Abstract
Mechanisms underlying chronic pain differ from those underlying acute pain. In chronic pain states, central nervous system (CNS) factors appear to play particularly prominent roles. In the absence of anatomical causes of persistent pain, medical sub-specialties have historically applied wide-ranging labels (e.g. fibromyalgia -FM, irritable bowel syndrome, interstitial cystitis, somatization) for what now is emerging as a single common set of CNS processes. The hallmark of these “centrally-driven” pain conditions is a diffuse hyperalgesic state identifiable using experimental sensory testing, and corroborated by functional neuroimaging. The characteristic symptoms of these central pain conditions include multifocal pain, fatigue, insomnia, memory difficulties, and a higher rate of co-morbid mood disorders. In contrast to acute and “peripheral” pain states that are responsive to NSAIDs and opioids, central pain conditions respond best to CNS neuromodulating agents such as serotonin-norepinephrine re-uptake inhibitors (SNRIs) and anticonvulsants.
Keywords: Central pain, Peripheral pain, Chronic pain, Neuroimaging, Osteoarthritis, Fibromyalgia
The beginning of central pain
Recent findings suggest that subsets of individuals with heretofore-considered “peripheral” or nociceptive pain states, such as knee osteoarthritis (KOA) and chronic low back pain (CLBP), may have prominent CNS contributions to their pain. The evidence for this includes both mechanistic studies (i.e. experimental pain testing, functional neuroimaging, and genetic studies) and therapeutic trials. Thus, any chronic pain condition may be a “mixed pain state” with variability at the level of the individual, regarding the degree to which peripheral vs. central factors are playing a role. This has tremendous implications for the treatment of chronic pain, because subsets of individuals with any rheumatologic disorder may have components of central pain, and the pharmacological and non-pharmacological approaches for treating this type of pain are quite different than those that are effective for treating “peripheral” pain due to damage or inflammation.
While there are clear descriptions of individuals with what we now call fibromyalgia going back centuries in the medical literature, Sir William Gowers coined the term of “fibrositis”, which was considered a form of muscular rheumatism caused by inflammation of fibrous tissue overlying muscles. Although other terms such as “psychogenic rheumatism” were proposed and used in the mid-20th century, the term fibrositis remained the most widely used term to describe individuals with chronic widespread pain and no alternative explanation.
Many investigators now believe that chronic pain is itself a disease, and the location of the body where it arises may not be as relevant as an individual’s genetically determined pain sensitivity, combined with neuroplastic changes that can occur in the central nervous system (CNS) that lead to augmented pain transmission. These heightened states of pain sensitivity can be associated with hyperalgesia (increased pain in response to normally painful stimuli) and or allodynia (pain in response to normally nonpainful stimuli). These states can be triggered by an initial peripheral injury or inflammatory process and may be regional or widespread. The concomitant influence of a separate outside stressors (i.e., infection or trauma) may also play a role in the chronicity of the disease (1, 2).
Several authors began to suggest that fibromyalgia was a misnomer because there was not inflammation of the muscles. Moldofsky and colleagues performed seminal studies showing that individuals with fibrositis suffered from objective sleep disturbances, and further showed that these same symptoms could be induced in healthy individuals deprived of sleep (3-6). Hudson and colleagues were arguably the first investigators to note the strong familial tendency to develop fibromyalgia, and proposed that this condition is a variant of depression, coining the term “affective spectrum disorder”(7, 8). In parallel during this same period of time, Yunus and colleagues similarly began to note the high frequency of associated “functional somatic syndromes” such as irritable bowel syndrome and headache with fibromyalgia, again steering the focus away from skeletal muscle (9). Nonetheless, the theories positing a pathophysiologic role of skeletal muscle took time to fade, persisting into the mid-1990’s (10-12).
Just as spastic colitis became irritable bowel syndrome, temporomandibular joint syndrome became temporomandibular disorder (when it was recognized that the problem was not in the joint), chronic EBV syndrome became chronic fatigue syndrome (CFS) (when it was realized that this syndrome occurs commonly after many viral illnesses and without infection with only this pathogen, and fibrositis became fibromyalgia.
Fibromyalgia appears to be more than simply what many clinicians identify as fibromyalgia (FM). There is now significant evidence that fibromyalgia is part of a much larger continuum that has been called many things, including functional somatic syndromes, medically unexplained symptoms, chronic multisymptom illnesses, somatoform disorders, and perhaps most appropriately, central pain or central sensitivity syndromes. Yunus et. al. showed FM to be associated with tension type headache, migraine and irritable bowel syndrome (IBS) (9). Together with primary dysmenorrhea, these entities were depicted by Yunus in a Venn diagram in 1984, emphasizing the epidemiological and clinical overlap between the syndromes. In this manuscript, the more recent term Central Sensitivity Syndromes (CSS) as proposed by Yunus is used, because we feel that this represents the best nosological term at present for these syndromes (13).
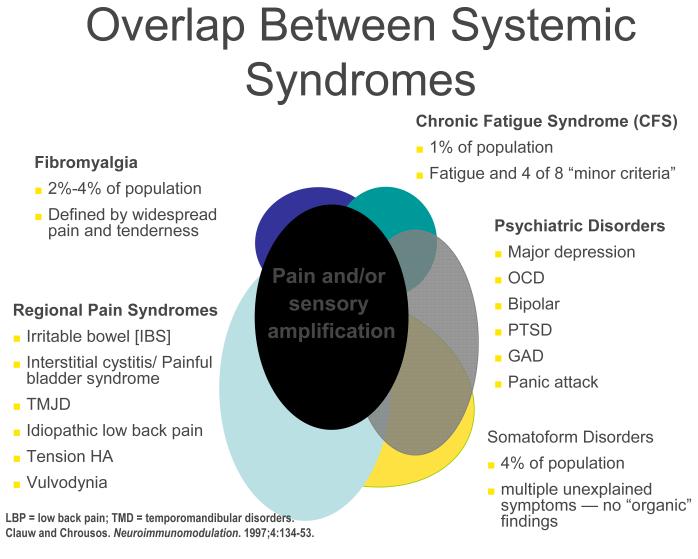
CSS disorders can overlap with a variety of psychiatric disorders. This overlap likely occurs at least in part because of same neurotransmitters (albeit in different brain regions) are operative in psychiatric conditions. The presence of co-morbid psychiatric disturbances is somewhat more common in individuals with CSS seen in tertiary care settings than primary care settings (14). Figure 1 demonstrates the overlap between FM, CFS, and a variety of regional pain syndromes as well as psychiatric disorders – and shows that the common underlying pathophysiological mechanism seen in most individuals with FM, and large subsets of individuals with these other syndromes, is central nervous system pain or sensory amplification.
Fig 1.
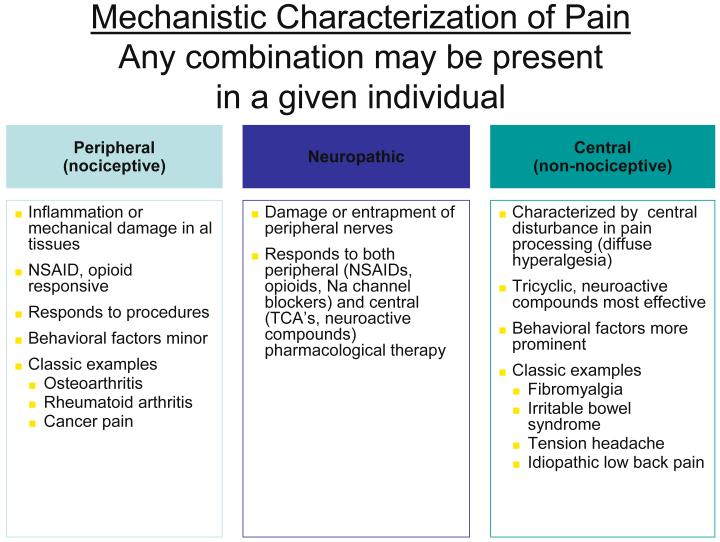
The use of research methods such as epidemiological and twin studies, experimental pain testing, functional imaging, and modern genetics has led to substantial advances in understanding several of these conditions, most notably FM, IBS, and TMJD. Together, these advances have led to an emerging recognition that chronic central pain itself is a “disease”, and that many of the underlying mechanisms operative in these heretofore “idiopathic” or “functional” pain syndromes may be similar, no matter whether the pain is present throughout the body (e.g. in FM), or localized to the low back, the bowel, or the bladder. Hence, the more contemporary terms used to describe conditions such as FM, IBS, TMJD, vulvodynia, and related entities include “central”, “neuropathic” or “non-nociceptive” pain (15, 16). Furthermore, many investigators feel that the neurobiological underpinnings of these conditions undermine the psychiatric construct of “somatization”, at least with respect to the notion that these phenomena are the somatic representation of psychological distress, with no “real” pathological basis. Figure 2 below shows a suggested schema for classifying pain syndromes based upon their underlying mechanism. It is important to recognize that even patients with a component of “peripheral” pain such as osteoarthritis or rheumatoid arthritis will often have elements of central pain that needs to be treated as such, which is why the fibromyalgia construct has moved well beyond simply relevance to functional somatic syndromes (17-19). The current thinking about these overlapping symptoms and syndromes includes:
In the general population, the presence and severity of these symptoms occur over a very wide continuum. All of our current diagnostic labels are at some level arbitrary because there is no objective tissue pathology or gold standard to which “disease” can be anchored.
There is a strong familial predisposition to these symptoms and illnesses, and studies clearly show that these somatic symptoms and syndromes are separable from depression and other psychiatric disorders (20, 21).
A variety of biological stressors seem to be capable of either triggering or exacerbating these symptoms and illnesses, including physical trauma, infections, early life trauma, deployment to war, as well as some types of psychological stress (e.g., there was no increase in somatic symptoms or worsening of fibromyalgia following the terrorist attacks of 9/11)(22-25).
Groups of individuals with these conditions (e.g. FM, IBS, headache, temporomandibular joint disorder) display diffuse hyperalgesia (increased pain in response to normally painful stimuli) and/or allodynia (pain in response to normally non-painful stimuli). Many of these conditions have also been shown to demonstrate more sensitivity to many stimuli other than pain (i.e., auditory, visual) and data suggest that these individuals have a fundamental problem with pain or sensory processing rather than an abnormality confined to the specific body region where the pain is being experienced. In fact, the expanded relevance of the fibromyalgia construct relates to the idea that all individuals (with and without pain) have different “volume control” settings on their pain and sensory processing. As such, their position on this bell-shaped curve of pain or sensory sensitivity determines to a large part whether they will have pain or other sensory symptoms over the course of their lifetime and how severe these symptoms will be.
Other shared underlying mechanisms have been identified in these illnesses that may be partly responsible for symptom expression in addition to pain and sensory amplification. These mechanisms will not be reviewed in detail in this manuscript but include the following: 1) neurogenic inflammation, especially of mucosal surfaces, leading to increased mast cells and the appearance of a mild inflammatory process; 2) dysfunction of the autonomic nervous system; and 3) hypothalamic pituitary dysfunction.
Individuals with these conditions typically do not respond to therapies that are effective when pain is due to damage or inflammation of tissues (e.g., NSAIDs, opioids, injections, surgical procedures). Similar types of therapies are efficacious for all of these conditions, including both pharmacological (e.g., tricyclic compounds such as amitriptyline) and nonpharmacological treatments (e.g., exercise and cognitive behavioral therapy).
Fig 2.
Epidemiology and clinical studies
The large numbers of studies that have directly compared the rate of FM in other related CSS, and vice-versa, or rates of co-morbidities between syndromes, will not be reviewed because these have been covered elsewhere. Instead, we will focus on describing several lines of research that have better clarified the overall picture with respect to the inter-relationships of these symptoms and syndromes.
Twin studies
Kato and colleagues, using a large Swedish twin registry, have performed a series of studies first showing the co-morbidities with chronic widespread pain, and then later examined a number of functional somatic syndromes and the relationship of these symptoms to those of depression and anxiety (18, 26). These studies clearly demonstrated that functional somatic syndromes such as FM, CFS, IBS, and headache have latent traits (e.g. multifocal pain, fatigue, memory and sleep difficulties) that are different than (but overlap somewhat with) psychiatric conditions such as anxiety and depression. Interestingly, the findings are exactly those found in functional neuroimaging studies, where, for example, individuals with FM alone primarily have increased activity in the regions of the brain that code for the sensory intensity of stimuli (e.g., the primary and secondary somatosensory cortices, posterior insula, thalamus) whereas the FM patients with co-morbid depression also have increased activation in brain regions coding for the affective processing of pain, such as the amygdala and anterior insula (27). The notion that there are two overlapping sets of traits, one being pain and sensory amplification, and the other being mood and affect, is also seen in other genetic studies of idiopathic pain syndromes (28, 29). Twin studies have also been useful in helping examine potential underlying mechanisms versus “epiphenomena”. Buchwald and colleagues have compared identical twins with and without symptoms and have found that, in many cases, the two twins share abnormalities in sleep or immune function, yet have markedly different symptom profiles. These investigators have likewise suggested that this is evidence of a problem with perceptual amplification in the affected twins (30).
The role of environmental stressors in triggering these illnesses
As with most illnesses that may have a genetic underpinning, environmental factors may play a prominent role in triggering the development of central sensitivity syndrome related conditions. Environmental “stressors” temporally associated with the development of either fibromyalgia or chronic fatigue syndrome include early life trauma, physical trauma (especially involving the trunk), certain infections such as Hepatitis C, Epstein Barr virus, parvovirus, Lyme disease, and emotional stress. The disorder is also associated with other regional pain conditions or autoimmune disorders (31-33). Of note, each of these stressors only leads to CWP or fibromyalgia in approximately 5 – 10% of individuals who are exposed; the overwhelming majority of individuals who experience these same infections or other stressful events regain their baseline state of health.
Wolfe’s “Symptom Inventory”
Although an early advocate of the FM construct, more recently, Wolfe has become critical of the construct, arguing that FM is not a discrete illness but rather the end of a continuum (34). A contrary opinion is that FM is both a discrete illness (i.e., an individual with FM) and the end of a continuum of pain processing. Wolfe has performed seminal work in showing that the degree of fibromyalgia symptoms an individual with any rheumatic disorder has (including individuals with osteoarthritis, rheumatoid arthritis, regional pain syndromes, etc.) as measured by his Symptom Inventory is closely correlated with their level of pain and/or disability, even if they do have a “peripheral” cause for their pain (35). The Symptom Inventory (or other measures of the current or lifetime level of somatic symptoms or “somatization”) is a very good measure for whether an individual has a CSS or an element of central sensitivity, no matter if they also have a peripheral cause for their pain or not. We predict that genetic factors such as those discussed below will be shown to be highly predictive of these measures, and that functional imaging (e.g., hyperactivity or increases in excitatory neurotransmitters in brain regions such the insula that code for the intensity of all sensory information) and other research methods will similarly show that these self-report measures will have strong biological underpinnings (36-39).
Genetic and familial predisposition
Pain is ultimately experienced in the brain, not in the peripheral tissues, and the function of pain processing systems throughout the body markedly influences who has pain and how much pain an individual experiences. Just as we know there is tremendous variability in pain sensitivity between strains of rodents, there is similarly great variability in pain sensitivity among humans. Evidence exists for a strong familial component to FM and all other CSS. Arguably, this component has been best studied in twin studies comparing a variety of functional somatic syndromes, and in fibromyalgia. Regarding the development of FM, Arnold and colleagues showed that the first degree relatives of individuals with FM had an eight-fold greater risk of developing FM compared with those in the general population (20). Family members of individuals with FM are more sensitive to pressure stimulation (i.e. have a lower pain threshold) than family members of controls, irrespective of the presence of pain. Furthermore, family members of individuals with FM are also much more likely to have other pain syndromes, such as IBS, TMJD, headaches, and other regional pain syndromes (40, 41). Similarly, strong genetic predisposition to chronic pain, and to nearly all of the CSS syndromes, has similarly been noted. These observations are congruent with the twin studies that suggest that approximately 50% of the risk of developing one of these disorders is genetic, and 50% environmental.
Zubieta et al first showed that the COMT Val158Met polymorphism was responsible for differential pain sensitivity in humans, working in part by modulating the endogenous-opioid system (42)-3}. Diatchenko and colleagues demonstrated that subsets of individuals could be identified based on the findings in 4 COMT single-nucleotide polymorphisms (SNPs), termed low pain sensitive (LPS), average pain sensitive(APS), and high pain sensitive (HPS) groups (43). These three subgroups are highly predictive of pain sensitivity in a variety of different experimental pain-inducing tasks (28). A prospective cohort of 240 pain-free individuals phenotyped at baseline and followed for 3 years demonstrated that individuals in the HPS group were 3 times as likely as the others to develop TMJ disorder (43). A differential effect of COMT in male and female patients was subsequently noted by van Meurs et al (44) is also not surprising given what is known about COMT. COMT is regulated by estrogen and has been shown to be at least partly responsible for sex differences in several different “phenotypic” characteristics of women and men, including pain sensitivity. Because these types of genetic factors may play a strong role in determining an individual’s experience with pain, it is equally as likely that genetic and epigenetic factors also play key roles in most rheumatic diseases and may help account for sex differences in patient reported outcomes noted in many rheumatologic disorders.
Other candidate genes have been identified which may play a role in the susceptibility to central pain mechanisms. This area is constantly evolving, with potential genes identified each year. Studies in a cohort of patients with chronic widespread pain did not show an association between the frequency of a GCH1 “pain-protective” haplotype of GTP cyclohydrolase when compared with controls and no significant associations were observed between the OPRM1 (mu opiod receptor) SNP (45).
Once fibromyalgia is established, by far the most consistently detected objective abnormalities involve pain and sensory processing systems. Evidence of augmented pain and sensory processing is the most reproducible pathogenic feature of these illnesses. Since FM is defined in part by tenderness, considerable work has been performed exploring the potential reason for this phenomenon. The results of two decades of psychophysical pressure pain testing in fibromyalgia have been very instructive (46).
One of the earliest findings in this regard is that tenderness in fibromyalgia is not confined to tender points, but instead extends throughout the entire body (47-49). Theoretically, such diffuse tenderness could be either primarily due to a psychological factor (e.g., hypervigilance, where individuals are too attentive to their surroundings), or neurobiological influence (e.g., the plethora of factors that can lead to temporary or permanent amplification of sensory input) factors.
Early studies typically used dolorimetry to assess pressure pain threshold, and concluded that tenderness was in large part related to psychological factors, because these measures of pain threshold were correlated with levels of distress (34, 49-52). To minimize the biases associated with “ascending” (i.e., the individual knows that the pressure will be predictably increased) measures of pressure pain threshold, Petzke and colleagues performed a series of studies using more sophisticated paradigms using random delivery of pressures (53-56). These studies showed that: 1) the random measures of pressure pain threshold were not influenced by levels of distress of the individual, whereas tender point count and dolorimetry exams were; 2) fibromyalgia patients were much more sensitive to pressure even when these more sophisticated paradigms were used; 3) fibromyalgia patients were not any more “expectant” or “hypervigilant” than controls; and 4) pressure pain thresholds at any four points in the body are highly correlated with the average tenderness at all 18 tender points and 4 “control points” (the thumbnail and forehead).
In addition to the heightened sensitivity to pressure noted in fibromyalgia, other types of stimuli applied to the skin are also judged as more painful or noxious by these patients. Fibromyalgia patients also display a decreased threshold to heat (56-59), and electrical stimuli (60).
Gerster and colleagues were the first to demonstrate that fibromyalgia patients also display a low noxious threshold to auditory tones, suggesting that this was a more global problem in sensory processing in some (61). A recent study by Geisser and colleagues used an identical random staircase paradigm to test fibromyalgia patients’ threshold to the loudness of auditory tones, and to pressure (59). This study found that fibromyalgia patients displayed low thresholds to both types of stimuli, and the correlation between the results of auditory and pressure pain threshold testing suggested that some of this was due to shared variance, and some unique to one stimulus or the other. The notion that fibromyalgia and related syndromes might represent biological amplification of all sensory stimuli has significant support from functional imaging studies that suggest that the insula is the most consistently hyperactive region. This region has been noted to play a critical role in sensory integration, with the posterior insula serving a purer sensory role, and the anterior insula being associated with the emotional processing of sensations (38, 62, 63).
These same findings of hyperalgesia and allodynia have been noted in most of the other conditions acknowledged to be part of this continuum, including IBS, TMJD, tension type headache, idiopathic low back pain, vulvodynia, and interstitial cystitis (25, 64-68). Brain imaging studies also the existence of central pain augmentation in FM, IBS, low back pain, and several other of these conditions (68-71).
The role of specific neurotransmitters
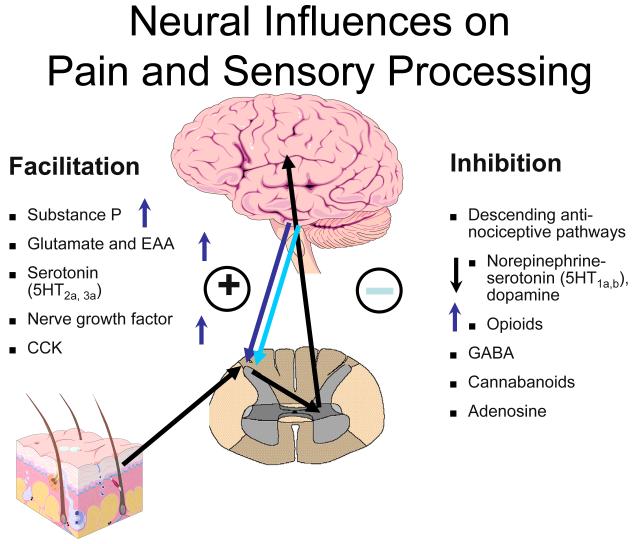
Rheumatologists might best understand pain and sensory processing by considering that this type of processing is controlled in a manner very similar to immune function. Just as high levels of pro-inflammatory cytokines, or low levels of anti-inflammatory cytokines, can move an individual towards hyperimmune function, there are neurotransmitters that are similarly known to either increase or decrease pain transmission in the CNS. Overall, the analogy of an increased “volume control” or gain“ setting on pain and sensory processing is supported by studies from a variety of sources. Similar to essential hypertension, where a variety of root causes can lead to elevated systemic blood pressure, these disorders represent ”essential hypertension of pain and sensory processing pathways“. Elevated levels of neurotransmitters that tend to be pronociceptive (i.e. on the left side of Figure 3 below) or reduced levels of neurotransmitters that inhibit pain transmission (i.e. on the right side of the figure) have a tendency to increase the volume control, and drugs that block neurotransmitters on the left or augment activity of those on the right will typically be found to be effective treatments, at least for a subset of individuals with this spectrum of illness.
Fig 3.
The arrows on Figure 3 indicate the direction of the abnormalities in these neurotransmitter levels (either in the CSF or brain) that have been identified to date in fibromyalgia. As noted, in FM, there is evidence for increases in the CSF levels of Substance P, glutamate, nerve growth factor, and brain derived neurotrophic factor, and low levels of the metabolites of serotonin, norepinephrine, and dopamine, any of which could lead to an ”increase in the volume control“ and augmented pain and sensory processing (72-75). The only neurotransmitter system that has been studied to date and not found to be out of line in a direction that would cause augmented pain transmission is the endogenous opioid system. Both CSF levels and brain activity by functional neuroimaging appears to be augmented, not reduced (as would cause augmented pain processing) in FM, which may be why opioidergic drugs do not work well to treat FM and related pain syndromes (76, 77).
Potential role of cytokines in these illnesses
Although the CSS conditions all were originally felt to be autoimmune or inflammatory diseases and then later felt not to be, recent findings are leading to a re-consideration of whether subtle inflammatory changes may be responsible for some of the symptoms seen. Immunological cascades have a role in the maintenance of central sensitivity and chronic pain which is enhanced through release of pro-inflammatory cytokines by CNS glial cells; thus, the traditional paradigm regarding inflammatory versus non-inflammatory pain may gradually become less dichromatic. As may be expected in any complex biological system, a delicate apparatus of checks and balances is at work in the spinal transmission of pain. Multiple inhibitory transmitters act at the spinal level to reduce the ”volume“ of pain transmission. Serotonin, norepinephrine, enkephalins, dopamine and gamma-ammino-butyric – acid (GABA) (75) are among the better known players in this balance.
Similar treatments work for many of the CSS entities
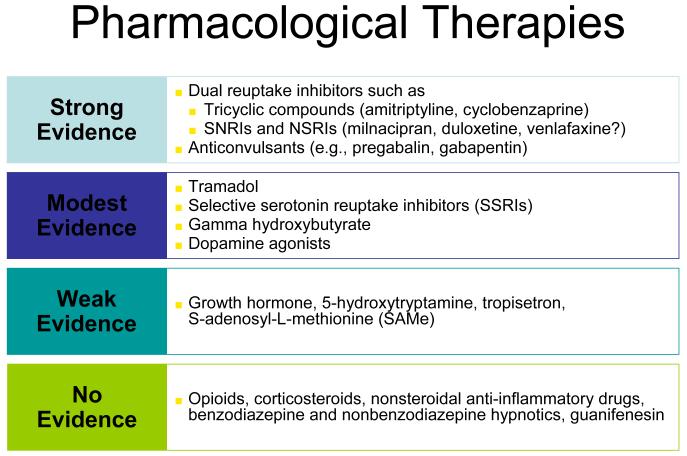
Several drug and non-drug therapies have been shown to be effective for nearly any of the CSS disorders, further reinforcing that this may well be a large overlapping disorder rather than several separate ones. Amongst classes of drugs, substantial data suggest that tricyclic compounds are effective for treating most of the conditions noted (78-80). Newer serotoninnorepinephrine re-uptake inhibitors such as duloxetine and tramadol have similarly been shown to be effective across a broad range of these conditions (81), and interestingly duloxetine had much earlier been shown to be helpful in treating the pain associated with depression, which is not surprising. The alpha-2-delta ligands such as pregabalin and gabapentin are also being shown to be efficacious in a wide range of these entities (81, 82).
Figure 4 lists the classes of drugs and their level of evidence in fibromyalgia, but in general those drugs with the highest level of evidence in fibromyalgia are also being shown to work in subsets of individuals with CSS. More importantly, drugs such as duloxetine are being shown to be effective in conditions such as osteoarthritis and low back pain, pointing out the these central mechanisms that are ”front and center“ in patients with syndromes such as fibromyalgia may be also palying prominent roles in conditions heretofore thought to be peripheral pain syndromes. But we have known for some time that hyperalgesia and other central factors, as well as various other indicators of a wide range of ”fibromyaglia-ness, is present in conditions such as osteoarthritis and low back pain.
Fig 4.
It is of note that any one of these classes of drugs only works well in about a third of patients, which is entirely consistent that this is a strongly genetic – but polygenic – disorder and thus will need different treatments in different individuals. Going back to the “essential hypertension of pain processing pathway” analogy, just as we use 8 – 10 classes of drugs acting in different body systems and at different molecular targets to control hypertension, and individuals may respond very well to one class of anti-hypertensive drug but not another, the same is likely true of CSS syndromes. Individuals may only respond to one of these classes of drugs or may often be on several classes of centrally-acting analgesics (e.g. a low dose of cyclobenzaprine at bedtime, pregabalin or gabapentin either just at bedtime or twice daily, and a serotoninnorepineprine reuptake inhibitor such as duloxetine or milnacipran during the day. However, our current pharmacological armamentarium is not nearly as well developed for central pain as for essential hypertension, which is likely one of the reason that these syndromes are often still difficult to treat.
Figure 4 also points out that classes of drugs that are quite effective for “peripheral” pain due to damage or inflammation in peripheral tissues such as NSAIDs and opiods are not effective analgesics in central pain states. There are even some data suggesting that giving opioids to individuals with central pain states may worsen their pain, by leading to opioid-induced hyperalgesia that could augment and worsen the baseline hyperagesia that may be playing a central pathogenic role in these conditions.
Just as many pharmacological therapies work across all or most of these conditions, similarly non-pharmacological therapies such as education, exercise, and cognitive behavioral therapy have been demonstrated to be effective across nearly all of the CSS conditions (83-85).
Opposing the notion of a true peripheral/nociceptive pain state: recent data from osteoarthritis (OA)
Until recently, pain in OA was largely been attributed to joint damage, and nearly all previous therapies were aimed at treating pain localized to the joint, including exercise, topical analgesics, oral nonsteroidal anti-inflammatory drugs (NSAIDs), injections of various preparations, opioids, and joint replacment. It has been evident for some time that peripheral factors can, at best, only partially explain the pain and other symptoms suffered by individuals with OA. Population-based studies consistently show a poor relationship between the degree of “pathology” in OA and reported pain intensity. In fact, in population-based studies approximately 30 – 40% of KOA patients with the most severe forms of radiographic knee OA (KOA; Kellgren-Lawrence grades III and IV) have no pain.(86, 87). Furthermore, many OA patients that theoretically have a pathological process confined to one or only a few joints experience symptoms that cannot be explained by the “peripheral” model of pathogenesis. Multifocal pain in areas not commonly affected by OA is common in individuals identified as having KOA.(88). Similarly, other somatic symptoms that would not be explained by a purely peripheral problem are often seen. For example, a recent study from our group showed that fatigue was a prominent problem in individuals with KOA, and in many individuals it was a more functionally limiting symptom than was pain (89). Insomnia likewise occurs in a sizable proportion of OA patients, and has been shown to improve along with pain in the small proportion of individuals with KOA that responded to opioids, suggesting that insomnia and pain may have overlapping biological underpinnings (90, 91).
Peripheral factors are clearly important in OA, as seen in a recent study by Neogi and colleagues that elegantly demonstrated that for individuals with asymmetric KOA, pain levels in each knee were strongly related to joint space narrowing in the affected knee (92). However, some individuals have central factors that are superimposed upon the more traditional peripheral factors leading to the need for a broader and more flexible approach to diagnosis and treatment of pain in OA. OA, like nearly all other chronic pain states is likely a “mixed pain state,” with individual variability in the relative balance of peripheral (i.e. nociceptive) and central elements of pain.
Thus, until recently it had been clear that entirely “peripheral” theories regarding OA were incomplete, and only recently have studies begun to explore the potential CNS contributions to this condition. Several groups have demonstrated that small groups of OA patients display diffuse hyperalgesia to mechanical or heat stimuli (93-95). Kosek demonstrated that individuals with OA of the hip had reduced descending analgesic activity, which partially normalized following hip arthroplasy(96, 97). Those having baseline hyperalgesia required more opiods in the perioperative setting. Harden and colleagues (98) compared 37 subjects with KOA and 35 controls on a battery of psychophysical tests and found that the OA subjects demonstrated lower overall mechanical pain thresholds compared to controls, as well as greater mechanical and thermal temporal summation than controls, whereas there were no differences among the subjects for any of the remaining experimental pain testing paradigms testing.
Most recently, Gwilym and colleagues used both experimental pain testing and functional neuroimaging procedures to show augmented CNS processing of pain in 20 OA patients (99). Perhaps the strongest evidence of CNS factors being important in OA is the finding that the drug duloxetine, a centrally acting analgesic, is efficacious in individuals with KOA (100).
Conclusion
In the past few decades, our understanding of chronic pain has evolved tremendously. The study of the heretofore considered “functional” or “psychosomatic” conditions such as fibromyalgia and IBS has taught us about the mechanisms that may underlie chronic pain or other somatic syndromes, in individuals without fibromyalgia per se. A better understanding of the underlying mechanisms and most effective treatment for this spectrum of illness is critical to rheumatologists, because as Wolfe has taught us, many patients with chronic pain disorders have variable degrees of “fibromyalgia-ness”. When this occurs, we need to treat both the peripheral and central elements of pain along with other somatic symptoms. The era of evidence-based, individualized analgesia in chronic pain is upon us.
Practice points.
Individuals with these illnesses exhibit core symptoms such as multifocal pain, fatigue, insomnia, cognitive or memory problems, and, in many cases, psychological distress .
Some individuals in the population only have one of these symptoms but more often individuals have many, and the precise location of the pain, and predominant symptom at any given point in time, changes over time.
In clinical practice it is useful to consider a fibromyalgia-like or central pain syndrome when individuals have multifocal pain combined with other somatic symptoms.
Studies demonstrate a strong familial predisposition to these symptoms and illnesses, and furthermore have shown that these somatic symptoms and syndromes are separable from depression and other psychiatric disorders
Research agenda.
Further studies are warranted to develop a better understanding of the shared underlying mechanisms that may be partly responsible for symptom expression in addition to pain and sensory amplification.
The promising development of efficacious agents for the treatment of CNS mediated pain mechanisms has created a need for well-defined guidelines indicating how and when to use these agents.
Table 1.
Clinical entities currently considered parts of the spectrum of Central Sensitivity Syndrome (CSS)
| Clinical syndromes |
|---|
| Fibromyalgia |
| Chronic fatigue syndrome (CFS) |
| Irritable Bowel Syndrome (IBS) and other functional GI disorders |
| Temporomandibular Joint Disorder (TMJD) |
| Restless Leg Syndrome (RLS) and Periodic Limb Movements in Sleep (PLMS) |
| Idiopathic Low Back Pain (LBP) |
| Multiple Chemical Sensitivity (MCS) |
| Primary Dysmenorrhea |
| Headache (tension > migraine, mixed) |
| Migraine |
| Interstitial Cystitis/Chronic Prostatitis/Painful Bladder Syndrome |
| Chronic pelvic pain and endometriosis |
| Myofascial Pain Syndrome / Regional Soft Tissue Pain Syndrome |
Acknowledgements
Dr. Phillips has received support from the American College of Rheumatology Research and Education Foundation and the National Institutes of Health, grant number UL1RR024986 from the National Center for Research Resources and grant number 1K23AR060241.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woolf CJ. Dissecting out mechanisms responsible for peripheral neuropathic pain: implications for diagnosis and therapy. Life Sci. 2004;74:2605–2610. doi: 10.1016/j.lfs.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31:206–219. doi: 10.1016/j.genhosppsych.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Bennett RM. Fibrositis: misnomer for a common rheumatic disorder. West J Med. 1981;134:405–413. [PMC free article] [PubMed] [Google Scholar]
- 4.Simms RW. Fibromyalgia is not a muscle disorder. Am J Med Sci. 1998 Jun;315(6):346–50. doi: 10.1097/00000441-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Moldofsky H, Lue FA. The relationship of alpha and delta EEG frequencies to pain and mood in ‘fibrositis’ patients treated with chlorpromazine and L-tryptophan. Electroencephalogr Clin Neurophysiol. 1980;50:71–80. doi: 10.1016/0013-4694(80)90324-7. [DOI] [PubMed] [Google Scholar]
- 6.Moldofsky H, England RS. Facilitation of somatosensory average-evoked potentials in hysterical anesthesia and pain. Arch Gen Psychiatry. 1975;32:193–197. doi: 10.1001/archpsyc.1975.01760200057005. [DOI] [PubMed] [Google Scholar]
- 7.Hudson JI, Hudson MS, Pliner LF, Goldenberg DL, Pope HG., Jr. Fibromyalgia and major affective disorder: a controlled phenomenology and family history study. Am J Psychiatry. 1985 Apr;142(4):441–6. doi: 10.1176/ajp.142.4.441. [DOI] [PubMed] [Google Scholar]
- 8.Hudson JI, Pope HG., Jr. Fibromyalgia and psychopathology: is fibromyalgia a form of “affective spectrum disorder”? J Rheumatol Suppl. 1989;19:15–22. [PubMed] [Google Scholar]
- 9.Yunus MB. Primary fibromyalgia syndrome: current concepts. Compr Ther. 1984 Aug;10(8):21–8. [PubMed] [Google Scholar]
- 10.Bengtsson A, Henriksson KG. The muscle in fibromyalgia--a review of Swedish studies. J Rheumatol Suppl. 1989;19:144–149. [PubMed] [Google Scholar]
- 11.Bennett RM. Beyond fibromyalgia: ideas on etiology and treatment. J Rheumatol Suppl. 1989;19:185–191. [PubMed] [Google Scholar]
- 12.Bennett RM. Physical fitness and muscle metabolism in the fibromyalgia syndrome: an overview. J Rheumatol Suppl. 1989;19:28–29. [PubMed] [Google Scholar]
- 13.Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum. 2008;37:339–352. doi: 10.1016/j.semarthrit.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Aaron LA, Bradley LA, Alarcon GS, Alexander RW, Triana-Alexander M, Martin MY, et al. Psychiatric diagnoses in patients with fibromyalgia are related to health care-seeking behavior rather than to illness. Arthritis Rheum. 1996;39:436–445. doi: 10.1002/art.1780390311. [DOI] [PubMed] [Google Scholar]
- 15.Clauw DJ. Fibromyalgia: update on mechanisms and management. J Clin Rheumatol. 2007;13:102–109. [PubMed] [Google Scholar]
- *16.Woolf CJ. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140:441–451. doi: 10.7326/0003-4819-140-8-200404200-00010. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda K, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA. 1998;280:981–988. doi: 10.1001/jama.280.11.981. [DOI] [PubMed] [Google Scholar]
- 18.Kato K, Sullivan PF, Evengard B, Pedersen NL. A population-based twin study of functional somatic syndromes. Psychol Med. 2009;39:497–505. doi: 10.1017/S0033291708003784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Arnold LM, Hudson JI, Hess EV, Ware AE, Fritz DA, Auchenbach MB, et al. Family study of fibromyalgia. Arthritis Rheum. 2004;50:944–952. doi: 10.1002/art.20042. [DOI] [PubMed] [Google Scholar]
- 21.Buskila D. Genetics of chronic pain states. Best Pract Res Clin Rheumatol. 2007;21:535–547. doi: 10.1016/j.berh.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Williams H. Making a difference in chronic pain management. Nurs Times. 2003;99:42–43. [PubMed] [Google Scholar]
- *23.Williams DA, Clauw DJ. Understanding fibromyalgia: lessons from the broader pain research community. J Pain. 2009;10:777–791. doi: 10.1016/j.jpain.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raphael KG, Natelson BH, Janal MN, Nayak S. A community-based survey of fibromyalgia-like pain complaints following the World Trade Center terrorist attacks. Pain. 2002;100:131–139. doi: 10.1016/s0304-3959(02)00273-7. [DOI] [PubMed] [Google Scholar]
- *25.Clauw DJ, Chrousos GP. Chronic pain and fatigue syndromes: overlapping clinical and neuroendocrine features and potential pathogenic mechanisms. Neuroimmunomodulation. 1997;4:134–153. doi: 10.1159/000097332. [DOI] [PubMed] [Google Scholar]
- 26.Kato K, Sullivan PF, Evengard B, Pedersen NL. Chronic widespread pain and its comorbidities: a population-based study. Arch Intern Med. 2006;166:1649–1654. doi: 10.1001/archinte.166.15.1649. [DOI] [PubMed] [Google Scholar]
- 27.Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577–1584. doi: 10.1002/art.21008. [DOI] [PubMed] [Google Scholar]
- 28.Diatchenko L, Nackley AG, Slade GD, Bhalang K, Belfer I, Max MB, et al. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain. 2006;125:216–224. doi: 10.1016/j.pain.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 29.Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders--pathways of vulnerability. Pain. 2006;123:226–230. doi: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Armitage R, Landis C, Hoffmann R, Lentz M, Watson N, Goldberg J, et al. Power spectral analysis of sleep EEG in twins discordant for chronic fatigue syndrome. J Psychosom Res. 2009 Jan;66(1):51–7. doi: 10.1016/j.jpsychores.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buskila D, Neumann L. Fibromyalgia syndrome (FM) and nonarticular tenderness in relatives of patients with FM. J Rheumatol. 1997;24:941–944. [PubMed] [Google Scholar]
- 32.McLean SA, Clauw DJ. Predicting chronic symptoms after an acute “stressor”--lessons learned from 3 medical conditions. Med Hypotheses. 2004;63:653–658. doi: 10.1016/j.mehy.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Clauw DJ, Schmidt M, Radulovic D, Singer A, Katz P, Bresette J. The relationship between fibromyalgia and interstitial cystitis. J Psychiatr Res. 1997;31:125–131. doi: 10.1016/s0022-3956(96)00051-9. [DOI] [PubMed] [Google Scholar]
- *34.Wolfe F. The relation between tender points and fibromyalgia symptom variables: evidence that fibromyalgia is not a discrete disorder in the clinic. Ann Rheum Dis. 1997;56:268–271. doi: 10.1136/ard.56.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolfe F, Rasker JJ. The Symptom Intensity Scale, fibromyalgia, and the meaning of fibromyalgia-like symptoms. J Rheumatol. 2006;33:2291–2299. [PubMed] [Google Scholar]
- 36.Williams DA, Schilling S. Advances in the assessment of fibromyalgia. Rheum Dis Clin North Am. 2009;35:339–357. doi: 10.1016/j.rdc.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *37.Harris RE, Sundgren PC, Pang Y, Hsu M, Petrou M, Kim SH, et al. Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in fibromyalgia. Arthritis Rheum. 2008;58:903–907. doi: 10.1002/art.23223. [DOI] [PubMed] [Google Scholar]
- 38.Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003;26:1–230. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- 39.Brooks JC, Tracey I. The insula: a multidimensional integration site for pain. Pain. 2007;128:1–2. doi: 10.1016/j.pain.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 40.Buskila D, Sarzi-Puttini P. Biology and therapy of fibromyalgia. Genetic aspects of fibromyalgia syndrome. Arthritis Res Ther. 2006;8(5):218. doi: 10.1186/ar2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kato K, Sullivan PF, Evengard B, Pedersen NL. Importance of genetic influences on chronic widespread pain. Arthritis Rheum. 2006;54:1682–1686. doi: 10.1002/art.21798. [DOI] [PubMed] [Google Scholar]
- *42.Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- *43.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 44.van Meurs JB, Uitterlinden AG, Stolk L, Kerkhof HJ, Hofman A, Pols HA, et al. A functional polymorphism in the catechol-O-methyltransferase gene is associated with osteoarthritis-related pain. Arthritis Rheum. 2009;60:628–629. doi: 10.1002/art.24175. [DOI] [PubMed] [Google Scholar]
- 45.Holliday KL, Nicholl BI, Macfarlane GJ, Thomson W, Davies KA, McBeth J. Do genetic predictors of pain sensitivity associate with persistent widespread pain? Mol Pain. 2009;5:56. doi: 10.1186/1744-8069-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staud R, Spaeth M. Psychophysical and neurochemical abnormalities of pain processing in fibromyalgia. CNS Spectr. 2008;13(3 Suppl 5):12–17. doi: 10.1017/s109285290002678x. [DOI] [PubMed] [Google Scholar]
- 47.Granges G, Littlejohn G. Pressure pain threshold in pain-free subjects, in patients with chronic regional pain syndromes, and in patients with fibromyalgia syndrome. Arthritis Rheum. 1993;36:642–646. doi: 10.1002/art.1780360510. [DOI] [PubMed] [Google Scholar]
- 48.Granges G, Littlejohn GO. A comparative study of clinical signs in fibromyalgia/fibrositis syndrome, healthy and exercising subjects. J Rheumatol. 1993;20:344–351. [PubMed] [Google Scholar]
- 49.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 50.Wolfe F, Russell IJ, Vipraio G, Ross K, Anderson J. Serotonin levels, pain threshold, and fibromyalgia symptoms in the general population. J Rheumatol. 1997;24:555–559. [PubMed] [Google Scholar]
- 51.Giesecke T, Williams DA, Harris RE, Cupps TR, Tian X, Tian TX, et al. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheum. 2003;48:2916–2922. doi: 10.1002/art.11272. [DOI] [PubMed] [Google Scholar]
- 52.Gracely RH, Grant MA, Giesecke T. Evoked pain measures in fibromyalgia. Best Pract Res Clin Rheumatol. 2003;17:593–609. doi: 10.1016/s1521-6942(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 53.Petzke F, Radbruch L, Zech D, Loick G, Grond S. Temporal presentation of chronic cancer pain: transitory pains on admission to a multidisciplinary pain clinic. J Pain Symptom Manage. 1999;17:391–401. doi: 10.1016/s0885-3924(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 54.Petzke F, Khine A, Williams D, Groner K, Clauw DJ, Gracely RH. Dolorimetry performed at 3 paired tender points highly predicts overall tenderness. J Rheumatol. 2001;28:2568–2569. [PubMed] [Google Scholar]
- 55.Petzke F, Radbruch L, Sabatowski R, Karthaus M, Mertens A. Slow-release tramadol for treatment of chronic malignant pain--an open multicenter trial. Support Care Cancer. 2001;9:48–54. doi: 10.1007/s005200000155. [DOI] [PubMed] [Google Scholar]
- 56.Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105:403–413. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 57.Gibson SJ, Littlejohn GO, Gorman MM, Helme RD, Granges G. Altered heat pain thresholds and cerebral event-related potentials following painful CO2 laser stimulation in subjects with fibromyalgia syndrome. Pain. 1994;58:185–193. doi: 10.1016/0304-3959(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 58.Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70:41–51. doi: 10.1016/s0304-3959(96)03295-2. [DOI] [PubMed] [Google Scholar]
- 59.Geisser ME, Gracely RH, Giesecke T, Petzke FW, Williams DA, Clauw DJ. The association between experimental and clinical pain measures among persons with fibromyalgia and chronic fatigue syndrome. Eur J Pain. 2007;11:202–207. doi: 10.1016/j.ejpain.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Arroyo JF, Cohen ML. Abnormal responses to electrocutaneous stimulation in fibromyalgia. J Rheumatol. 1993;20:1925–1931. [PubMed] [Google Scholar]
- 61.Gerster JC, Hadj-Djilani A. Hearing and vestibular abnormalities in primary fibrositis syndrome. J Rheumatol. 1984;11:678–680. [PubMed] [Google Scholar]
- 62.Tracey I. Neuroimaging of pain mechanisms. Curr Opin Support Palliat Care. 2007;1:109–116. doi: 10.1097/SPC.0b013e3282efc58b. [DOI] [PubMed] [Google Scholar]
- 63.Craig KD. Social communication of pain enhances protective functions: a comment on Deyo, Prkachin and Mercer (2004) Pain. 2004;107:5–6. doi: 10.1016/s0304-3959(03)00264-1. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez MA, Afari N, Buchwald DS. Evidence for overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009;182:2123–2131. doi: 10.1016/j.juro.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 1995 Dec;63(3):341–51. doi: 10.1016/0304-3959(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 66.Langemark M, Bach FW, Jensen TS, Olesen J. Decreased nociceptive flexion reflex threshold in chronic tension-type headache. Arch Neurol. 1993;50:1061–1064. doi: 10.1001/archneur.1993.00540100056015. [DOI] [PubMed] [Google Scholar]
- 67.Ness TJ. Pelvic pain in women and men: recent findings. Curr Opin Anaesthesiol. 2005;18:555–562. doi: 10.1097/01.aco.0000182567.70798.a7. [DOI] [PubMed] [Google Scholar]
- 68.Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613–623. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- 69.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 70.Drossman DA. Brain imaging and its implications for studying centrally targeted treatments in irritable bowel syndrome: a primer for gastroenterologists. Gut. 2005;54:569–573. doi: 10.1136/gut.2004.058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rapps N, van Oudenhove L, Enck P, Aziz Q. Brain imaging of visceral functions in healthy volunteers and IBS patients. J Psychosom Res. 2008;64:599–604. doi: 10.1016/j.jpsychores.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 72.Giovengo SL, Russell IJ, Larson AA. Increased concentrations of nerve growth factor in cerebrospinal fluid of patients with fibromyalgia. J Rheumatol. 1999;26:1564–1569. [PubMed] [Google Scholar]
- 73.Sarchielli P, Di Filippo M, Nardi K, Calabresi P. Sensitization, glutamate, and the link between migraine and fibromyalgia. Curr Pain Headache Rep. 2007;11:343–351. doi: 10.1007/s11916-007-0216-2. [DOI] [PubMed] [Google Scholar]
- 74.Russell TS, Percy JS. The presence of chronic widespread pain in the general population. J Rheumatol. 1994;21:579–580. [PubMed] [Google Scholar]
- 75.Russell IJ. Neurochemical pathogenesis of fibromyalgia. Z Rheumatol. 1998;57(Suppl 2):63–6. doi: 10.1007/s003930050238. [DOI] [PubMed] [Google Scholar]
- 76.Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27:10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baraniuk JN, Whalen G, Cunningham J, Clauw DJ. Cerebrospinal fluid levels of opioid peptides in fibromyalgia and chronic low back pain. BMC Musculoskelet Disord. 2004;5:48. doi: 10.1186/1471-2474-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bryson HM, Wilde MI. Amitriptyline. A review of its pharmacological properties and therapeutic use in chronic pain states. Drugs Aging. 1996;8:459–476. doi: 10.2165/00002512-199608060-00008. [DOI] [PubMed] [Google Scholar]
- 79.van Ophoven A, Hertle L. Long-term results of amitriptyline treatment for interstitial cystitis. J Urol. 2005;174:1837–1840. doi: 10.1097/01.ju.0000176741.10094.e0. [DOI] [PubMed] [Google Scholar]
- 80.Lynch M. Pain: the fifth vital sign. Comprehensive assessment leads to proper treatment. Adv Nurse Pract. 2001;9:28–36. [PubMed] [Google Scholar]
- *81.Arnold LM, Crofford LJ, Martin SA, Young JP, Sharma U. The effect of anxiety and depression on improvements in pain in a randomized, controlled trial of pregabalin for treatment of fibromyalgia. Pain Med. 2007;8:633–638. doi: 10.1111/j.1526-4637.2007.00332.x. [DOI] [PubMed] [Google Scholar]
- 82.Arnold BS, Alpers GW, Suss H, Friedel E, Kosmutzky G, Geier A, et al. Affective pain modulation in fibromyalgia, somatoform pain disorder, back pain, and healthy controls. Eur J Pain. 2008;12:329–338. doi: 10.1016/j.ejpain.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 83.Bergman S. Management of musculoskeletal pain. Best Pract Res Clin Rheumatol. 2007;21:153–166. doi: 10.1016/j.berh.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 84.Deuster PA. Exercise in the prevention and treatment of chronic disorders. Womens Health Issues. 1996;6:320–331. doi: 10.1016/s1049-3867(96)00059-x. [DOI] [PubMed] [Google Scholar]
- 85.Williams CS, Zimmerman S, Sloane PD, Reed PS. Characteristics associated with pain in long-term care residents with dementia. Gerontologist. 2005;45(Spec No 1(1)):68–73. doi: 10.1093/geront/45.suppl_1.68. [DOI] [PubMed] [Google Scholar]
- 86.Creamer P, Hochberg MC. Why does osteoarthritis of the knee hurt--sometimes? Br J Rheumatol. 1997;36:726–728. doi: 10.1093/rheumatology/36.7.726. [DOI] [PubMed] [Google Scholar]
- 87.Clauw DJ, Witter J. Pain and rheumatology: thinking outside the joint. Arthritis Rheum. 2009;60:321–324. doi: 10.1002/art.24326. [DOI] [PubMed] [Google Scholar]
- 88.Chan KW, Ngai HY, Ip KK, Lam KH, Lai WW. Co-morbidities of patients with knee osteoarthritis. Hong Kong Med J. 2009;15:168–172. [PubMed] [Google Scholar]
- 89.Murphy SL, Smith DM, Clauw DJ, Alexander NB. The impact of momentary pain and fatigue on physical activity in women with osteoarthritis. Arthritis Rheum. 2008;59:849–856. doi: 10.1002/art.23710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Allen KD, Renner JB, Devellis B, Helmick CG, Jordan JM. Osteoarthritis and sleep: the Johnston County Osteoarthritis Project. J Rheumatol. 2008;35:1102–1107. [PMC free article] [PubMed] [Google Scholar]
- 91.Turk DC, Cohen MJ. Sleep as a marker in the effective management of chronic osteoarthritis pain with opioid analgesics. Semin Arthritis Rheum. 39:477–490. doi: 10.1016/j.semarthrit.2008.10.006. [DOI] [PubMed] [Google Scholar]
- *92.Neogi T, Felson D, Niu J, Nevitt M, Lewis CE, Aliabadi P, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Driscoll SL, Jayson MI. Proceedings: Pain threshold (PT) analysis in patients with osteoarthritis of the hip. Ann Rheum Dis. 1975;34:195–196. doi: 10.1136/ard.34.2.195-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bajaj P, Graven-Nielsen T, Arendt-Nielsen L. Osteoarthritis and its association with muscle hyperalgesia: an experimental controlled study. Pain. 2001;93:107–114. doi: 10.1016/S0304-3959(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 95.Farrell M, Gibson S, McMeeken J, Helme R. Pain and hyperalgesia in osteoarthritis of the hands. J Rheumatol. 2000;27:441–447. [PubMed] [Google Scholar]
- 96.Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain. 2000;88:69–78. doi: 10.1016/S0304-3959(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 97.Kosek E, Ordeberg G. Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. Eur J Pain. 2000;4:229–238. doi: 10.1053/eujp.2000.0175. [DOI] [PubMed] [Google Scholar]
- 98.Harden RN, Bruehl S, Stanos S, Brander V, Chung OY, Saltz S, et al. Prospective examination of pain-related and psychological predictors of CRPS-like phenomena following total knee arthroplasty: a preliminary study. Pain. 2003;106:393–400. doi: 10.1016/j.pain.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 99.Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, Chessell I, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61:1226–1234. doi: 10.1002/art.24837. [DOI] [PubMed] [Google Scholar]
- *100.Chappell AS, Ossanna MJ, Liu-Seifert H, Iyengar S, Skljarevski V, Li LC, et al. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, placebo-controlled trial. Pain. 2009;146:253–260. doi: 10.1016/j.pain.2009.06.024. [DOI] [PubMed] [Google Scholar]