Abstract
OBJECTIVE
To assess the efficacy and safety of MK-0941, a glucokinase activator (GKA), when added to stable-dose insulin glargine in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
In this double-blind study, 587 patients taking stable-dose insulin glargine (±metformin ≥1,500 mg/day) were randomized (1:1:1:1:1) to MK-0941 10, 20, 30, or 40 mg or matching placebo t.i.d. before meals (a.c.). This study included an initial 14-week, dose-ranging phase followed by a 40-week treatment phase during which patients were to be uptitrated as tolerated to 40 mg (or placebo) t.i.d. a.c. The primary efficacy end point was change from baseline in A1C at Week 14.
RESULTS
At Week 14, A1C and 2-h postmeal glucose (PMG) improved significantly versus placebo with all MK-0941 doses. Maximal placebo-adjusted least squares mean changes from baseline in A1C (baseline A1C 9.0%) and 2-h PMG were −0.8% and −37 mg/dL (−2 mmol/L), respectively. No significant effects on fasting plasma glucose were observed at any dose versus placebo. By 30 weeks, the initial glycemic responses noted at 14 weeks were not sustained. MK-0941 at one or more doses was associated with significant increases in the incidence of hypoglycemia, triglycerides, systolic blood pressure, and proportion of patients meeting criteria for predefined limits of change for increased diastolic blood pressure.
CONCLUSIONS
In patients receiving stable-dose insulin glargine, the GKA MK-0941 led to improvements in glycemic control that were not sustained. MK-0941 was associated with an increased incidence of hypoglycemia and elevations in triglycerides and blood pressure.
Glucokinase (GK) plays an integral role in glucose homeostasis and offers a potential therapeutic target for the treatment of type 2 diabetes (1). GK functions as a glucose sensor in pancreatic β-cells and as a mediator of hepatic glucose disposal. Increased or decreased GK activity, linked to specific genetic mutations, is associated with hypoglycemia and hyperglycemia, respectively (2). Glucokinase activators (GKAs) interact with the same region of the GK enzyme that is commonly affected by naturally occurring GK-activating mutations in humans (3). Treatment with different GKAs has been shown to increase insulin secretion, reduce hepatic glucose production, and improve glycemic excursion in normal and diabetic animals (4–6). In single- and multiple-dose studies, GKAs reduced fasting and postprandial glucose in patients with type 2 diabetes (7,8) and healthy adults (9).
MK-0941 is an oral, selective, allosteric GKA that activates GK (hexokinase subtype IV), with ≥100-fold selectivity over other hexokinase isoforms (Supplementary Table 1). In single-dose studies of MK-0941, mean fasting plasma glucose (FPG) decreased by ∼30 mg/dL relative to baseline in healthy subjects and by ∼100 mg/dL in patients with type 2 diabetes. Given its relatively short duration of action (∼4-h postdose) and potential to induce hypoglycemia when administered in the fasted state, MK-0941 was dosed prior to each main meal. In one study, reduction in 24-h weighted mean glucose (WMG) after dosing MK-0941 40 mg t.i.d. before meals (a.c.). for a single day was comparable to the reduction observed with 8 IU of rapid-acting insulin t.i.d. a.c. In another study, patients taking stable-dose insulin glargine had a reduction in 24-h WMG of ∼48 mg/dL after receiving MK-0941 t.i.d. a.c. for 11 days (initial 4-day titration period up to 40 mg t.i.d., as tolerated, followed by 1 week of treatment). When added to metformin, MK-0941 administered b.i.d. a.c. decreased 24-h WMG by ∼41 mg/dL after 13 days (including an 8-day titration phase). Overall, in phase I studies in subjects with and without type 2 diabetes, MK-0941, administered either b.i.d. or t.i.d. a.c. for up to 13 days, had robust glucose-lowering effects and was generally well tolerated, except for an expected increase in the incidence of hypoglycemia.
In chronic oral toxicity studies in animals with MK-0941, cataracts were observed in rats and dogs at drug exposures of 3 and 1.5 times, respectively, the maximum predicted human exposure based on doses planned for further clinical development. No cataracts were observed in either species at drug exposures equal to the maximum predicted human exposure. A potential mechanism may be related to the severe and sustained hypoglycemia observed in affected animals. Nevertheless, on the basis of the preclinical findings, ophthalmologic examinations using LOCS III (Lens Opacities Classification System III) (10) were implemented in the MK-0941 clinical development program.
Patients with type 2 diabetes taking basal insulin often require the addition of multiple premeal injections with short- or rapid-acting insulin to optimize postprandial glucose control. Given the pharmacodynamic similarities between premeal insulin and MK-0941, the drug was considered a potential oral alternative to injections of short-acting insulin. The purpose of the current study was to assess the efficacy and safety of MK-0941 in patients with type 2 diabetes and inadequate glycemic control taking insulin glargine (either alone or in combination with metformin).
RESEARCH DESIGN AND METHODS
This multinational, randomized, double-blind, placebo-controlled, parallel-group, 54-week study was conducted at 101 clinical sites in 27 countries. The study consisted of a 14-week dose-ranging phase (MK-0941 10, 20, 30, or 40 mg or placebo t.i.d. a.c.), followed by a 40-week continuation phase, during which patients who did not receive glycemic rescue therapy (described below) and were not already at the maximum dose of MK-0941 (40 mg t.i.d. a.c.) had their MK-0941 dose or matching placebo uptitrated potentially to a maximum of 40 mg t.i.d. a.c. The study was conducted in accordance with principles of good clinical practice and was approved by the appropriate institutional review boards and regulatory agencies.
All patients provided written informed consent. Patients with type 2 diabetes (aged 21–70 years) were eligible to participate if they were taking any insulin at a total daily dose of ≥15 units alone or in combination with oral monotherapy or combination therapy (with the exception of current/recent thiazolidinedione therapy or injectable incretin-based therapy). Patients were excluded for type 1 diabetes, a history of ketoacidosis, a history of severe hypoglycemic episodes, uncontrolled hypertension, New York Heart Association class II–IV cardiac functional classification, or new or worsening signs/symptoms (within past 3 months) of cardiovascular disease. Patients received counseling on a weight-maintaining diet consistent with local guidelines.
Patients taking insulin glargine ≥15 units/day at a stable dose for at least 6 weeks prior to the screening visit, with an A1C between 7.5 and 11.0%, and who met all entry criteria entered directly into a 2-week, single-blind, placebo run-in period. All other patients were switched to a stable-dose regimen of insulin glargine ≥15 units/day during a conversion period of up to 10 weeks (consisting of a 2- to 4-week dose-adjustment phase and a 6-week stable-dose phase). Patients were instructed to inject their insulin glargine at the same time each evening. At the start of the conversion period, patients taking concurrent metformin ≥1,500 mg/day were allowed to continue their metformin dose, whereas those taking metformin <1,500 mg/day or other concurrent oral antihyperglycemic agents had their treatment discontinued. After this period, eligible patients entered the 2-week placebo run-in period. Patients were not randomized if they experienced a severe hypoglycemic episode during the prerandomization period.
After the 2-week placebo run-in period, eligible patients (n = 587) were randomized to MK-0941 10, 20, 30, or 40 mg or matching placebo t.i.d. a.c. (1:1:1:1:1) using a computer-generated allocation schedule. Randomization was stratified according to metformin use (the percentage of randomized patients taking metformin was to be ≤70%) and their baseline cortical cataract LOCS III score from the eye with the worse score (≤0.5 or >0.5). During the first 2 weeks’ postrandomization, patients were to be uptitrated to either their dose assigned at randomization or a lower dose that maximally controlled glucose without causing unacceptable hypoglycemia. The goal of uptitration was to attain preprandial fingerstick glucose values in the 80–110 mg/dL (4.4–6.1 mmol/L) range. During the following 12-week dose-stable period, MK-0941 could be downtitrated to avoid hypoglycemia. To maintain blinding, mock titration was performed in patients randomized to placebo.
Patients were to monitor their fingerstick glucose throughout the study by performing a minimum of two premeal determinations per day, with additional monitoring during titration periods or at an investigator-determined frequency. Patients taking their assigned MK-0941 dose (or a lower dose that maximally controlled glucose without causing unacceptable hypoglycemia) for at least 4 weeks were to receive glycemic rescue therapy if they met the following criteria: from baseline through Week 6, multiple fasting or preprandial fingerstick glucose >270 mg/dL (15 mmol/L); after Week 6 up to Week 22, glucose >240 mg/dL (13.3 mmol/L); after Week 22 up to Week 38, glucose >200 mg/dL (11.1 mmol/L); and after Week 38 to Week 54, glucose >170 mg/dL (9.4 mmol/L) without a reasonable explanation. Rescue therapy consisted of increasing the daily insulin dose by >10% (and >2 units) above the stable dose of insulin glargine determined during the prerandomization period. After rescue, patients could not have their MK-0941 dose/matching placebo further uptitrated during the study.
For 2-h postmeal glucose (PMG) assessment, a standard meal tolerance test was administered at baseline (prior to first dose of study medication) and at Week 14 (study medication taken 5 min prior to the meal). Patients were instructed to consume the meal within 15 min. The meal consisted of one nutrition bar and one nutrition drink (∼460 kcal; 75 g carbohydrate, 9 g fat, and 18 g protein). Blood was collected at −5, 0, 60, and 120 min from the meal start.
End points
The primary efficacy end point was change in A1C from baseline at Week 14. Secondary efficacy end points included 2-h PMG and FPG. Lipids were also assessed.
Safety and tolerability were evaluated by review of adverse events (AEs), physical examinations, body weight, vital signs, and electrocardiograms (ECGs). For hypoglycemia, patients were counseled with regard to symptoms, fingerstick evaluation, treatment, and reporting as described previously (11). Any type of event (symptomatic or asymptomatic) assessed by the investigator as hypoglycemia was reported as an AE of hypoglycemia; documentation of a glucose value at the time the patient had symptoms was not required for symptomatic hypoglycemia. Asymptomatic episodes were those without symptoms of hypoglycemia but with a fingerstick glucose ≤70 mg/dL (3.9 mmol/L). Events of hypoglycemia were classified as not requiring assistance, requiring the (nonmedical) assistance of others, and meeting the protocol-specified definition of severity (i.e., requiring medical intervention or exhibiting markedly depressed level of consciousness, including loss of consciousness or seizure). LOCS III assessments were to be made prior to randomization, during the double-blind study period, and 3 months after the patient had either completed or discontinued from the study. All laboratory measurements were performed at central laboratories (PPD Inc., Highland Heights, KY, and Zaventem, Belgium). ECGs were read centrally by technicians (Quintiles, Durham, NC) blinded to treatment assignment.
Statistical analysis
The full analysis set, used for all efficacy analyses at Week 14, included all randomized patients who took at least one dose of study medication and had a baseline or at least one postrandomization measurement. Efficacy analyses at Week 30 included only patients with data through Week 30. The longitudinal data analysis (LDA) method proposed by Zeger and Liang (12) was used to evaluate efficacy end points. This repeated-measures model included terms for treatment, metformin use stratum, cortical cataract LOCS III score stratum, study visit, and respective interaction terms between study visit and the other covariates. Data obtained after initiation of glycemic rescue therapy were excluded. Missing data were handled by the LDA model. For triglycerides, a nonparametric method using an ANCOVA based on Tukey normalized scores on the percent change from baseline was used. Within-treatment effects were estimated using medians and between-treatment effects were estimated using the Hodges–Lehmann estimate (13) with a corresponding confidence interval based on Wilcoxon rank-sum test. Missing data for triglycerides were imputed using the last observation carried forward method.
Safety analyses included all randomized patients who took at least one dose of study medication. For hypoglycemia and cataract AEs and changes from baseline in LOCS III scores and body weight at Week 14, between-group differences (MK-0941 vs. placebo) were assessed. The AE analyses used the Miettinen and Nurminen method (14), stratified by metformin use and cortical cataract LOCS III score. LOCS III and body weight analyses used LDA. Analyses of hypoglycemia and body weight excluded data after initiation of rescue therapy to avoid the confounding influence of increasing insulin doses. All other safety analyses included data after initiation of rescue therapy.
RESULTS
Once all enrolled patients had either completed 14 weeks of treatment or discontinued from the study, the data were unblinded and reviewed by the sponsor. After review of the efficacy and safety results through Week 14, as well as the data from the cohort of patients who had completed 30 weeks of treatment, the study was terminated (2 March 2010) owing to lack of sustained glycemic efficacy.
Baseline characteristics were generally well balanced across treatment groups (Supplementary Table 2). For the 587 randomized patients (50% men), mean age was 56 years, mean BMI was 32 kg/m2, 62% were enrolled on concomitant metformin, and mean insulin glargine dose was 45 units/day. Disease-related characteristics were a mean baseline A1C of 9.0% and mean duration of diabetes of 12 years. The proportion of patients completing the trial through Week 14 ranged from 83% (40-mg group) to 97% (20-mg group) (Supplementary Fig. 1). The most common reasons for discontinuation were withdrawal of consent, AEs, and lost to follow-up. The proportion of patients receiving glycemic rescue therapy was 6.1, 9.2, 4.3, 6.8, and 3.4% in the placebo, 10-, 20-, 30-, and 40-mg groups, respectively. At Week 14, the proportion of patients reaching their allocated MK-0941 dose was 85, 70, 68, and 36% and the mean total daily dose was 29, 53, 72, and 82 mg in the 10-, 20-, 30-, and 40-mg t.i.d. a.c. groups, respectively.
For patients who completed through Week 30 (n = 41–51/group), baseline demographics and physical characteristics were similar relative to the randomized cohort, whereas baseline glycemic parameters appeared to be better (data not shown). Mean baseline A1C ranged from 8.5 to 8.8% across groups in this cohort. Following the protocol-specified uptitration to 40 mg t.i.d. a.c. in MK-0941–treated patients after Week 14, the mean total daily dose at Week 30 was 85, 94, 93, and 102 mg in the 10-, 20-, 30-, and 40-mg t.i.d. a.c. groups, respectively.
Efficacy results
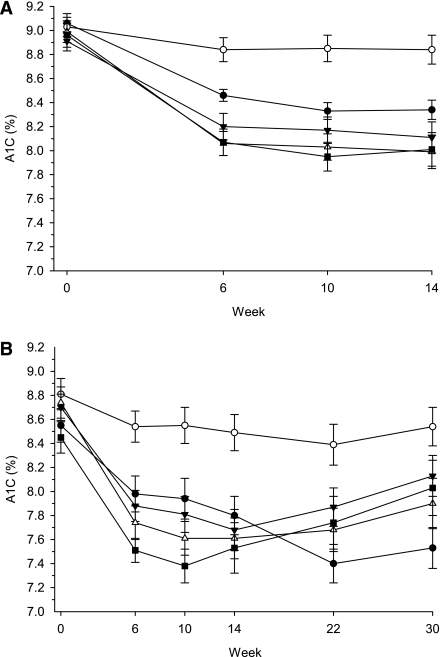
At Week 14, least squares (LS) mean changes in A1C from baseline ranged from −0.5 to −0.8% (P < 0.001 all MK-0941 doses vs. placebo), with an apparent dose-dependent reduction observed between 10 and 30 mg t.i.d. (Table 1). At doses >10 mg t.i.d., a numerically, larger, placebo-adjusted reduction from baseline in A1C was observed in patients receiving concomitant metformin (data not shown). Maximal A1C efficacy was observed by Week 10 with all MK-0941 doses (Fig. 1A). Similar results over time were noted in the cohort of patients who completed 14 weeks of treatments (n = 81–93/group; data not shown).
Table 1.
Fasting and postmeal glycemic responses to treatment at Week 14
| Parameter | Placebo | MK-0941 dose (mg t.i.d. a.c.) |
|||
|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | ||
| n | 111 to 114 | 112 to 118 | 113 to 117 | 113 to 117 | 113 to 118 |
| A1C (%) | |||||
| Change from baseline | −0.1 (−0.3 to 0.1) | −0.6 (−0.8 to −0.4) | −0.7 (−0.9 to −0.5) | −0.9 (−1.1 to −0.7) | −0.8 (−1.0 to −0.6) |
| Change from placebo | — | −0.5 (−0.8 to −0.2)* | −0.6 (−0.9 to −0.4)* | −0.8 (−1.1 to −0.5)* | −0.8 (−1.0 to −0.5)* |
| FPG (mg/dL) | |||||
| Change from baseline | −11.8 (−21.4 to −2.1) | −10.0 (−19.9 to 0.0) | −1.5 (−10.9 to 7.9) | −21.1 (−30.9 to −11.2) | −5.0 (−14.9 to 4.8) |
| Change from placebo | — | 1.8 (−11.6 to 15.2) | 10.3 (−2.7 to 23.2) | −9.3 (−22.6 to 4.0) | 6.7 (−6.6 to 20.0) |
| 2-h PMG (mg/dL) | |||||
| Change from baseline | −2.4 (−16.0 to 11.2) | −39.0 (−52.9 to −25.1) | −29.2 (−42.5 to −15.9) | −37.4 (−51.2 to −23.6) | −39.3 (−53.1 to −25.5) |
| Change from placebo | — | −36.6 (−55.6 to −17.5)* | −26.7 (−45.4 to −8.1)** | −35.0 (−54.0 to −16.0)* | −36.9 (−55.9 to −17.9)* |
Change from baseline or change from placebo data are expressed as LS mean change (95% CI).
*P ≤ 0.001 for the between-group difference relative to placebo.
**P ≤ 0.05 for the between-group difference relative to placebo.
Figure 1.
A1C over time: (A) through Week 14 (primary efficacy time point) in the full analysis set and (B) through Week 30 in patients with results at Week 30 (n per group is 43 for placebo, 45 for 10 mg, 52 for 20 mg, 44 for 30 mg, and 41 for 40 mg). For both figures, ○ = placebo, • = MK-0941 10 mg t.i.d. a.c., ▼ = MK-0941 20 mg t.i.d. a.c., ▵ = MK-0941 30 mg t.i.d. a.c., ▪ = MK-0941 40 mg t.i.d. a.c. Data are expressed as mean ± SE.
Despite the protocol-specified increase in MK-0941 dose at Week 14 for patients not randomized to 40 mg t.i.d. a.c., the reductions in A1C observed through Week 14 appeared to deteriorate through Week 30 (Fig. 1B). The largest and most rapid deterioration in A1C response was observed in patients randomized to the highest dose of MK-0941 (40 mg t.i.d. a.c.).
Relative to placebo, MK-0941 significantly decreased 2-h PMG, with placebo-adjusted LS mean changes from baseline ranging from −27 mg/dL (−1.5 mmol/L) to −37 mg/dL (2.1 mmol/L). For FPG, no significant difference relative to placebo was observed at Week 14 (Table 1).
The effect of MK-0941 on plasma lipids was generally neutral, except for a modest median percent increase (up to 19% relative to placebo) in triglycerides (Table 2). Two AEs related to increased triglycerides were reported (one each in the placebo and the 40-mg groups). Small mean increases from baseline in body weight were observed with MK-0941 relative to placebo, with a statistically significant difference between the 40-mg and placebo groups (Table 2).
Table 2.
Lipid, body weight, and BP responses to treatment at Week 14
| Parameter | Placebo | MK-0941 dose (mg t.i.d. a.c.) |
|||
|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | ||
| n | 111 to 114 | 112 to 118 | 113 to 117 | 113 to 117 | 113 to 118 |
| Total cholesterol | |||||
| % Change from baseline | 4.3 (1.2 to 7.4) | 4.7 (1.5 to 7.9) | 6.2 (3.1 to 9.4) | 5.1 (1.9 to 8.3) | 7.4 (4.2 to 10.5) |
| Change from placebo | — | 0.4 (−4.0 to 4.7) | 1.9 (−2.4 to 6.2) | 0.8 (−3.5 to 5.1) | 3.0 (−1.3 to 7.4) |
| LDL cholesterol | |||||
| % Change from baseline | 7.8 (3.2 to 12.4) | 2.6 (−2.2 to 7.5) | 5.6 (0.8 to 10.3) | 5.5 (0.7 to 10.2) | 5.5 (0.7 to 10.3) |
| Change from placebo | — | −5.2 (−11.7 to 1.4) | −2.2 (−8.7 to 4.3) | −2.3 (−8.8 to 4.2) | −2.3 (−8.9 to 4.3) |
| HDL cholesterol | |||||
| % Change from baseline | −0.2 (−2.9 to 2.5) | 2.3 (−0.5 to 5.1) | −0.1 (−2.8 to 2.6) | −0.9 (−3.7 to 1.8) | 1.9 (−0.9 to 4.6) |
| Change from placebo | — | 2.5 (−1.3 to 6.3) | 0.1 (−3.7 to 3.8) | −0.7 (−4.5 to 3.1) | 2.1 (−1.7 to 5.9) |
| Non-HDL cholesterol | |||||
| % Change from baseline | 6.3 (2.2 to 10.4) | 6.1 (1.9 to 10.4) | 9.3 (5.2 to 13.4) | 8.2 (4.0 to 12.4) | 9.8 (5.6 to 14.0) |
| Change from placebo | — | −0.2 (−5.9 to 5.6) | 3.0 (−2.6 to 8.7) | 1.9 (−3.8 to 7.6) | 3.5 (−2.2 to 9.3) |
| Triglycerides | |||||
| % Change from baseline† | 8.0 (−1.6 to 17.5) | 15.3 (6.0 to 24.6) | 27.6 (15.5 to 39.7) | 12.1 (3.4 to 20.8) | 18.2 (8.8 to 27.5) |
| Change from placebo† | — | 7.8 (−3.4 to 18.7) | 19.3 (7.2 to 31.2)* | 6.1 (−4.7 to 16.8) | 12.7 (1.9 to 24.3)** |
| Body weight (kg) | |||||
| Change from baseline | −0.2 (−0.7 to 0.3) | 0.0 (−0.5 to 0.6) | 0.3 (−0.2 to 0.8) | 0.4 (−0.1 to 0.9) | 0.6 (0.1 to 1.1) |
| Change from placebo | — | 0.2 (−0.5 to 0.9) | 0.5 (−0.2 to 1.2) | 0.6 (−0.1 to 1.3) | 0.8 (0.0 to 1.5)** |
| SBP (mmHg) | |||||
| Change from baseline | −1.3 (−3.7 to 1.2) | 1.7 (−0.8 to 4.2) | −1.2 (−3.6 to 1.2) | 0.8 (−1.7 to 3.3) | 2.5 (−0.1 to 5.0) |
| Change from placebo | — | 3.0 (−0.4 to 6.4) | 0.1 (−3.2 to 3.4) | 2.0 (−1.3 to 5.4) | 3.7 (0.3 to 7.2)** |
| DBP (mmHg) | |||||
| Change from baseline | −0.5 (−2.0 to 1.0) | 0.4 (−1.1 to 2.0) | −1.0 (−2.5 to 0.5) | 0.5 (−1.0 to 2.0) | 0.8 (−0.8 to 2.3) |
| Change from placebo | — | 1.0 (−1.1 to 3.0) | −0.5 (−2.5 to 0.5) | 1.0 (−1.0 to 3.1) | 1.3 (−0.8 to 3.4) |
Change from baseline or change from placebo data are expressed as LS mean change or LS mean percent change (95% CI) with LDA unless otherwise indicated.
†Data are median percent change (95% CI) using ANCOVA with last observation carried forward analysis; n per group ranged from 82 to 96 for triglycerides.
*P ≤ 0.001 for the between-group difference relative to placebo.
**P ≤ 0.05 for the between-group difference relative to placebo.
Safety results
The incidences of AEs overall and drug related were modestly higher with MK-0941 relative to placebo through Week 14 primarily owing to the increased incidence of hypoglycemia (Table 3). The incidence of serious AEs was higher with MK-0941 compared with placebo. No deaths were reported. Discontinuations as a result of AEs were highest in the 40-mg group. One patient (40-mg group) had an AE (musculoskeletal chest pain) that was considered serious and drug related and that resolved after discontinuation.
Table 3.
AE summary through Week 14
| Placebo | MK-0941 dose (mg t.i.d. a.c.) |
||||
|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | ||
| n | 115 | 119 | 117 | 117 | 119 |
| ≥1 AEs | 58 (50.4) | 73 (61.3) | 75 (64.1) | 71 (60.7) | 73 (61.9) |
| Drug-related AEs† | 17 (14.8) | 20 (16.8) | 36 (30.8) | 32 (27.4) | 35 (29.7) |
| SAEs | 1 (0.9) | 4 (3.4) | 2 (1.7) | 4 (3.4) | 4 (3.4) |
| Drug-related SAEs† | 0 | 0 | 0 | 0 | 1 (0.8) |
| Discontinued owing to AEs | 3 (2.6) | 4 (3.4) | 3 (2.6) | 3 (2.6) | 6 (5.1) |
| Discontinued owing to drug-related AEs† | 1 (0.9) | 0 | 2 (1.7) | 1 (0.9) | 4 (3.4) |
| Discontinued owing to SAEs | 1 (0.9) | 0 | 0 | 0 | 1 (0.8) |
| Discontinued owing to drug-related SAEs† | 0 | 0 | 0 | 0 | 1 (0.8) |
| Specific AEs occurring with an incidence of ≥5% in any group | |||||
| Hypoglycemia | 40 (34.8) | 46 (38.7) | 61 (52.1)* | 54 (46.2) | 63 (53.4)* |
| Cataracts | 5 (4.3) | 8 (6.7) | 1 (0.9) | 5 (4.3) | 2 (1.7) |
| Diarrhea | 3 (2.6) | 6 (5.0) | 5 (4.3) | 3 (2.6) | 3 (2.5) |
| Influenza | 0 (0) | 6 (5.0) | 2 (1.7) | 8 (6.8) | 4 (3.4) |
| Nasopharyngitis | 1 (0.9) | 3 (2.5) | 9 (7.7) | 3 (2.6) | 6 (5.1) |
| Upper respiratory tract infection | 2 (1.7) | 4 (3.4) | 5 (4.3) | 7 (6.0) | 4 (3.4) |
| Headache | 2 (1.7) | 2 (1.7) | 10 (8.5) | 3 (2.6) | 4 (3.4) |
Data are n (%). For all end points except for hypoglycemia, this summary includes data after initiation of glycemic rescue therapy. For hypoglycemia, this summary excludes data after initiation of glycemic rescue therapy to eliminate the confounding effect of increased insulin dose. SAE, serious adverse event.
†Considered by the investigator to be drug related.
*P ≤ 0.01 vs. placebo (analysis of between-group difference relative to placebo was prespecified for hypoglycemia AEs).
Specific AEs with an incidence ≥5% are shown in Table 3. For hypoglycemia, the incidence was significantly (P ≤ 0.01) higher in the MK-0941 20- and 40-mg groups relative to the placebo group. The proportion of patients experiencing episodes of hypoglycemia with a concurrent glucose ≤50 mg/dL (2.8 mmol/L) was 8, 15, 16, 15, and 14% in the placebo, 10-, 20-, 30-, and 40-mg groups, respectively. Four patients discontinued treatment because of hypoglycemia (one patient each in the 10- and 20-mg groups and two in the 40-mg group). Two episodes of hypoglycemia requiring nonmedical assistance occurred in two patients in the 10-mg group and one episode of severe hypoglycemia (requiring medical assistance) occurred in a patient in the 20-mg group.
The incidence of AEs of new or worsening cataracts was not different among groups (Table 3). For the multiple comparisons of each dose relative to placebo for each of the four categories of the LOCS III (cortical cataract, nuclear color, nuclear opalescence, and posterior subscapular cataract), no significant differences were observed between MK-0941 and placebo except for a significant LS mean increase in nuclear color from baseline in the worse eye with 20-mg relative to placebo (0.2 [95% CI 0.1–0.3] vs. 0.0 [–0.1 to 0.1], respectively; P = 0.004).
At Week 14, there were small mean increases from baseline in systolic blood pressure (SBP) with MK-0941, with a statistically significant difference between the 40-mg and placebo groups (Table 2). For diastolic blood pressure (DBP), no significant differences in LS mean change from baseline in DBP were observed between any MK-0941 dose and placebo. The proportion of patients meeting predefined limits of change for increased SBP (last value increased by ≥15 mmHg) was 12.3% in the placebo group and 19.5, 14.5, 17.4, and 14.7% in the 10-, 20-, 30-, and 40-mg groups, respectively. For increased DBP (last value increased by ≥10 mmHg), the proportion was 9.6% in the placebo group and 19.5, 16.2, 13.0, and 19.0% in the 10-, 20-, 30-, and 40-mg groups, respectively (95% CIs for the difference in proportions between the 10- or the 40-mg group relative to placebo excluded zero). Hypertension AEs occurred at rates between 1 and 2% in each group. One AE of increased DBP was reported in the 20-mg group, and one AE of “increased BP” was reported in the 40-mg group. There were no clinically relevant differences between groups in ECGs or in routine hematology, serum chemistry, or urinalysis.
CONCLUSIONS
This clinical trial represents the first published clinical assessment of efficacy and safety after prolonged treatment with a GKA. The addition of MK-0941 to insulin in patients with type 2 diabetes resulted in significant reductions in A1C at Week 14. The A1C effects appeared to be dose related up to 30 mg t.i.d. a.c. The lack of separation in the observed efficacy between the higher MK-941 doses may be explained by the inability to uptitrate patients in the higher dose groups to their allocated dose because of risk or occurrence of hypoglycemia. Alternatively, the higher doses might have been at the top of the dose response. It is important to note that the study was not powered to assess small between-dose differences in A1C. The observed reduction in A1C appeared to be the result of changes in PMG because no significant effect on FPG was observed. An FPG response was not expected owing to the short duration of action of MK-0941. As the trial continued beyond Week 14, the A1C response deteriorated, despite the protocol-specified dose uptitration after Week 14. Since hepatic glucose production, insulin secretion, and insulin sensitivity were not measured during this trial, the mechanism underlying the lack of sustained glycemic effect with MK-0941 cannot be determined.
MK-0941 led to an increased incidence of hypoglycemia AEs through Week 14, which was not unexpected considering the mechanism of action of this drug. The increase in the incidence of hypoglycemia is consistent with activating mutations of the GK enzyme that are associated with varying degrees of hypoglycemia (2,3). Other safety-related outcomes (changes in triglycerides and BP) were not expected, based on earlier studies with this agent.
Elevated plasma triglycerides with MK-0941 were also noted in a separate 6-week phase IIa trial conducted concurrently with the current study, but this finding contrasts with results from earlier studies (Supplementary Table 1). Published evidence has revealed an association between GK activation and lipid perturbations. Overexpression of GK in the liver by 6.4-fold led to a 38% reduction in plasma glucose and a 190% increase in plasma triglycerides in rats (15). In humans, increased hepatic GK expression was associated with increased de novo lipogenesis and liver triglyceride content (16). Furthermore, polymorphisms in the GK regulatory protein gene are associated with elevated plasma triglycerides, free fatty acids, and VLDL–triglyceride levels and reduced plasma glucose levels in humans (17–22). We can speculate that increased triglycerides with chronic MK-0941 use may be associated with increased GK activity within the liver that ultimately favor de novo lipogenesis and attendant triglyceride production (18).
In the current study, the efficacy of MK-0941 appeared to wane sooner in those treated with the highest dose, in which increases in triglycerides and body weight were observed. This higher dose exposure and activation of GK may have led to multiple metabolic perturbations that affected the glucose-lowering response. Of interest, these negative metabolic effects were not observed in wild type mice and β-cell–specific GK haploinsufficient mice treated with a GKA for up to 40 months (23,24). The differences between studies may be related to target organ (i.e., liver vs. pancreas) specificity of the GKA molecules and/or species specificity. The present results are consistent, however, with the development of hyperglycemia, hyperinsulinemia, hypertriglyceridemia, and increased body weight in transgenic mice overexpressing hepatic GK (25). The present results coupled with the findings of Peter et al. (16) indicate that continued activation of hepatic GK has deleterious metabolic effects and that nonspecific activation of GK may not be an appropriate approach for the treatment of type 2 diabetes.
Some negative effects of MK-0941 on BP were observed in this study. In addition, elevations in SBP were observed in the aforementioned concurrent phase IIa trial (Supplementary Table 1). A mechanistic explanation for BP changes associated with GK activation is unknown. Recent findings in dogs with another GKA found an increased catecholamine tone (J. Ehrhart, personal communication) that if verified as a finding in humans, may explain, at least in part, the BP findings observed with chronic MK-0941 use.
In the current study, no meaningful between-group differences in incidences of cataract AEs were observed. A small increase in the nuclear color LOCS III dimension observed with the 20-mg dose of MK-0941 compared with placebo may have been a spurious finding because no other significant between-group differences in LOCS III scores were observed. Because profound and persistent hypoglycemia with MK-0941 potentially mediates cataract formation in animals, the present results suggest that the mild-to-moderate, transient episodes of hypoglycemia and rare incidence of severe hypoglycemia reported with MK-0941 in this study would not be expected to lead to clinically meaningful eye-related changes.
In summary, the addition of MK-0941 to stable-dose insulin glargine led to significant improvements in glycemic control that were not sustained with continued treatment. MK-0941 was associated with an increased incidence of hypoglycemia and elevations in triglycerides and SBP. It is unknown whether the efficacy and safety profiles observed with MK-0941 were compound specific or mechanism based. A better understanding of the GK mechanism and its downstream metabolic effects is needed to determine whether GK activation with other compounds may be a viable treatment target for type 2 diabetes.
Supplementary Material
Acknowledgments
This study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Whitehouse Station, New Jersey.
G.E.M., M.A., Y.S., E.L., H.A., M.J.D., K.D.K., and B.J.G. are employees of or were employed during the time of this study by Merck Sharp & Dohme Corp. As employees, these authors may have stocks or stock options in the company. R.S. was a clinical investigator for one of the sites participating in this study and is a member of an advisory committee of Merck Sharp & Dohme Corp.; he has no financial associations with any pharmaceutical company. No other potential conflicts of interest relevant to this article were reported.
G.E.M. conceived/designed or planned the study, drafted the manuscript, and provided substantive revisions to the manuscript. R.S. provided substantive revisions to the manuscript. M.A. collected and assembled data, performed or supervised analyses, and drafted the manuscript. Y.S. conceived/designed or planned the study, performed or supervised analyses, and provided substantive revisions to the manuscript. E.L. performed or supervised analyses and provided substantive revisions to the manuscript. H.A. conceived/designed or planned the study, collected and assembled data, and provided substantive revisions to the manuscript. M.J.D. drafted the manuscript. K.D.K. conceived/designed or planned the study and provided substantive revisions to the manuscript. B.J.G. performed or supervised analyses and provided substantive revisions to the manuscript and is the guarantor of the article. All authors interpreted results and approved the final manuscript.
The authors wish to acknowledge Drs. Bei Zhang, Elizabeth Migoya, and Julie Ehrhart of Merck Sharp & Dohme Corp. for their contributions to the early scientific development of MK-0941.
Footnotes
Clinical trial reg. no. NCT00767000, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1200/-/DC1.
G.E.M. and M.A. are currently affiliated with Johnson & Johnson Pharmaceutical Research & Development, Raritan, New Jersey. E.L. is currently affiliated with Forest Laboratories, Jersey City, New Jersey.
A complete list of MK-0941 Study 007 investigators can be found in the Supplementary Data online.
References
- 1.Matschinsky FM, Magnuson MA, Zelent D, et al. The network of glucokinase-expressing cells in glucose homeostasis and the potential of glucokinase activators for diabetes therapy. Diabetes 2006;55:1–12 [PubMed] [Google Scholar]
- 2.Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov 2009;8:399–416 [DOI] [PubMed] [Google Scholar]
- 3.Osbak KK, Colclough K, Saint-Martin C, et al. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum Mutat 2009;30:1512–1526 [DOI] [PubMed] [Google Scholar]
- 4.Grimsby J, Sarabu R, Corbett WL, et al. Allosteric activators of glucokinase: potential role in diabetes therapy. Science 2003;301:370–373 [DOI] [PubMed] [Google Scholar]
- 5.Camacho RC, Qureshi SA, Yang X, Eiki J, Zhang BB. Stimulation of insulin secretion and enhancement of insulin action, in vivo, by a small molecule glucokinase activator (Abstract). Diabetes 2009;58(Suppl. 1):A388 [Google Scholar]
- 6.Ohyama S, Takano H, Satow A, et al. A small molecule glucokinase activator lowers blood glucose in rats desensitized to sulfonylurea agents (Abstract). Diabetes 2009;58(Suppl. 1):A397 [Google Scholar]
- 7.Bonadonna RC, Heise T, Arbet-Engels C, et al. Piragliatin (RO4389620), a novel glucokinase activator, lowers plasma glucose both in the postabsorptive state and after a glucose challenge in patients with type 2 diabetes mellitus: a mechanistic study. J Clin Endocrinol Metab 2010;95:5028–5036 [DOI] [PubMed] [Google Scholar]
- 8.Zhi J, Zhai S, Mulligan ME, et al. A novel glucokinase activator RO4389620 improved fasting and postprandial plasma glucose in type 2 diabetic patients (Abstract). Diabetologia 2008;51(Suppl. 1):S23 [Google Scholar]
- 9.Migoya EM, Miller J, Maganti J, Gottesdiener K, Wagner JA. The glucokinase activator MK-0599 lowers plasma glucose concentrations in healthy non-diabetic subjects (Abstract). Diabetes 2009;58(Suppl. 1):A31 [Google Scholar]
- 10.Chylack LT, Jr, Wolfe JK, Singer DM, et al. ; The Longitudinal Study of Cataract Study Group The Lens Opacities Classification System III. Arch Ophthalmol 1993;111:831–836 [DOI] [PubMed] [Google Scholar]
- 11.Vilsbøll T, Rosenstock J, Yki-Järvinen H, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2010;12:167–177 [DOI] [PubMed] [Google Scholar]
- 12.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121–130 [PubMed] [Google Scholar]
- 13.Hodges JL, Lehmann EL. The efficiency of some nonparametric competitors of the t-test. Ann Math Stat 1956;27:324–335 [Google Scholar]
- 14.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med 1985;4:213–226 [DOI] [PubMed] [Google Scholar]
- 15.O’Doherty RM, Lehman DL, Télémaque-Potts S, Newgard CB. Metabolic impact of glucokinase overexpression in liver: lowering of blood glucose in fed rats is accompanied by hyperlipidemia. Diabetes 1999;48:2022–2027 [DOI] [PubMed] [Google Scholar]
- 16.Peter A, Stefan N, Cegan A, et al. Hepatic glucokinase expression is associated with lipogenesis and fatty liver in humans. J Clin Endocrinol Metab 2011;96:E1126–E1130 [DOI] [PubMed] [Google Scholar]
- 17.Sparsø T, Andersen G, Nielsen T, et al. The GCKR rs780094 polymorphism is associated with elevated fasting serum triacylglycerol, reduced fasting and OGTT-related insulinaemia, and reduced risk of type 2 diabetes. Diabetologia 2008;51:70–75 [DOI] [PubMed] [Google Scholar]
- 18.Orho-Melander M, Melander O, Guiducci C, et al. Common missense variant in the glucokinase regulatory protein gene is associated with increased plasma triglyceride and C-reactive protein but lower fasting glucose concentrations. Diabetes 2008;57:3112–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozian DH, Barthel A, Cousin E, et al. Glucokinase-activating GCKR polymorphisms increase plasma levels of triglycerides and free fatty acids, but do not elevate cardiovascular risk in the Ludwigshafen Risk and Cardiovascular Health Study. Horm Metab Res 2010;42:502–506 [DOI] [PubMed] [Google Scholar]
- 20.Saxena R, Voight BF, Lyssenko V, et al. ; Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of BioMedical Research Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 2007;316:1331–1336 [DOI] [PubMed] [Google Scholar]
- 21.Vaxillaire M, Cavalcanti-Proença C, Dechaume A, et al. ; DESIR Study Group The common P446L polymorphism in GCKR inversely modulates fasting glucose and triglyceride levels and reduces type 2 diabetes risk in the DESIR prospective general French population. Diabetes 2008;57:2253–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beer NL, Tribble ND, McCulloch LJ, et al. The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum Mol Genet 2009;18:4081–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura A, Terauchi Y, Ohyama S, et al. Impact of small-molecule glucokinase activator on glucose metabolism and β-cell mass. Endocrinology 2009;150:1147–1154 [DOI] [PubMed] [Google Scholar]
- 24.Nakumura A, Shimazaki H, Ohyama S, et al. Effect of long-term treatment with a small-molecule glucokinase activator on glucose metabolism, lipid profiles and hepatic function. J Diabetes Invest 2011;2:276–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferre T, Riu E, Franckhauser S, Agudo J, Bosch F. Long-term overexpression of glucokinase in the liver of transgenic mice leads to insulin resistance. Diabetologia 2003;46:1662–1668 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.