Abstract
OBJECTIVE
Using a mouse model for human-like lipoprotein metabolism, we observed previously that reduction of the hepatic triglyceride (TG) content resulted in a decrease in plasma cholesteryl ester transfer protein (CETP) and an increase in HDL levels. The aim of the current study was to investigate the effects of prolonged caloric restriction in obese patients with type 2 diabetes mellitus, resulting in a major reduction in hepatic TG content, on plasma CETP and HDL levels.
RESEARCH DESIGN AND METHODS
We studied 27 obese (BMI: 37.2 ± 0.9 kg/m2) insulin-dependent patients with type 2 diabetes mellitus (14 men and 13 women, aged 55 ± 2 years) who received a 16-week very low calorie diet (VLCD). At baseline and after a 16-week VLCD, plasma lipids, lipoproteins, and CETP were measured. Furthermore, functionality of HDL with respect to inducing cholesterol efflux from human monocyte cells (THP-1) was determined.
RESULTS
A 16-week VLCD markedly decreased plasma CETP concentration (−18%; P < 0.01) and increased plasma apolipoprotein (apo)AI levels (+16%; P < 0.05), without significantly affecting plasma HDL-cholesterol and HDL-phospholipids. Although a VLCD results in HDL that is less lipidated, the functionality of HDL with respect to inducing cholesterol efflux in vitro was unchanged.
CONCLUSIONS
The marked decrease in hepatic TG content induced by a 16-week VLCD is accompanied by a decrease in plasma CETP concentration and an increase in apoAI levels, without improving the cholesterol efflux properties of HDL in vitro.
Patients with type 2 diabetes mellitus display a typical atherogenic dyslipidemia marked by increased plasma triglycerides (TG) and VLDL-cholesterol concentrations and decreased HDL-cholesterol levels. Furthermore, hepatic steatosis, which is also strongly associated with cardiovascular disease risk (1,2), is frequently observed in patients with type 2 diabetes mellitus (3–5).
We demonstrated previously that the HDL-raising effect of various classical lipid-lowering drugs was caused by a reduction in plasma cholesteryl ester transfer protein (CETP) that mediates the net transfer of cholesteryl esters from HDL to (V)LDL. In APOE*3-Leiden.CETP mice, a well-established animal model for human-like lipoprotein metabolism, statins (6), fibrates (7), and niacin (8) decrease the hepatic lipid content (i.e., both TG and cholesterol), resulting in a decreased hepatic CETP expression accompanied by decreased plasma CETP levels and a consequently increased plasma HDL. Recently, we showed that a similar mechanism may account for the HDL-raising effect of pioglitazone in humans. In patients with type 2 diabetes mellitus, pioglitazone decreased hepatic TG content (9), accompanied by a decrease in plasma CETP concentration and increase in HDL level (10). In contrast, metformin did not affect either hepatic TG, plasma CETP, or HDL levels (10).
Lifestyle interventions such as diet-induced weight reduction and exercise are very important in the treatment of obese patients with type 2 diabetes mellitus. Recently, we reported that a 16-week very low calorie diet (VLCD) in obese patients with type 2 diabetes mellitus significantly decreased plasma total cholesterol and TG levels and markedly reduced hepatic TG content (11), but the potential beneficial effect of a VLCD on plasma CETP and HDL levels has not been studied. Therefore, using plasma samples from that study (11), we investigated whether prolonged caloric restriction reduces CETP concentration and thereby increases HDL levels in obese patients with type 2 diabetes mellitus.
RESEARCH DESIGN AND METHODS
Patients
The study protocol has been described in detail previously (11). Twenty-seven obese patients with insulin-dependent type 2 diabetes mellitus (14 men and 13 women) were included (mean ± SEM: age: 55 ± 2 years, BMI: 37.2 ± 0.9 kg/m2, HbA1c: 7.8 ± 0.2%). At baseline patients used 82 ± 11 units of insulin per day with or without concomitant use of metformin and/or sulfonyl ureum derivates. Exclusion criteria were as follows: smoking, unstable weight during 3 months before inclusion, or any other chronic disease. In an article published previously (11), we described only 12 out of the 27 patients from whom proton magnetic resonance spectroscopy (1H-MRS) scans could be obtained. The local ethics committee approved this protocol. All patients gave written informed consent, and the study was performed in accordance with the Declaration of Helsinki.
Study design
Patients were studied before the start and after completion of the 16-week VLCD. Three weeks before start of the VLCD all oral blood glucose–lowering medication was stopped and insulin therapy was intensified. The day before the start of the VLCD intervention, only short-acting insulin was prescribed. Patients did not use any blood glucose–lowering medication, including insulin, during the 16-week VLCD. The VLCD consisted of three liquid food shakes (Modifast Intensive; provided by Nutrition & Santé, Antwerp, Belgium) containing a total of 450 kcal/day and all essential micro- and macronutrients. Thirteen of the twenty-seven subjects simultaneously followed an exercise program in addition to the VLCD. Because exercise had no effect on outcome parameters (Supplementary Table 1), data of all subjects were pooled for the present analyses.
Hepatic TG content
Hepatic TG content was measured in supine position using 1H-MRS on a 1.5 Tesla whole-body MR scanner (Gyroscan ACS/NT15; Philips, Best, the Netherlands), exactly as described previously (11).
Plasma (apo)lipoprotein and CETP analyses
All plasma samples were obtained after an overnight fast before the start (i.e., after stopping all glucose-lowering medication including insulin) and after the 16-week VLCD protocol, stored in aliquots at −80°C, and analyzed after thawing once in a single laboratory (Leiden, the Netherlands). To ascertain that we could make adequate correlations, all analyses were performed within the same blood samples in the same assay runs. Plasma cholesterol and TG concentrations were determined using enzymatic kits (no. 236691 and 11488872, respectively; Roche Molecular Biochemicals, Indianapolis, IN). Plasma phospholipids were determined using the phospholipids B kit (Wako Chemicals, Neuss, Germany). Plasma CETP concentration was quantified using kit CETP ELISA Daiichi (Daiichi Pure Chemicals, Tokyo, Japan). HDL fractions were obtained after precipitation of apolipoprotein (apo)B-lipoproteins from 50 μL plasma by adding 25 μL 36% polyethylene glycol 6000 (PEG6000, no.81260; Sigma Aldrich) The HDL-cholesterol and phospholipids were determined as described above. Plasma apoAI and apoB100 levels were determined with the Human ApoAI ELISA kit (no. 3710–1H; Mabtech AB, Nacka, Sweden) and Human ApoB ELISA kit (no. 3715–1H; Mabtech AB), respectively.
Cholesterol efflux study
Cholesterol efflux to total plasma and apoB-depleted human plasma were determined using the human monocyte cell line THP-1 as cholesterol donor. THP-1 cells were obtained from European Collection of Cell Cultures and maintained in medium A (RPMI 1640 with 25 mmol/L HEPES buffer, supplemented with 10% fetal bovine serum, 1% l-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin) at 37°C in 5% CO2. Before the experiment, THP-1 cells were seeded into 24-well plates at density of 5 × 105 cells per well and differentiated into macrophages with 0.1 μmol/L phorbol 12-myristate-13-acetate (no. P1585; Sigma Aldrich) within 3 days. Macrophages were washed three times with PBS and incubated in medium B (RPMI 1640 with 25 mmol/L HEPES buffer, supplemented with 2% fetal bovine serum, 1% l-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin, 50 μg/mL acetyl-LDL, and 10 μCi/mL [1α,2α(n)-3H]-cholesterol (no. NET139001MC; Perkin Elmer, the Netherlands) for 1 day at 37°C in 5% CO2. After incubation, cells were washed three times with PBS and the efflux assay was started by adding total human plasma or apoB-depleted human plasma diluted to 1% in medium C (RPMI 1640 with 25 mmol/L HEPES buffer, supplemented with 1% l-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin, and 0.5 mg/mL BSA). The whole assay was carried out in triplicate. To be able to normalize results between series of experiments and to correct for plate-to-plate variation, efflux to a standard preparation of HDL (50 μg protein/mL) was determined in triplicate. After 4-h incubation, medium was collected and centrifuged. Subsequently, [3H]cholesterol was quantified by liquid scintillation counting. Total cellular 3H-cholesterol was determined after extraction of the cells with 0.1 mol/L NaOH. Cholesterol efflux rate was calculated by dividing the 3H activity in the medium by the sum of the 3H activity in the medium and the cell extract. Background values (the efflux in the absence of plasma) were subtracted.
Statistics
Data are expressed as means ± SEM. Paired t tests were used for the statistical comparisons between measurements at baseline and after 16 weeks of caloric restriction. For correlation analysis, Pearson correlation analysis was used. A P value < 0.05 was considered statistically significant.
RESULTS
In line with our previous observations in 12 patients (11), a subset of the 27 patients who were included in the current study, the VLCD profoundly reduced bodyweight from 113.1 ± 3.7 to 87.7 ± 2.9 kg (P < 0.05) and decreased BMI from 37.2 ± 0.9 to 28.9 ± 0.8 kg/m2 (P < 0.05). In addition, in the 12 patients from whom 1H-MRS scans could be obtained, hepatic TG content reduced considerably from 21.2 ± 4.2 to 3.0 ± 0.9% (n = 12; P < 0.001) as reported previously (11).
Plasma CETP and (apo)lipoproteins
When compared with baseline, VLCD decreased plasma CETP concentration (−18.2%; P < 0.01). In addition, VLCD reduced plasma levels of total cholesterol (−13.1%; P < 0.001), TG (−45.1%; P < 0.001), phospholipid (−15.2%; P < 0.0001), LDL-cholesterol (−15.8%; P < 0.01), and apoB100 (−13.9%; P < 0.01). VLCD did not alter plasma HDL-cholesterol and HDL-phospholipids, but increased apoAI (+16.2%; P < 0.05) (Table 1). The change in body weight after 16 weeks of VLCD did not correlate with the change in either plasma TG (R2 = 0.0000; P = 0.9952), total cholesterol (R2 = 0.0471; P = 0.2769), phospholipid (R2 = 0.0305; P = 0.3837), LDL-cholesterol (R2 = 0.0320; P = 0.3717), HDL-cholesterol (R2 = 0.0086; P = 0.6460), or HDL-phospholipid (R2 = 0.0013; P = 0.8570).
Table 1.
Plasma CETP and (apo)lipoprotein levels in obese patients with type 2 diabetes mellitus and hepatic steatosis in response to 16 weeks of VLCD
| Plasma parameters | Baseline | After VLCD | Δ (%) | P value |
|---|---|---|---|---|
| CETP (μg/mL) | 2.48 ± 0.15 | 2.03 ± 0.14 | −18.2 | 0.0021 |
| Total cholesterol (mmol/L) | 5.76 ± 0.30 | 5.00 ± 0.22 | −13.1 | 0.0007 |
| Triglycerides (mmol/L) | 2.41 ± 0.28 | 1.32 ± 0.10 | −45.1 | 0.0003 |
| Phospholipids (mmol/L) | 2.69 ± 0.11 | 2.28 ± 0.07 | −15.2 | 0.0000 |
| LDL-cholesterol (mmol/L) | 3.99 ± 0.27 | 3.36 ± 0.20 | −15.8 | 0.0028 |
| ApoB100 (mg/dL) | 130 ± 6 | 111 ± 5 | −13.9 | 0.0016 |
| HDL-cholesterol (mmol/L) | 0.84 ± 0.04 | 0.91 ± 0.06 | — | NS |
| HDL-phospholipids (mmol/L) | 0.98 ± 0.03 | 0.98 ± 0.05 | — | NS |
| ApoAI (mg/dL) | 135 ± 10 | 156 ± 11 | +16.2 | 0.0174 |
Data are presented as means ± SEM (n = 27). Δ values are calculated by comparing values obtained after VLCD with those obtained at baseline from obese patients with type 2 diabetes mellitus. P values are calculated using paired Student t test. NS, not significant.
Cholesterol efflux
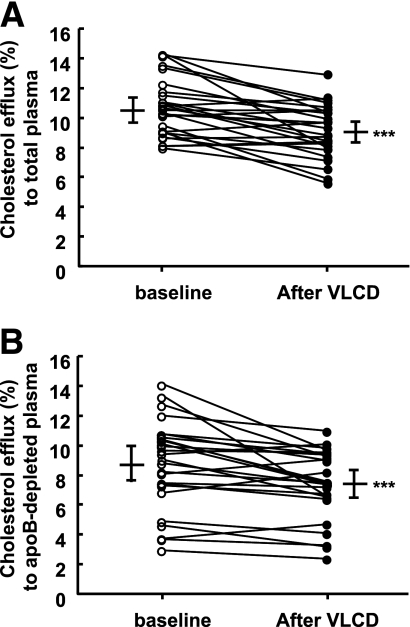
VLCD decreased cholesterol efflux from THP-1 cells to total plasma obtained from patients compared with plasma from baseline (−14.5%; P < 0.001; Fig. 1A). Similarly, the capacity of apoB-depleted plasma obtained after VLCD to promote cholesterol efflux was lower than that of apoB-depleted plasma obtained at baseline (−14.9%; P < 0.001; Fig. 1B).
Figure 1.
Cholesterol efflux to total plasma and apoB-depleted plasma. THP-1 cells were loaded with [3H]cholesterol and incubated for 4 h at 37°C with total plasma (1% vol/vol; A) or apoB-depleted plasma (1% vol/vol; B), obtained before and after VLCD from 27 obese patients with type 2 diabetes mellitus. Cholesterol efflux rate is calculated by dividing 3H-activity in the medium by the sum of the 3H-radioactivity in the medium and cell extract. Data are means ± SEM. P values are calculated using paired Student t test. ***P < 0.001 as compared with baseline.
Correlation analysis showed that cholesterol efflux to total plasma positively correlated with plasma total cholesterol (R2 = 0.2416; P < 0.001) and plasma total phospholipid (R2 = 0.3499; P < 0.001). Cholesterol efflux to total plasma positively correlated with non-HDL-cholesterol (R2 = 0.2339; P < 0.001) and non-HDL-phospholipid (R2 = 0.2855; P < 0.001) rather than HDL-cholesterol or HDL-phospholipid (both P > 0.05; Supplementary Fig. 1). Moreover, no significant correlation was observed between cholesterol efflux to plasma and apoAI (Supplementary Fig. 2).
CONCLUSIONS
A main finding from the current study is that prolonged caloric restriction by a 16-week VLCD in obese patients with type 2 diabetes mellitus and hepatic steatosis, which considerably reduces hepatic TG content (−85%) (11), also markedly decreases plasma CETP concentration (−18.2%). This observation corroborates our recent finding that a reduction of the hepatic lipid content (−30.5%), as induced by pioglitazone, also associates with a reduction in plasma CETP concentration (−11.6%) in patients with type 2 diabetes mellitus (10). However, the potency of prolonged caloric restriction to reduce hepatic lipid content and plasma CETP concentration exceeds that of pioglitazone treatment considerably.
These data are in full accordance with our previous observations that lowering hepatic lipids (i.e., TG as well as cholesterol) in APOE*3-Leiden.CETP mice by classical lipid-lowering drugs decreased hepatic CETP mRNA expression, resulting in decreased plasma CETP concentration (6–8). Because CETP expression is regulated by liver X receptor α (LXRα) for which oxysterols are natural ligands (12) and the liver cholesterol level determines LXRα activation (13), we concluded from those studies that a decrease in hepatic cholesterol content, associated with a decrease in cholesterol derivatives, reduces hepatic LXRα activation, thereby downregulating CETP mRNA transcription. Although it is unknown whether hepatic TG levels reflect levels of hepatic cholesterol and oxysterols in the current study, since we cannot assess hepatic (oxy)sterols noninvasively in humans, hepatic TG and cholesterol levels were highly correlated (r = 0.867) in 33 Chinese subjects (Dr. P. Parini, personal communication). Therefore, it is likely that the reduction in plasma CETP concentration induced by VLCD also reduces hepatic LXRα-activated CETP mRNA transcription, thereby reducing plasma CETP. Our data corroborate those of Laimer et al. (14) who showed that substantial weight loss in morbidly obese women induced by laparoscopic gastric banding surgery also decreased plasma CETP mass (−8.3%) at 1 year after surgery.
The effect of caloric restriction on HDL levels is still under debate. Although a single study reported that an average of 6 years of caloric restriction in 18 subjects increased HDL-cholesterol (15), other studies demonstrated that both 6 months and 2 years of caloric restriction in eight subjects in fact decreased plasma HDL-cholesterol (16,17). Moreover, caloric restriction had conflicting effects on HDL-cholesterol among different diet groups even in one study: HDL-cholesterol was either increased or unaffected (18). The current study is the first to show that caloric restriction by a VLCD increased the plasma level of the main HDL protein constituent apoAI (+16.2%), which is supposed to be a good (negative) predictor of cardiovascular disease risk (19). However, the VLCD did not increase HDL-cholesterol levels. Our previous studies in mice (6–8) and in patients with type 2 diabetes mellitus (10) showed that reduction of hepatic lipids and plasma CETP, as induced by drugs, were in fact related to increased plasma HDL-cholesterol levels. In fact, treatment of only 20 patients with type 2 diabetes mellitus with pioglitazone resulted in a significant increase in HDL-cholesterol despite less pronounced decreases in hepatic lipid and plasma CETP (10), suggesting that our present study including 27 patients would not be underpowered to detect a potential effect on HDL-cholesterol. It is known that the LXRα target ATP binding cassette A1 (ABCA1) plays a crucial role in HDL maturation by mediating the lipidation of plasma apoAI (20). Furthermore, hepatic ABCA1 is the main contributor to the loading of HDL with cholesterol as evidenced by studies in mice that selectively lack ABCA1 from the liver (21). Collectively, it is thus conceivable that the dramatic reduction in hepatic lipids largely downregulates the hepatic expression of ABCA1, resulting in reduced lipidation of plasma apoAI with liver-derived cholesterol, thereby counteracting the expected rise in HDL-cholesterol as a result of the reduction in CETP.
The reduced ratio of HDL-cholesterol over apoAI as induced by VLCD may be expected to result in an increased ability of total plasma and apoB-depleted plasma to induce cholesterol efflux from cholesterol-laden macrophages. However, we observed that the VLCD actually reduced the capacity of total plasma and apoB-depleted plasma to induce cholesterol efflux from THP-1 cells, even after discarding the three subjects showing the largest decrease in cholesterol efflux from the analysis. Correlation analysis revealed that HDL constituents (cholesterol, phospholipid, and apoAI) did not correlate with cholesterol efflux to plasma. Instead, the decrease in cholesterol efflux to plasma was mainly related to the decrease in total phospholipid levels in plasma. Collectively, these data indicate that the total lipoprotein surface area in plasma, rather than the HDL level, determines the capacity of plasma to induce cholesterol efflux.
Our findings are in line with those of a recent study showing that the cholesterol efflux capacity of plasma was independent of total HDL-cholesterol or apoAI levels (22). In fact, independently of HDL-cholesterol, sera with high efflux capacity had a significant increase in ABCA1-mediated efflux as a result of the presence of preβ-1 HDL. Likewise, another study showed that, although both pioglitazone and statins increase HDL-cholesterol, pioglitazone but not statins increases the cholesterol efflux capacity of plasma, and no correlation was noted between the change in HDL-cholesterol and the change in cholesterol efflux capacity (23). Overall, the cholesterol efflux capacity of plasma is thus not simply related to HDL-cholesterol or apoAI, although the capacity of HDL to mediate cholesterol efflux from macrophages has recently been established to strongly inversely associate with carotid intima-media thickness and likelihood of angiographic coronary artery disease (23). The fact that we were unable to detect a correlation between cholesterol efflux and HDL-cholesterol or apoAI thus probably indicates that VLCD does not improve the functionality of HDL with respect to mediating cholesterol efflux.
A limitation of the current study may be the small study group. Although we initially included 27 patients for the VLCD intervention, hepatic TG quantification by MRS was possible in only 12 patients, mainly related to limitations for maximum weight and circumference of the MRI scanner. Second, the design of the current human study does not permit to assess causal relationships between hepatic lipid content and plasma CETP or apoAI level. Nonetheless, the results are in accordance with data obtained from mechanistic studies in a mouse model relevant for human lipoprotein metabolism (6–8).
In conclusion, this study indicates that prolonged caloric restriction, which considerably reduces hepatic TG content, also markedly decreases plasma CETP concentration. This is in full accordance with our previous findings in APOE*3-Leiden.CETP mice, in which we showed that classical lipid-lowering drugs concurrently lowered hepatic lipids resulting in decreased plasma CETP concentration. Furthermore, the VLCD increased plasma apoAI levels without improving functionality of HDL with respect to cholesterol efflux from macrophages.
Supplementary Material
Acknowledgments
This research was supported by the Center for Translational Molecular Medicine, project PREDICCt (Grant 01C-104), by the Netherlands Heart Foundation (Grant 2007B081), and by Roba Metals B.V., IJsselstein, the Netherlands. P.C.N.R. is an established investigator of the Netherlands Heart Foundation (Grant 2009T038). No other potential conflicts of interest relevant to this article were reported.
Y.W. researched data, contributed to the discussion, and wrote the manuscript. M.S., J.T.J., S.H., and H.J.L. researched data, contributed to the discussion, and reviewed and edited the manuscript. A.d.R., A.E.M., H.P., J.A.R., J.W.A.S., and I.M.J. contributed to the discussion and reviewed and edited the manuscript. P.C.N.R. contributed to the discussion and wrote the manuscript.
Footnotes
Clinical trial reg. no. ISRCTN76920690, www.isrctn.org.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0685/-/DC1.
References
- 1.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis 2007;191:235–240 [DOI] [PubMed] [Google Scholar]
- 2.Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia 2008;51:1947–1953 [DOI] [PubMed] [Google Scholar]
- 3.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab 2003;285:E906–E916 [DOI] [PubMed] [Google Scholar]
- 4.Gupte P, Amarapurkar D, Agal S, et al. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol 2004;19:854–858 [DOI] [PubMed] [Google Scholar]
- 5.Clark JM, Diehl AM. Hepatic steatosis and type 2 diabetes mellitus. Curr Diab Rep 2002;2:210–215 [DOI] [PubMed] [Google Scholar]
- 6.de Haan W, van der Hoogt CC, Westerterp M, et al. Atorvastatin increases HDL cholesterol by reducing CETP expression in cholesterol-fed APOE*3-Leiden.CETP mice. Atherosclerosis 2008;197:57–63 [DOI] [PubMed] [Google Scholar]
- 7.van der Hoogt CC, de Haan W, Westerterp M, et al. Fenofibrate increases HDL-cholesterol by reducing cholesteryl ester transfer protein expression. J Lipid Res 2007;48:1763–1771 [DOI] [PubMed] [Google Scholar]
- 8.van der Hoorn JW, de Haan W, Berbée JF, et al. Niacin increases HDL by reducing hepatic expression and plasma levels of cholesteryl ester transfer protein in APOE*3Leiden.CETP mice. Arterioscler Thromb Vasc Biol 2008;28:2016–2022 [DOI] [PubMed] [Google Scholar]
- 9.van der Meer RW, Rijzewijk LJ, de Jong HW, et al. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation 2009;119:2069–2077 [DOI] [PubMed] [Google Scholar]
- 10.Jonker JT, Wang Y, de Haan W, et al. Pioglitazone decreases plasma cholesteryl ester transfer protein mass, associated with a decrease in hepatic triglyceride content, in patients with type 2 diabetes. Diabetes Care 2010;33:1625–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammer S, Snel M, Lamb HJ, et al. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol 2008;52:1006–1012 [DOI] [PubMed] [Google Scholar]
- 12.Luo Y, Tall AR. Sterol upregulation of human CETP expression in vitro and in transgenic mice by an LXR element. J Clin Invest 2000;105:513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang XC, Agellon LB, Walsh A, Breslow JL, Tall A. Dietary cholesterol increases transcription of the human cholesteryl ester transfer protein gene in transgenic mice. Dependence on natural flanking sequences. J Clin Invest 1992;90:1290–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laimer MW, Engl J, Tschoner A, et al. Effects of weight loss on lipid transfer proteins in morbidly obese women. Lipids 2009;44:1125–1130 [DOI] [PubMed] [Google Scholar]
- 15.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA 2004;101:6659–6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verdery RB, Walford RL. Changes in plasma lipids and lipoproteins in humans during a 2-year period of dietary restriction in Biosphere 2. Arch Intern Med 1998;158:900–906 [DOI] [PubMed] [Google Scholar]
- 17.Walford RL, Harris SB, Gunion MW. The calorically restricted low-fat nutrient-dense diet in Biosphere 2 significantly lowers blood glucose, total leukocyte count, cholesterol, and blood pressure in humans. Proc Natl Acad Sci USA 1992;89:11533–11537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tapsell L, Batterham M, Huang XF, et al. Short term effects of energy restriction and dietary fat sub-type on weight loss and disease risk factors. Nutr Metab Cardiovasc Dis 2010;20:317–325 [DOI] [PubMed] [Google Scholar]
- 19.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet 2001;358:2026–2033 [DOI] [PubMed] [Google Scholar]
- 20.Wang N, Silver DL, Costet P, Tall AR. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J Biol Chem 2000;275:33053–33058 [DOI] [PubMed] [Google Scholar]
- 21.Timmins JM, Lee JY, Boudyguina E, et al. Targeted inactivation of hepatic ABCA1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest 2005;115:1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol 2010;30:796–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011;364:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.