in both groups (Table 1), and a fitness score of less than 28 is defined as “very low.” It was challenging to apply the Steptest at 35 weeks’ gestation. Only 90 women in the intervention group and 76 in the control group were able to complete the test. Most refused due to low back pain or minor contractions. We found a lower fitness score in the control group in the third trimester; this might be explained by less training, but it could also be explained by a higher GWG. The information is further limited because the participation rate for the test was low. The Steptest is mainly designed as an initial screening tool and not for assessing personal changes. We can conclude that entering the project, the majority of the women in our study had very low fitness scores.
Compliance
Compliance with the dietetic counseling was very satisfactory, whereas attendance for physical activity was more difficult to maintain when measured as attendance at the aerobic classes. The relatively low attendance was probably due to the weekly frequency and because a large proportion of the women had minor or major ailments in pregnancy that made them refrain from participation or they chose to train by themselves with swimming or other activities. In addition, many women reported that the scheduled aerobic classes conflicted with their working hours or family obligations.
Benefits of participation in the intervention and control groups
When comparing rates of complications in the LiP study with the background population of obese pregnant Danish women (2), we found a lower prevalence of cesarean section, preeclampsia, and GDM in both groups, but the differences were not significant. The prevalence of cesarean section, preeclampsia, and GDM in Danish obese women is 28, 7.6, and 8.7%, respectively (5). Average birth weight in our intervention group was almost 150 g higher compared with the control group, which was an unexpected and paradoxical finding. Because the GWG was lower in the intervention group, we speculate whether an explanation could be improved placental function due to the intervention. However, we had no information about body composition of the neonates and whether the higher weight was due to lean or fat body mass. Previous studies have shown that exercise in pregnancy increases placental size and volume and thereby the nutrient supply, which might affect fetal growth (1,24). Because the intervention group had marginally improved physical fitness and self-reported physical activities, the higher birth weight in the intervention group could be explained by placental development due to exercise. Also, smoking was not fully balanced between the groups, with more smokers in the control group, probably affecting fetal growth negatively. The difference in smokers was not statistically significant, and in regression models, smoking was not a significant confounder on birth weight or GWG. Still, this might be due to small numbers. Women in the control group were followed up more closely than obese women not included in the study and received the same information about purpose and content as did the intervention group. As a result, their behavior likely changed toward a healthier lifestyle, as borne out by their responses when asked about their physical activities and dietary habits. Such crossover from the control group could explain some of the small differences between our groups.
The GWG in our control group was lower than in the control groups of comparable studies. In a Danish study by Wolff et al. (16), a mean GWG of 6.6 kg was found in the intervention group compared with 13.3 kg in the control group after 10 individual dietary counseling sessions during pregnancy. The study of Claesson et al. (15), from Sweden, showed a GWG of 8.7 kg in the intervention group and 11.3 kg in the control group. This study was not randomized, but even when adjusting for different maternal characteristics, the difference in GWG between their groups was bigger than the difference in the LiP Study. We speculate that the close follow-up in the control group in itself had a behavioral influence on the GWG; therefore, our control group might rather be characterized as a “passive intervention group.”
Our results demonstrate that intervention with obese pregnant women is challenging. Because we did not find any clinical difference between the two groups, we cannot answer from this study whether the lower prevalence of obstetric complications compared with the background is due to selection bias or to the intervention and follow-up program. According to the initial power calculations, 180 women were needed in each arm to have a power of 85%, but we ended up with approximately 150 participants, which reduced the power to 77%.
Future research
We succeeded in limiting GWG in our intervention group, and 65% had a GWG of ≤9 kg. Considering the selected and highly motivated group of women and the intensive nature of the intervention, it is a concern that 35% exceeded the IOM weight gain recommendations. The limitations of these recommendations are that they are based on observational studies, and so far, no interventional studies conducted during pregnancy have fully adhered to the recommendations. To target the sensitive developmental period in early pregnancy, prepregnancy interventions may need to be considered to prevent obesity and to limit short-term obstetric and neonatal complications. Prepregnancy BMI is a stronger predictor for maternal and infant adverse outcomes than GWG (25). It could be speculated that the effects of an altered maternal metabolism in obese women already in early pregnancy is only partly modifiable due to epigenetic changes. Ongoing and future interventional studies, with varying design and composition of intensity and frequency targeting different populations, should give us further information about whether and how dietary counseling and physical activity in pregnancy can limit GWG. These studies should be powered to address whether intervention reduces the risk of certain obstetric and neonatal complications in obese women. Follow-up in childhood and the risk of obesity in the offspring should give us further information about the role of obesity “programming” in pregnancy.
Lifestyle intervention in pregnant obese women has the potential to improve fetomaternal outcome by limiting GWG. Active intervention using increased physical activity as well as dietary change is better than passive intervention, as in our control group, which appears better than no intervention at all. Further research is necessary to identify the nature, timing, intensity, frequency, and duration of such interventions and what benefits, if any, these confer on health in later life.
Acknowledgments
The study was supported by Trygfonden, The Health Insurance Foundation (Helsefonden), the Faculty of Health Sciences, University of Southern Denmark, the Danish Diabetes Association, Odense University Hospital, the NoVo Foundation, the Danish Medical Association Research Foundation, Aase og Ejnar Danielsens Fond, CMA Medico, and Ferrosan A/S. No other potential conflicts of interest relevant to this article were reported.
C.A.V. researched data and wrote the manuscript. D.M.J. researched data, contributed to discussion, and edited the manuscript. P.O. and H.B.-N. contributed to discussion and edited the manuscript. J.S.J. researched data, contributed to discussion, and edited the manuscript.
Assistance with data collection and contributions to the study from midwife and physiotherapist Pia Ingerslev and dietician Charlotte Wolff (Department of Gynecology and Obstetrics, Aarhus University Hospital) and midwife Cecilie Thomsen, dieticians Berit Knold and Rikke Nestor, and physiotherapists Maria Bødker and Dorte Larsen (Department of Gynecology and Obstetrics, Odense University Hospital) is gratefully acknowledged. The authors also acknowledge the editorial assistance of Prof. Ronald F. Lamont, University of Southern Denmark.
Parts of this study were presented at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011, and at the 43rd Annual Meeting of the Diabetes in Pregnancy Study Group, Cambridge, U.K., 22–24 September 2011.
Footnotes
Clinical trial reg no. NCT00530439, clinicaltrials.gov.
References
- 1.Nelson SM, Matthews P, Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Hum Reprod Update 2010;16:255–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danish National Board of Health. New data from the National Board of Health (Danish) [Internet], 2008. Available from http://www.sst.dk/publ/tidsskrifter/nyetal/pdf/2008/09_08.pdf Accessed 5 October 2011
- 3.Rode L, Nilas L, Wøjdemann K, Tabor A. Obesity-related complications in Danish single cephalic term pregnancies. Obstet Gynecol 2005;105:537–542 [DOI] [PubMed] [Google Scholar]
- 4.Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev 2008;9:140–150 [DOI] [PubMed] [Google Scholar]
- 5.Ovesen P, Rasmussen S, Kesmodel U. Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstet Gynecol 2011;118:305–312 [DOI] [PubMed] [Google Scholar]
- 6.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol 2007;196:322–328, e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schack-Nielsen L, Michaelsen KF, Gamborg M, Mortensen EL, Sørensen TI. Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. Int J Obes (Lond) 2010;34:67–74 [DOI] [PubMed] [Google Scholar]
- 8.Andersen CS, Gamborg M, Sørensen TI, Nohr EA. Weight gain in different periods of pregnancy and offspring's body mass index at 7 years of age. Int J Pediatr Obes 2011;6:e179–e186 [DOI] [PubMed] [Google Scholar]
- 9.Ehrlich SF, Hedderson MM, Feng J, Davenport ER, Gunderson EP, Ferrara A. Change in body mass index between pregnancies and the risk of gestational diabetes in a second pregnancy. Obstet Gynecol 2011;117:1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clausen TD, Mathiesen ER, Hansen T, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 2008;31:340–346 [DOI] [PubMed] [Google Scholar]
- 11.Lauenborg J, Hansen T, Jensen DM, et al. Increasing incidence of diabetes after gestational diabetes: a long-term follow-up in a Danish population. Diabetes Care 2004;27:1194–1199 [DOI] [PubMed] [Google Scholar]
- 12.Institute of Medicine Weight gain during pregnancy: reexamining the guidelines. Washington, D.C., The National Academy Press, 2009 [PubMed] [Google Scholar]
- 13.Jensen DM, Ovesen P, Beck-Nielsen H, et al. Gestational weight gain and pregnancy outcomes in 481 obese glucose-tolerant women. Diabetes Care 2005;28:2118–2122 [DOI] [PubMed] [Google Scholar]
- 14.Hinkle SN, Sharma AJ, Dietz PM. Gestational weight gain in obese mothers and associations with fetal growth. Am J Clin Nutr 2010;92:644–651 [DOI] [PubMed] [Google Scholar]
- 15.Claesson IM, Sydsjö G, Brynhildsen J, et al. Weight gain restriction for obese pregnant women: a case-control intervention study. BJOG 2008;115:44–50 [DOI] [PubMed] [Google Scholar]
- 16.Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obes (Lond) 2008;32:495–501 [DOI] [PubMed] [Google Scholar]
- 17.Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr 2010;91:373–380 [DOI] [PubMed] [Google Scholar]
- 18.Polley BA, Wing RR, Sims CJ. Randomized controlled trial to prevent excessive weight gain in pregnant women. Int J Obes Relat Metab Disord 2002;26:1494–1502 [DOI] [PubMed] [Google Scholar]
- 19.Phelan S, Phipps MG, Abrams B, Darroch F, Schaffner A, Wing RR. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: the Fit for Delivery Study. Am J Clin Nutr 2011;93:772–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinlivan JA, Lam LT, Fisher J. A randomised trial of a four-step multidisciplinary approach to the antenatal care of obese pregnant women. Aust N Z J Obstet Gynaecol 2011;51:141–146 [DOI] [PubMed] [Google Scholar]
- 21.Zacho M. Development of the new Steptest (Danish) (provisional report) [Internet], 2008. Available from http://www.steptest.dk Accessed 5 October 2011
- 22.Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 1996;85:843–848 [DOI] [PubMed] [Google Scholar]
- 23.Heitmann BL. Ten-year trends in overweight and obesity among Danish men and women aged 30-60 years. Int J Obes Relat Metab Disord 2000;24:1347–1352 [DOI] [PubMed] [Google Scholar]
- 24.Clapp JF., 3rd The effects of maternal exercise on fetal oxygenation and feto-placental growth. Eur J Obstet Gynecol Reprod Biol 2003;110 (Suppl. 1):S80–S85 [DOI] [PubMed] [Google Scholar]
- 25.Nohr EA, Vaeth M, Baker JL, Sørensen TIa, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr 2008;87:1750–1759 [DOI] [PubMed] [Google Scholar]