Abstract
OBJECTIVE
To assess performance of nonfasting tests to screen children for dysglycemia (prediabetes or diabetes).
RESEARCH DESIGN AND METHODS
This was a cross-sectional study of 254 overweight or obese (BMI ≥85th percentile) children aged 10–17 years. Subjects came for two visits to a clinical research unit. For visit one, they arrived fasting and a 2-h glucose tolerance test and HbA1c and fructosamine testing were performed. For visit two, they arrived nonfasting and had a random plasma glucose, a 1-h 50-g nonfasting glucose challenge test (1-h GCT), and urine dipstick performed. The primary end point was dysglycemia (fasting plasma glucose ≥100 mg/dL or a 2-h postglucose ≥140 mg/dL). Test performance was assessed using receiver operating characteristic (ROC) curves and calculations of area under the ROC curve.
RESULTS
Approximately one-half of children were female, 59% were white, and 30% were black. There were 99 (39%) cases of prediabetes and 3 (1.2%) cases of diabetes. Urine dipstick, HbA1c (area under the curve [AUC] 0.54 [95% CI 0.47–0.61]), and fructosamine (AUC 0.55 [0.47–0.63]) displayed poor discrimination for identifying children with dysglycemia. Both random glucose (AUC 0.66 [0.60–0.73]) and 1-h GCT (AUC 0.68 [0.61–0.74]) had better levels of test discrimination than HbA1c or fructosamine.
CONCLUSIONS
HbA1c had poor discrimination, which could lead to missed cases of dysglycemia in children. Random glucose or 1-h GCT may potentially be incorporated into clinical practice as initial screening tests for prediabetes or diabetes and for determining which children should undergo further definitive testing.
Rates of type 2 diabetes in the U.S. pediatric population are rising (1,2). Because type 2 diabetes can be asymptomatic at diagnosis and requires tight glycemic, blood pressure, and lipid control to delay the onset of microvascular and macrovascular complications, screening for type 2 diabetes in children is endorsed by the American Diabetes Association (ADA) and the American Academy of Pediatrics (3,4). There is also increasing interest in screening for prediabetes in the pediatric population (5,6), given its increasing prevalence (7) and the documented link between prediabetes in childhood and development of diabetes in young adulthood (8).
The ADA guidelines (3) recommend that children with a BMI ≥85th percentile for age and sex and additional risk factors should be screened every 2 years starting at age 10 years or at onset of puberty and that either a fasting plasma glucose (FPG) or a 2-h glucose tolerance test be performed. However, these recommendations were based on expert opinion and were not evidence based, as acknowledged by the ADA in its Position Statement.
Both FPG and 2-h postload glucose have traditionally been used as the gold standard tests for diagnosing diabetes, but both tests require that the individual be fasting, which is an important barrier to screening. Studies have found that only a minority (4–21%) of pediatric providers in the primary care setting have screening practices consistent with the ADA guidelines (9,10) and instead use nonfasting tests, such as HbA1c, random glucose, or urinalysis.
Furthermore, in 2009, the screening and diagnosis landscape changed when an international expert committee of individuals from the ADA and their international counterparts recommended that HbA1c be exclusively used for the diagnosis of diabetes with eventual phase out of additional glucose measurements such as FPG and the 2-h postload glucose (11).
Despite the frequent use of nonfasting tests by physicians in clinical practice and the recent guideline advocating HbA1c as a diagnostic test for diabetes among children, there is a critical lack of data about the performance of nonfasting tests in children. Therefore, the objective of our study was to assess the test performance of nonfasting screening tests, including HbA1c, urinalysis, fructosamine, a nonfasting 1-h glucose challenge test (1-h GCT), and a random blood glucose for identifying adolescents aged 10–17 years with dysglycemia. Empiric information about test performance for nonfasting diabetes tests is critical for the development of evidence-based screening recommendations for prediabetes and diabetes in overweight and obese children.
RESEARCH DESIGN AND METHODS
Our study population consisted of a convenience sample of overweight or obese adolescents aged 10–17 years without known diabetes. The majority of children were recruited from pediatric primary care clinics (85%) with a smaller fraction (15%) from pediatric specialty clinics in the southeast Michigan area. Patients and families were recruited by research assistants in the clinic or responded to flyers posted in pediatric clinics and received an incentive for participating in the study. We excluded those who were pregnant and those who were on medications known to affect glucose metabolism.
Children came in for two visits to the Michigan Clinical Research Unit, where a history and physical exam were performed. For the first visit, they had a formal 2-h oral glucose tolerance test (OGTT) performed. They were fasting for a minimum of 12 h and had an intravenous catheter placed. Blood was drawn at baseline and every 30 min up to 2 h following a glucose ingestion of 1.75 g/kg to a maximum of 75 g. Testing was also conducted for HbA1c and fructosamine. Within 1–3 weeks, participants returned for a second visit in a nonfasting state. They had a random glucose test (glucose measured in a nonfasting state), a 1-h GCT (glucose measured 1 h after ingestion of 50 g of glucola in a nonfasting state), and urinalysis (measured 1 h after ingestion of 50 g glucola). We elected to use a uniform 50-g challenge (which was well tolerated by the children), given that it would be difficult for primary care providers to calculate weight-specific doses. Visit one was generally conducted in the morning, with mean time of administration of glucola 9:34 a.m., and visit two was generally conducted in the afternoon, with random plasma glucose (PG) drawn at a mean time of 1:23 p.m. The majority of families did not have access to their results until after visit two; therefore, it is unlikely that the children and their families were sensitized to the issue of diabetes.
Glucose was measured using the glucose hexokinase method, and HbA1c was measured using an NGSP-certified assay in whole blood (Pointe Scientific) using anti-human HbA1c monoclonal antibody. Glucose, fructosamine, and HbA1c were all measured using the Cobas Mira Plus Chemistry Analyzer. Urine dipstick was performed using Chemstrip 10 strips manufactured by Roche. Laboratory measurements were performed by the Michigan Diabetes Research and Training Center Core Laboratories.
Study definitions
Measured height and weight were converted to BMI percentiles according to the 2000 Centers for Disease Control and Prevention growth curves (12). Overweight was defined as an age-, sex-, and race-specific BMI ≥85th and <95th percentile, and obesity was similarly defined as a BMI ≥95th percentile. We elected to use definitions of prediabetes and diabetes based on the FPG and the 2-h PG. We acknowledge limitations of these definitions, given studies which have shown low concordance (13) and lack of reproducibility (14,15). However, these definitions have traditionally been used throughout the pediatric literature (16–18), which is important for comparison of our data with previous literature. Furthermore, these tests were the ADA-recommended gold standard tests prior to 2010 (19). With the 2010 revised guidelines (20), individuals can still be classified as having prediabetes or diabetes according to these tests, even if they are not recommended as first-line tests.
Based on the OGTT, considered the gold standard test, normal glucose metabolism was defined as a 2-h postload glucose level of <140 mg/dL and an FPG of <100 mg/dL. Prediabetes was defined as either impaired glucose tolerance (IGT) (2-h postload glucose ≥140 and <200 mg/dL) or impaired fasting glucose (IFG) (FPG ≥100 and <126 mg/dL). Diabetes was defined as a 2-h postload glucose ≥200 mg/dL or FPG ≥126 mg/dL (19). Because of the low number of children with diabetes (n = 3), we created a combined outcome of dysglycemia (prediabetes or diabetes) (n = 102). Any evidence of glucose (trace or above) was classified as a positive urine dipstick result. In sensitivity analyses, we assessed the degree to which the nonfasting tests predicted IGT and IFG separately, and we provide results of testing for prediabetes alone, excluding those from the three individuals with diabetes (Supplementary Fig. 1C).
We assessed sensitivity, specificity, positive likelihood ratio, and positive and negative predictive values at various thresholds of each test, with the exception of the urine dipstick test, for which the result was either positive or negative. Positive likelihood ratios represent the ratio of sensitivity to 1-specificity; positive predictive value represents the probability of having the disease given a positive test result, and negative predictive value represents the probability of not having the disease given a negative test result. We then used receiver operating characteristic (ROC) analysis, which assesses the trade-offs between sensitivity and specificity at various test cutoffs or thresholds for each of the four remaining nonfasting tests. We tested the homogeneity of ROC areas across the nonfasting tests.
Previous studies in adults have found that the number of hours since the last meal (postprandial time) can impact nonfasting test performance (21). Therefore, we also assessed test performance in children by postprandial time (<2 h and ≥2 h) and by time of day (a.m. vs. p.m.) for random glucose and 1-h GCT.
The prevalence of type 2 diabetes in children is low (22). For example, the recent SEARCH for Diabetes in Youth study used gold standard methods for defining type 2 diabetes and reported a prevalence of 0.04% among U.S. adolescents aged 10–19 years in 2001 (23). Therefore, our sample size calculations were based on the combined outcome of prediabetes and diabetes. Our goal was a sample size of 250, assuming a 35% prevalence of prediabetes in the population, a sensitivity of 65%, and a width of 20% for a two-sided 95% CI. Statistical analyses were performed using Stata 10.0 (Stata-Corp, College Station, TX). Sample sizes stratified by race and sex were too small to make reasonable conclusions. This study was approved by the University of Michigan institutional review board.
RESULTS
Table 1 shows demographic characteristics of the 254 children in the study. There were slightly more female than male subjects, and close to one-third of the population was black. There was a higher proportion of obese children in the sample compared with overweight children. Of the population, 39% had prediabetes, and only 1.2% of children were classified as having diabetes. Although there was a higher prevalence of prediabetes in blacks (46%) compared with whites (36%) and male subjects (44.6%) compared with female subjects (36.1%), these comparisons were not statistically significant. The majority of children in the study were pubertal, regardless of glucose tolerance status.
Table 1.
Characteristics of the study population
| Overall population | Normal glucose tolerance | IFG only | IGT only | IFG and IGT | Diabetes | |
|---|---|---|---|---|---|---|
| n | 254 | 152 | 46 | 36 | 17 | 3 |
| Age (years), mean (SD) | 13.6 (2.1) | 13.6 (2.2) | 14.0 (1.9) | 13.5 (2.0) | 13.0 (1.9) | 12.1 (2.2) |
| Sex | ||||||
| Female | 52.4 (133) | 55.9 (85) | 45.7 (21) | 55.6 (20) | 35.3 (6) | 33.3 (1) |
| Male | 47.6 (121) | 44.1 (67) | 54.3 (25) | 44.4 (16) | 64.7 (11) | 66.7 (2) |
| Race | ||||||
| White | 59.1 (150) | 63.2 (96) | 47.8 (22) | 50.0 (18) | 70.6 (12) | 66.7 (2) |
| Black | 29.9 (76) | 27.0 (41) | 45.7 (21) | 33.3 (12) | 5.9 (1) | 33.3 (1) |
| Other | 11.0 (28) | 9.8 (15) | 6.5 (3) | 16.7 (6) | 23.5 (4) | — |
| Weight status | ||||||
| Overweight (BMI ≥85th percentile and <95th percentile) | 21.6 (55) | 25.7 (39) | 19.6 (9) | 8.3 (3) | 17.7 (3) | 33.3 (1) |
| Obese (BMI ≥95th percentile) | 78.4 (199) | 74.3 (113) | 80.4 (37) | 91.7 (33) | 82.3 (14) | 66.7 (2) |
| Family history of type 2 diabetes* | ||||||
| Yes | 76.2 (166) | 70.5 (93) | 84.2 (32) | 81.3 (26) | 92.9 (13) | — |
| No | 23.8 (52) | 29.5 (39) | 15.8 (6) | 18.7 (6) | 7.1 (1) | 100.0 (2) |
| Tanner stage** | ||||||
| Median (range) | 4 (1–5) | 4 (1–5) | 4 (1–5) | 4 (1–5) | 3 (1–5) | 1.5 (1–2) |
| Prepubertal | 6.6 (13) | 6.8 (8) | 2.9 (1) | 6.7 (2) | 8.3 (1) | 50.0 (1) |
| Pubertal (Tanner stage 2 or higher) | 93.4 (184) | 93.2 (110) | 97.1 (34) | 93.3 (28) | 91.7 (11) | 50.0 (1) |
Data are % (n) unless otherwise noted.
*For those with self-reported family history.
**For those with self-reported pubertal measures.
Table 2 shows performance results of the five different nonfasting tests for identifying children with dysglycemia. Urine dipstick, for which there was only one threshold (positive vs. negative), had a very low sensitivity despite a high specificity. For the other tests, higher test thresholds resulted in a lower sensitivity and a higher specificity and lower test thresholds in a higher sensitivity and a lower specificity.
Table 2.
Test characteristics of HbA1c, urinalysis, fructosamine, random glucose, and 1-h GCT for predicting dysglycemia (prediabetes or diabetes)
| Threshold | Sensitivity (%) | Specificity (%) | Positive likelihood ratio | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|
| Urinalysis (n = 218) | |||||
| Positive | 2 | 97 | 0.75 | 34 | 59 |
| HbA1c (%) (AUC 0.54 [95% CI 0.47–0.61]; n = 254) | |||||
| 4.4 | 100 | 0 | 1.00 | 40 | |
| 4.7 | 99 | 2 | 1.01 | 41 | 75 |
| 5.0 | 93 | 9 | 1.03 | 41 | 67 |
| 5.3 | 70 | 36 | 1.08 | 42 | 63 |
| 5.5 | 45 | 57 | 1.04 | 41 | 60 |
| 5.7 | 32 | 74 | 1.23 | 45 | 62 |
| 6.0 | 15 | 92 | 1.86 | 56 | 62 |
| 6.5 | 7 | 98 | 3.48 | 70 | 61 |
| 7.0 | 2 | 100 | 100 | 60 | |
| Fructosamine (mmol/L) (AUC 0.55 [0.47–0.63]; n = 222) | |||||
| 0 | 100 | 0 | 1.00 | 40 | |
| 1.25 | 71 | 29 | 0.99 | 40 | 59 |
| 1.45 | 47 | 60 | 1.17 | 44 | 63 |
| 1.65 | 24 | 88 | 2.08 | 58 | 63 |
| 1.85 | 12 | 99 | 7.91 | 84 | 62 |
| 2.05 | 6 | 100 | 100 | 61 | |
| Random glucose (mg/dL) (AUC 0.66 [0.60–0.73]; n = 243) | |||||
| 50 | 100 | 0 | 1.00 | 40 | |
| 60 | 99 | 1 | 1.00 | 40 | 50 |
| 70 | 98 | 5 | 1.03 | 41 | 78 |
| 80 | 97 | 17 | 1.17 | 44 | 89 |
| 90 | 87 | 33 | 1.30 | 47 | 79 |
| 100 | 55 | 67 | 1.66 | 53 | 69 |
| 110 | 30 | 88 | 2.38 | 62 | 65 |
| 120 | 14 | 96 | 3.45 | 70 | 62 |
| 130 | 7 | 99 | 5.18 | 78 | 61 |
| 140 | 3 | 99 | 4.44 | 75 | 60 |
| 1-h OGTT (mg/dL) (AUC 0.68 [0.61–0.74]; n = 241) | |||||
| 60 | 100 | 0 | 1.00 | 40 | |
| 70 | 99 | 2 | 1.01 | 41 | 75 |
| 80 | 96 | 11 | 1.08 | 42 | 80 |
| 90 | 90 | 25 | 1.20 | 45 | 78 |
| 100 | 77 | 42 | 1.34 | 48 | 73 |
| 110 | 63 | 63 | 1.68 | 53 | 71 |
| 120 | 44 | 81 | 2.28 | 61 | 68 |
| 130 | 31 | 92 | 4.05 | 73 | 66 |
| 140 | 21 | 92 | 2.70 | 65 | 63 |
| 150 | 13 | 97 | 3.86 | 72 | 62 |
| 160 | 9 | 98 | 4.45 | 75 | 62 |
| 170 | 4 | 100 | 100 | 61 |
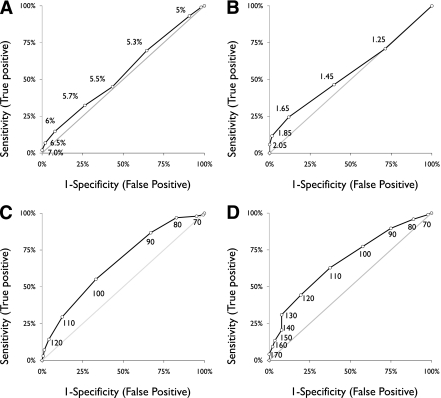
Figure 1A–D displays ROC curves at various cutoffs for each of the four remaining nonfasting tests. Discrimination was equally poor for HbA1c and fructosamine, as indicated by relatively low likelihood ratios across thresholds and low values for area under the curve (AUC). In contrast, random glucose and 1-h GCT had levels of test discrimination closer to an acceptable range. Compared with HbA1c, there was no significant difference in AUC for fructosamine, but AUC was significantly higher for both random glucose (P = 0.01) and 1-h GCT (P < 0.01). We did not find any differences in test performance by postprandial time or by time of day (a.m. vs. p.m.) for the 1-h GCT or the random glucose (data not shown).
Figure 1.
ROC curve for predicting dysglycemia (prediabetes or diabetes) at various thresholds of nonfasting tests. A: HbA1c (AUC 0.54 [95% CI 0.47–0.61]; n = 254). B: Fructosamine (AUC 0.55 [0.47–0.63]; n = 222). C: Random glucose (AUC 0.66 [0.60–0.73]; n = 243). D: 1-h GCT (AUC 0.68 [0.61–0.74]; n = 241).
ROC curves were also created for identifying individuals with IGT and IFG as separate outcomes (Supplementary Fig. 1A and B). Although AUC appeared to be quite similar for most tests, AUC for the 1-h GCT was substantially higher for predicting IGT (0.72) compared with IFG (0.58), which is consistent with the fact that both types of tests are stimulated glucose tests. Test performance was slightly lower when only those with prediabetes were included in the analysis (Supplementary Fig. 1C).
CONCLUSIONS
We systematically evaluated a variety of nonfasting screening tests used in the clinical setting for identifying dysglycemia in a population of overweight and obese adolescents and found reasonable test performance for the 1-h GCT and the random glucose. In contrast, test performance for HbA1c, fructosamine, and urinalysis was poor. Our findings have direct relevance for future recommendations for screening and diagnosis of dysglycemia among overweight and obese children and adolescents.
The poor test performance of HbA1c is notable given recent changes in diagnostic guidelines for prediabetes and diabetes (11), which advocate the use of HbA1c for identifying children and adults with diabetes (HbA1c ≥6.5%) and prediabetes (HbA1c 5.7–6.4%). We found that HbA1c had less than acceptable test performance for children with dysglycemia. Although the majority of patients in our study had prediabetes, two of three children with diabetes would have been missed using the ADA recommendation of 6.5%, with HbA1c levels of 5.1 and 5.2%.
Our findings are consistent with recent studies of HbA1c test performance in children. Using a nationally representative sample from National Health and Nutrition Examination Survey (NHANES) 1999–2006 (24), we found that an HbA1c threshold of 6.0% had low sensitivity for identifying overweight or obese children with prediabetes; sensitivity was 1.1% for identifying children with IFG (based on an FPG ≥100 mg/dL) and 0% for identifying children with IGT (based on a 2-h postload glucose ≥140 mg/dL), despite having high specificity for both outcomes (99%). In addition, the AUC for HbA1c was relatively low at 0.61 for predicting IFG and 0.53 for predicting IGT. Another study of a large cohort of obese children has also reported lower sensitivity for HbA1c (25). In contrast, adult studies have shown higher sensitivities for dysglycemia in the range of (20–25%) and higher levels of discrimination (26).
To our knowledge, the HbA1c guidelines have yet to be reviewed or endorsed by pediatric organizations such as the American Academy of Pediatrics. If the guidelines were to be endorsed by these organizations, this would most likely result in increased uptake of the test by providers, which could potentially lead to a significant proportion of missed cases of prediabetes and diabetes in the pediatric population.
Either the nonfasting 1-h GCT or the random glucose represent promising screening tests for use in the pediatric primary care setting, as these are tests that clinicians can easily order the same day of the visit. This would exclude a substantial proportion of children who would have to return for testing, and subsequently, only the subset of children with glucose levels surpassing specified thresholds of either nonfasting test would then have to undergo more formal testing with an FPG or a 2-h OGTT to confirm the diagnosis. Possible test thresholds to consider for the pediatric population for the 1-h GCT include cutoffs of 110 or 120 mg/dL, which would result in sensitivities of 63 and 44% and false-positive rates of 37 and 19%, respectively. Comparable thresholds using random glucose levels could include cutoffs of 100 or 110 mg/dL, which would result in sensitivities of 55 and 30% and false-positive rates of 33 and 12%, respectively.
Studies in adults have also found random glucose and 1-h GCT to be promising tests to use for screening for prediabetes and diabetes in adults. The Screening for Impaired Glucose Tolerance Study evaluated test performance of random glucose and 1-h GCT and found that both tests showed reasonable discrimination for identifying dysglycemia, with estimates of AUC of 0.72 for random PG and 0.82 for 1-h GCT, although their definition of dysglycemia was slightly different (IGT, IFG ≥110 mg/dL, or diabetes) (21,27). Similarly, studies in pregnant women to screen for gestational diabetes mellitus have reported reasonable test performance, hence, the use of this test as an initial screen to determine which women need full 3-h OGTT tests to look for gestational diabetes mellitus (28).
We found a very low sensitivity of urine dipstick for detecting dysglycemia, despite the fact that it was measured 1 h after ingestion of 50 g glucola. Sensitivity was even lower than for adult studies, which have reported sensitivities ranging from 18 to 64% (29). Because the renal glucose threshold is ~180 mg/dL, the sensitivity of this test for detecting diabetes would be expected to be better for diabetes than for prediabetes. However, in our study urine dipstick detected only two cases of prediabetes and failed to identify any of the three individuals with diabetes. Urinalysis is reportedly still being used for diabetes screening in the pediatric primary care setting (9); therefore, providers should perhaps be dissuaded from using this test in clinical practice.
We found no differences in test performance based on postprandial time or time of day, which contrasts with studies in adults that have reported improvements in test performance for random blood glucose with a longer postprandial period (>2 h) prior to the test (21). We also assessed test performance for predicting IFG and IGT as separate outcomes. Regardless of the outcome measure, test performance remained poor for HbA1c and fructosamine. However, when using IGT, we found significant improvements in test performance for the 1-h GCT. Both IGT and IFG predict risk for developing diabetes, but because of convenience previous screening recommendations have prioritized the use of FPG. Studies have shown that a stimulated glucose is a better predictor than FPG of progression from IGT to diabetes (30). Furthermore, FPG will often miss cases of IGT and diabetes (31,32), further lending support for the use of random glucose or the 1-h GCT test in clinical practice.
Strengths of our study include the systematic evaluation of the performance of nonfasting tests in a pediatric population, the use of a gold standard 2-h OGTT for classifying children with dysglycemia, a reasonable study sample size, and the substantial proportion of minority children who were included in the study. We also acknowledge limitations of our study. According to the ADA, two positive tests are required to make a diagnosis of prediabetes or diabetes (20), and studies have reported a lack of reproducibility of the diagnosis of prediabetes using the 2-h OGTT in children (15). Therefore, children in our cohort with 2-h postload glucose levels >200 mg/dL may not have been classified as having diabetes if a second test had been performed, which could result in improvements in test performance. Although it would have been ideal to perform two 2-h OGTTs, we elected to perform one given the additional burden on participants. Furthermore, clinical research studies of adults, including the Diabetes Prevention Project (33), or the Screening for Impaired Glucose Tolerance Study (27), have classified participants’ glucose status using just one test.
With the new guidelines, HbA1c would now be considered the gold standard for diabetes, but studies have demonstrated significant differences in diabetes and prediabetes prevalence depending on the definition of diabetes used (FPG vs. 2-h postload glucose vs. HbA1c) (34). Although it may be logical to use a chronic measure of hyperglycemia such as HbA1c rather than an acute measure such as glucose, longitudinal studies in children are needed to understand which tests are most predictive of later development of diabetes. We elected to compare the nonfasting tests against the definitions of diabetes that have been historically used in the pediatric literature for purposes of comparability with other pediatric studies.
Our population was a convenience sample of overweight and obese children from southeast Michigan rather than a systematic sample from a well-defined target population. Although we had adequate representation of black and white children, there were no Hispanic children in the sample. Furthermore, given the sample size, we did not have the power to assess test performance according to each Tanner stage. Therefore, generalizations must be made accordingly. We had to power our study to the outcome of dysglycemia given the low prevalence of childhood diabetes. However, we note that this is reflective of the epidemiology of type 2 diabetes in the U.S., which still has a very low prevalence in the adolescent population (0.02%), particularly compared with adults (23). The ADA screening guidelines were established in 2000 (3) based on reports of dramatic increases in type 2 diabetes among adolescents, but subsequent population-based epidemiologic studies have not revealed a large burden of childhood type 2 diabetes. Identification of children with prediabetes, which has a much higher burden in the pediatric population, could be considered a useful by-product of diabetes screening, which would provide the opportunity for directing targeted interventions at children at highest risk for developing diabetes.
Because of the high burden of childhood obesity, the CDC estimates that approximately 2.5 million children in the U.S. potentially qualify for diabetes screening based on the ADA screening guidelines (35), highlighting the need for effective and practical strategies for screening. Random glucose and particularly 1-h GCT, given its possible greater predictive capacity for incident diabetes (36), represent promising screening tests for use in the pediatric primary care setting. Future studies are needed to assess the feasibility, acceptability, and cost-effectiveness of these alternative screening strategies compared with current recommendations and to assess the impact of systematic prediabetes and diabetes screening on pediatric health outcomes.
Supplementary Material
Acknowledgments
J.M.L. was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (K08-DK-082386) and the Clinical Sciences Scholars Program at the University of Michigan. This project was supported by Michigan Clinical Research Unit Grant UL1RR024986, Michigan Institute for Clinical & Health Research Pilot and Collaborative Grant UL1RR024986, a Blue Cross Blue Shield Foundation of Michigan Investigator Initiated Research grant, and grants from the University of Michigan (Elizabeth Kennedy Award/Elizabeth Crosby Funds/Office of the Vice President for Research). This study made use of the Laboratory core(s) of the Michigan Diabetes Research and Training Center Core Laboratories, funded by National Institutes of Health Grant 5P60-DK-20572 from the NIDDK.
No potential conflicts of interest relevant to this article were reported.
J.M.L. researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. A.G. researched data and reviewed and edited the manuscript. E.-L.W. and J.L. researched data and reviewed and edited the manuscript. J.G.G. contributed to discussion and reviewed and edited the manuscript.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0827/-/DC1.
References
- 1.Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr 1996;128:608–615 [DOI] [PubMed] [Google Scholar]
- 2.Lipton RB, Drum M, Burnet D, et al. Obesity at the onset of diabetes in an ethnically diverse population of children: what does it mean for epidemiologists and clinicians? Pediatrics 2005;115:e553–e560 [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association Type 2 diabetes in children and adolescents. Pediatrics 2000;105:671–680 [DOI] [PubMed] [Google Scholar]
- 4.Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics 2007;120(Suppl. 4):S193–S228 [DOI] [PubMed] [Google Scholar]
- 5.Kaufman FR. Type 2 diabetes in children and youth. Rev Endocr Metab Disord 2003;4:33–42 [DOI] [PubMed] [Google Scholar]
- 6.Botero D, Wolfsdorf JI. Diabetes mellitus in children and adolescents. Arch Med Res 2005;36:281–290 [DOI] [PubMed] [Google Scholar]
- 7.Li C, Ford ES, Zhao G, Mokdad AH. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005-2006. Diabetes Care 2009;32:342–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS. Fasting plasma glucose levels within the normoglycemic range in childhood as a predictor of prediabetes and type 2 diabetes in adulthood: the Bogalusa Heart Study. Arch Pediatr Adolesc Med 2010;164:124–128 [DOI] [PubMed] [Google Scholar]
- 9.Rhodes ET, Finkelstein JA, Marshall R, Allen C, Gillman MW, Ludwig DS. Screening for type 2 diabetes mellitus in children and adolescents: attitudes, barriers, and practices among pediatric clinicians. Ambul Pediatr 2006;6:110–114 [DOI] [PubMed] [Google Scholar]
- 10.Anand SG, Mehta SD, Adams WG. Diabetes mellitus screening in pediatric primary care. Pediatrics 2006;118:1888–1895 [DOI] [PubMed] [Google Scholar]
- 11.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002;246:1–190 [PubMed] [Google Scholar]
- 13.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 2002;19:708–723 [DOI] [PubMed] [Google Scholar]
- 14.Mooy JM, Grootenhuis PA, de Vries H, et al. Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn Study. Diabetologia 1996;39:298–305 [DOI] [PubMed] [Google Scholar]
- 15.Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab 2008;93:4231–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med 2002;346:802–810 [DOI] [PubMed] [Google Scholar]
- 17.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care 2009;32:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolan LM, Bean J, D’Alessio D, et al. Frequency of abnormal carbohydrate metabolism and diabetes in a population-based screening of adolescents. J Pediatr 2005;146:751–758 [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2009;32(Suppl. 1):S62–S67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziemer DC, Kolm P, Foster JK, et al. Random plasma glucose in serendipitous screening for glucose intolerance: screening for impaired glucose tolerance study 2. J Gen Intern Med 2008;23:528–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JM. Why young adults hold the key to assessing the obesity epidemic in children. Arch Pediatr Adolesc Med 2008;162:682–687 [DOI] [PubMed] [Google Scholar]
- 23.Liese AD, D’Agostino RB, Jr, Hamman RF, et al. ; SEARCH for Diabetes in Youth Study Group The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics 2006;118:1510–1518 [DOI] [PubMed] [Google Scholar]
- 24.Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr 2011;158:947–952 [DOI] [PMC free article] [PubMed]
- 25.Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson DE, Rhee MK, Herrick K, Ziemer DC, Twombly JG, Phillips LS. Screening for diabetes and pre-diabetes with proposed A1C-based diagnostic criteria. Diabetes Care 2010;33:2184–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips LS, Ziemer DC, Kolm P, et al. Glucose challenge test screening for prediabetes and undiagnosed diabetes. Diabetologia 2009;52:1798–1807 [DOI] [PubMed] [Google Scholar]
- 28.Carr SR. Screening for gestational diabetes mellitus. A perspective in 1998. Diabetes Care 1998;21(Suppl. 2):B14–B18 [PubMed] [Google Scholar]
- 29.Engelgau MM, Aubert RE, Thompson TJ, Herman WH. Screening for NIDDM in nonpregnant adults. A review of principles, screening tests, and recommendations. Diabetes Care 1995;18:1606–1618 [DOI] [PubMed] [Google Scholar]
- 30.Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997;46:701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mannucci E, Bardini G, Ognibene A, Rotella CM. Screening for diabetes in obese patients using the new diagnostic criteria. Diabetes Care 1998;21:468–469 [PubMed] [Google Scholar]
- 32.Barzilay JI, Spiekerman CF, Wahl PW, et al. Cardiovascular disease in older adults with glucose disorders: comparison of American Diabetes Association criteria for diabetes mellitus with WHO criteria. Lancet 1999;354:622–625 [DOI] [PubMed] [Google Scholar]
- 33.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care 2010;33:562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fagot-Campagna A, Saaddine JB, Engelgau MM. Is testing children for type 2 diabetes a lost battle? Diabetes Care 2000;23:1442–1443 [DOI] [PubMed] [Google Scholar]
- 36.Abdul-Ghani MA, Stern MP, Lyssenko V, Tuomi T, Groop L, Defronzo RA. Minimal contribution of fasting hyperglycemia to the incidence of type 2 diabetes in subjects with normal 2-h plasma glucose. Diabetes Care 2010;33:557–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.