Abstract
OBJECTIVE
To assess the role of adiposity on the pharmacodynamics of basal insulins NPH, detemir, and glargine in type 2 diabetes mellitus (T2DM), as estimated by glucose infusion rate (GIR) and endogenous glucose production (EGP) rate in the euglycemic clamp.
RESEARCH DESIGN AND METHODS
We examined the variables that best predicted GIR and EGP in 32-h clamp studies after treatment with subcutaneous injection of 0.4 units/kg NPH, detemir, and glargine in 18 T2DM subjects (crossover).
RESULTS
A multiple regression analysis revealed that BMI best predicted GIR variation during the clamp. BMI was inversely correlated with GIR in all three insulin treatments, but was statistically significant in detemir treatment only. BMI correlated positively with residual suppression of EGP in detemir, but not with glargine and NPH treatments.
CONCLUSIONS
Adiposity blunts the pharmacodynamics of all basal insulins in T2DM. However, as adiposity increases, the effect of detemir is lower versus NPH and glargine.
We have shown that the pharmacokinetics and pharmacodynamics (PD) of basal insulins NPH, detemir, and glargine, when examined (euglycemic clamp), differ in subjects with type 2 diabetes mellitus (T2DM) (1). The primary PD end point parameter, the glucose infusion rate (GIR) over the 32 h of clamp study, was greater with glargine versus NPH and detemir.
We carried out the present analysis to determine the independent variable(s) that best predicted the PD insulin effect, as measured by GIR in clamp studies, after treatments with basal insulins NPH, detemir, and glargine.
RESEARCH DESIGN AND METHODS
After approval by the local ethical committee and informed written consent, 18 T2DM subjects on insulin and/or oral hypoglycemic agents were recruited (Supplementary Table 1) and studied (1).
Stepwise regression analyses were done to identify independent variables that best predicted GIR response. As a set of independent variables, we obtained the age of patients, duration of diabetes, plasma C-peptide, and BMI, which we included in the multiple regression analysis. GIR was the dependent variable. Pearson product moment correlation was used to assess association between changes in GIR and other parameters. Data were analyzed by a mixed-model analysis including sequence, treatment, and BMI (<29 and >29 kg/m2, i.e., below and above the median BMI of the overall group) as fixed factors and patients (nested within sequence) as a random effect followed by Bonferroni adjustments for multiple comparisons. Data in the text were expressed as means ± SD and as means ± SE in Fig. 1. All tests were two-sided tests with a nominal significance level of 0.05. Statistical analysis was performed using NCSS 2007 (NCSS, LLC, Kaysville, UT, www.ncss.com).
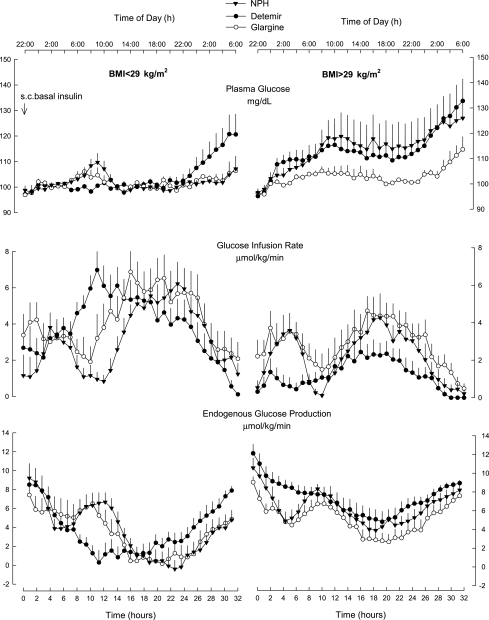
Figure 1.
Plasma glucose, rates of glucose infusion to maintain euglycemia, and endogenous glucose production in 18 subjects with T2DM given subcutaneous (s.c.) injection of basal insulin NPH or detemir or glargine (1). Left panels: nine subjects with BMI <29 kg/m2; right panels, nine subjects with BMI >29 kg/m2.
RESULTS
A multiple regression analysis revealed a significant model (F4,13 = 4.44, P = 0.017; adjusted R2 = 0.45). The significant predictor variable was BMI (standardized coefficient = −0.575), which accounted for 39% of variation in GIR variable (P = 0.013) (Supplementary Tables 2 and 3). Bivariate correlations of BMI and GIR split by treatment showed an inverse correlation that was significant only with detemir (r = −0.68, P = 0.003), but not with glargine (r = −0.41, P = 0.11) and NPH (r = −0.37, P = 0.15) (Supplementary Fig. 1). In addition, a positive and significant correlation was found between BMI and residual endogenous glucose production (EGP) with NPH and detemir (r = 0.66, P = 0.005, and r = 0.62, P = 0.006, respectively) but not with glargine (r = 0.35, P = 0.17) (Supplementary Fig. 1). An inverse and significant association with duration of action was found for NPH and detemir but not for glargine (Supplementary Table 4).
On the basis of BMI status, with all three basal insulins, GIR was lower in people with BMI >29 kg/m2 compared with people with BMI <29 kg/m2 (Fig. 1), but statistical significance was achieved with detemir treatment only (598 ± 604 and 1,564 ± 649 mg/kg, respectively; P = 0.03) and not with NPH (1,058 ± 859 and 1,282 ± 532 mg/kg) and glargine (1,408 ± 563 and 1,668 ± 807 mg/kg) (both P > 0.2). In addition, residual EGP was greater in people with BMI >29 vs. <29 kg/m2, although significance was achieved with detemir (7.8 ± 1.8 vs. 4.1 ± 2.1 μmol/kg/min, P = 0.001) but not with glargine (5.3 ± 1.3 vs. 3.8 ± 2.7 μmol/kg/min, P = 0.231) and tended to be different with NPH (6.6 ± 2.2 vs. 4.0 ± 2.1 μmol/kg/min, P = 0.068).
CONCLUSIONS
In the euglycemic clamp during intravenous infusion of regular insulin, there is an inverse correlation between BMI and GIR in T2DM (2), indicating that the greater the adiposity, the lower the insulin sensitivity. It is reasonable to translate this information to the clinical situation of the subcutaneous injected long-acting insulin, but no study so far has examined the question directly.
We recently reported that GIR area under the curve (AUC)0–32 h (a measure of insulin effect on glucose metabolism after subcutaneous injection of long-acting insulins) is greater with glargine than with NPH and detemir (1). However, in that study (1), the factor(s) responsible for GIR variations between T2DM subjects and between the three basal insulins were not assessed.
The results of the present analysis indicate that BMI is a factor that may well predict the PD of basal insulins NPH, detemir, and glargine, as indicated by the inverse correlation between BMI and PD (GIR AUC0–32 h). In addition, the results indicate that the PD of the three basal insulins is differentially affected by BMI (i.e., at increasing BMI, the PD was lower for detemir than for NPH and glargine). Likewise, EGP, the well-known primary determinant of fasting blood glucose (3), was more suppressed by glargine and NPH compared with detemir in people with greater BMI. Although all three basal insulins showed a lower suppression of EGP for increasing BMI, the correlations were significant only for NPH and detemir, suggesting that glargine has a greater effect in restraining EGP at increasing adiposity. The reasons for the lower effect of detemir at higher BMI are unclear. We acknowledge that these conclusions have limitations because of the small sample size of the study; nevertheless, the results might explain findings of recent clinical studies.
A previous crossover study, longer than 2 months, reported higher dose requirements of the long-acting insulin analogs compared with NPH insulin, in a group (n = 20) of T2DM subjects with BMI >30 kg/m2 (4). Results of studies examining larger groups of T2DM subjects for ≥6 months indicated that higher detemir insulin doses are required versus NPH (5) and glargine for similar efficacy on glycemic control (6–9). However, none of those studies ascertained the impact of BMI on insulin dose requirements. A recent post hoc analysis (10) of a study comparing insulin glargine (once a day) and detemir (twice a day) in T2DM (9) titrated to reach similar glycemic control has shown that the higher dose of insulin detemir needed was directly correlated with BMI (10).
In conclusion, BMI largely explains the PD effect of insulins NPH, detemir, and glargine in T2DM subjects previously reported (1). In fact, as BMI increases, the effect of detemir is lower versus NPH and glargine (lower GIR and greater EGP). The current study suggests that it is obesity that primarily drives the larger detemir versus NPH and glargine doses needed in T2DM.
Supplementary Material
Acknowledgments
This analysis was performed through an independent investigator-designed project, neither shared with, nor supported by, any pharmaceutical company. The study is registered as EudraCT 2007-004571-18.
F.P., P.L., P.R., and C.G.F. received grants from various companies (sanofi-aventis, Eli Lilly, Bristol-Myers Squibb, Novartis, and Merck Sharp & Dohme) for participating at meetings and congresses. G.B.B. received honoraria for scientific advising and consulting from the following companies: sanofi-aventis, MannKind, and Eli Lilly. No other potential conflicts of interest relevant to this article were reported.
F.P. wrote the study protocol, performed clamps, researched data, and reviewed and edited the manuscript. P.L. performed clamps and glucose turnover measurements, analyzed data, and reviewed and edited the manuscript. P.R. enrolled patients, performed clamps, researched data, and reviewed and edited the manuscript. P.Ca. performed clamps and laboratory assays and reviewed and edited the manuscript. A.M.A., S.M., and P.Ci. performed clamps and reviewed and edited the manuscript. G.B.B. provided the study concept and design, supervised the protocol development and the research, contributed to discussion, and reviewed and edited the manuscript. C.G.F. performed clamps, analyzed data, performed statistical analysis, and wrote the manuscript.
A preliminary report of this study was presented at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
This study is dedicated to the individuals with T2DM who volunteered for our studies.
Footnotes
Clinical trial reg. no. EudraCT 2007-004571-18, http://eudract.ema.europa.eu/.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1064/-/DC1.
References
- 1.Lucidi P, Porcellati F, Rossetti P, et al. Pharmacokinetics and pharmacodynamics of therapeutic doses of basal insulins NPH, glargine, and detemir after 1 week of daily administration at bedtime in type 2 diabetic subjects: a randomized cross-over study. Diabetes Care 2011;34:1312–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell PJ, Carlson MG. Impact of obesity on insulin action in NIDDM. Diabetes 1993;42:405–410 [PubMed] [Google Scholar]
- 3.Gerich JE. Control of glycaemia. Baillieres Clin Endocrinol Metab 1993;7:551–586 [DOI] [PubMed] [Google Scholar]
- 4.Schmid C, Krayenbühl P, Wiesli P. Increased insulin dose requirement of long-acting insulin analogues in obese patients with type 2 diabetes. Diabetologia 2009;52:2668–2669 [DOI] [PubMed] [Google Scholar]
- 5.Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006;29:1269–1274 [DOI] [PubMed] [Google Scholar]
- 6.Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia 2008;51:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollander P, Cooper J, Bregnhøj J, Pedersen CB. A 52-week, multinational, open-label, parallel-group, noninferiority, treat-to-target trial comparing insulin detemir with insulin glargine in a basal-bolus regimen with mealtime insulin aspart in patients with type 2 diabetes. Clin Ther 2008;30:1976–1987 [DOI] [PubMed] [Google Scholar]
- 8.Swinnen SG, DeVries JH. Higher dose requirements with insulin detemir in type 2 diabetes: three cases and a review of the literature. Diabetes Res Clin Pract 2009;84:e24–e26 [DOI] [PubMed] [Google Scholar]
- 9.Swinnen SG, Dain MP, Aronson R, et al. A 24-week, randomized, treat-to-target trial comparing initiation of insulin glargine once-daily with insulin detemir twice-daily in patients with type 2 diabetes inadequately controlled on oral glucose-lowering drugs. Diabetes Care 2010;33:1176–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holleman F, Wang E, Bolli GB. Detemir is associated with a higher insulin dose compared to insulin glargine across a wide BMI range (Abstract). Diabetes 2011;60 (Suppl. 1):A287
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.