Abstract
Cardiovascular disease is the leading cause of death in the United States despite a steady reduction in mortality over the last 4 decades. Much of this success is attributed to public health efforts to reduce cardiovascular risk factors, such as national dietary changes and reductions of smoking, as well as more aggressive treatment of clinical disease, including recognition and treatment of hypertension and hypercholesterolemia. The rising rates of obesity and diabetes, especially among adolescents and young adults, raise concern for impending increases in mortality due to these two factors. Some national vital statistics data have shown a leveling of CVD death rates among adults in the fifth decade of life. Major public health efforts have recently begun to address childhood obesity now as an attempt at true primary prevention. This article reviews the dyslipidemia associated with obesity in childhood and outlines a proposed approach to management.
Keywords: obesity, dyslipidemia, metabolic syndrome, epidemiology, children, adolescents, waist circumference, insulin resistance
Introduction
Cardiovascular disease (CVD) remains the leading cause of death in the United States despite a steady reduction in mortality over the last 4 decades, attributed to public health efforts to reduce cardiovascular risk factors and more aggressive treatment of clinical disease.(1) Concealed in the reduced overall mortality are increasing mortality rates due to obesity and diabetes.(1) When CVD mortality in young adults ages 35–54 yr is considered separately, there is an unsettling plateau in the net rate of CVD deaths that can be traced to the steady rise in childhood obesity and diabetes over the last 4 decades.(2) This is being interpreted as the leading edge of a new wave of cardiovascular disease (CVD) mortality.(3) Current adolescent overweight is forecast to increase future adult obesity by 5% to 15% by 2035, resulting in more than 100,000 excess prevalent cases of CVD.(4)
Concern about clinical events in middle aged and older adults has been the primary focus in cardiovascular disease but research over the past 2 decades has clearly shown that the atherosclerotic process has its beginnings in childhood.(5,6) The natural history of hypercholesterolemia in pediatrics has traditionally focused on the identification of children and adolescents with severe elevation in total (TC) and LDL cholesterol (LDL-C) levels, usually Familial Heterozygous Hypercholesterolemia (FH) (7,8). This lipid pattern occurs in 1 in 500 individuals and is inherited as an autosomal dominant. It is not associated with obesity. Children with FH have severely elevated TC and LDL-C levels from birth, and are at established high risk for premature cardiovascular disease.(7,8) The focused approach on children with severe LDL elevation in the context of FH comes from the desire to identify youth and young adults who are at greatly elevated risk for premature coronary artery disease.(7) However, the number of children with LDL elevations who meet criteria for drug treatment is small. Ford et al., reported the rate of hypercholesterolemia from a nationally representative sample of US adolescents from 1999–2006 (9) using the defined levels from the last NHLBI guidelines for management of hypercholesterolemia in childhood published in 1992 (7) (TC ≥ 200 mg/dL and LDL-C ≥ 130 mg/dL) and found 5.2% with elevated LDL-C and 10.7% with elevated TC. They next examined the proportion of US adolescents with an elevated LDL-C that would trigger pharmacological treatment based on recent AAP recommendations (9):1) LDL-C > 190 mg/dL with no other risk factors (likely genetic origin); 2) LDL-C > 160 mg/dL with ≥ 2 additional risk factors (obesity, hypertension, smoking or family history of premature CVD); and 3) LDL-C >130 mg/dL among youth with diabetes mellitus. Only 0.8% of US teens had an LDL-C value elevated enough to consider starting drug treatment, and among these, less than half had LDL-C > 190 mg/dL.(9) From these findings, the number of children who will meet criteria for drug treatment for hypercholesterolemia is small.
Trends and Prevalence in Obesity among Pediatric Populations
Unfortunately, the pediatric obesity epidemic has resulted in a second and much larger population of children with abnormal lipids, those with secondary combined dyslipidemia. Childhood obesity rates have tripled in the past 30 years with now as many as 17% of US adolescents and 16% of US children, 2–11yrs of age, defined as obese.(11) Alarmingly, prevalence rates among Black and Hispanic children and adolescents in the US are even higher.(11) Severe obesity is formally defined by the 2007 CDC/AMA/HHS Childhood Obesity Expert Committee Recommendations as a BMI > 99th percentile for age. In a recent report from a national sample of US children and adolescents (12), almost 4%, or nearly 2.7 million of today’s youth were severely obese.. This study also examined the change in the prevalence of severe obesity from the 1980s to present, and found alarming disparities with Hispanic and African American children and adolescents, as well as those in the lowest poverty group showing the greatest increase in rates of severe obesity over the last 3 decades.
Abdominal obesity is a special concern because of its known association with insulin resistance and the metabolic syndrome. In adults, the metabolic syndrome is defined as 3 or more of the following risk factors: elevated waist circumference, triglyceride levels, BP, and/or fasting glucose, and reduced HDL-cholesterol. In the U.S., the metabolic syndrome is said to affect between 34% and 39% of adults including 7% of men and 6% of women in the 20- to 30-year old age group. As yet, there is no consensus on use of the diagnosis of the metabolic syndrome in children but the cluster of findings seen in adults often occurs with obesity in children.(13) In the first study to define the metabolic syndrome among U.S. adolescents, abdominal obesity (defined as by waist circumference above the >90th percentile for age/gender) was found to be strongly associated with other cardiovascular risk factors defined in the metabolic syndrome, as in adults.(14) Li et al, examined changes in measures of abdominal obesity from NHANES data in 2 national cohorts of US children from 1988–1994 and from 1999–2004. (15) Waist circumference had increased at a relatively higher rate than BMI over that time period. Mean waist circumference for the population had increased from 2% to 8% depending on age and gender. Comparing these 2 cohorts, the overall relative increase in abdominal obesity for boys was 65.4% (10.5% to 17.4%) and for girls, 69.4% (10.5% to 17.8%). This is important when considering the implications of abdominal obesity for identifying overweight or obese youth who may also have increased visceral fat. Visceral fat is strongly associated with the atherogenic dyslipidemia seen in the metabolic syndrome and insulin resistance. The rate of the metabolic syndrome found among US teens has also increased along with this trend in abdominal obesity.(15)
Pathophysiology of Dyslipidemia and Pediatric Obesity
Childhood obesity appears with a powerful array of cardiovascular risk factors including combined dyslipidemia, insulin resistance and hypertension(17) and has been shown to be associated with pathologic evidence of accelerated atherosclerosis in autopsy studies in this age group.(18) The dyslipidemia pattern associated with childhood obesity consists of a combination of elevated triglycerides (TG), decreased high density lipoprotein cholesterol (HDL-C), and top normal to mildly elevated low density lipoprotein cholesterol (LDL-C).(8) Normal lipid values in childhood have been developed from major epidemiologic studies including the Bogalusa Heart Study and the Lipid Research Clinics Study and are shown in Table 1.(7,8) Normal TG levels are <100 mg/dL in children younger than age 10 years and <130 mg/dL at ages 10–18 years.(7,8) In the dyslipidemia associated with obesity, TG levels are usually between 100 and 400 mg/dL.(8) Recent NHANES data indicate this pattern is highly prevalent, present in 42.9%of children with BMI>95th%ile.(19) Insulin resistance, another common feature in obese children and adolescents, contributes significantly to development of the combined dyslipidemia of obesity by enhancing hepatic delivery of nonesterified free fatty acids for triglyceride (TG) production and sequestration into triglyceride-rich lipoproteins. (20) High TG levels are processed into small, dense LDL and small, less stable HDL; there is both an increase in small, dense LDL and in overall LDL particle number and a reduction in total HDL-C and in large HDL particles with analysis by nuclear magnetic resonance spectroscopy (NMR). (21, 22) The combined dyslipidemia pattern seen with traditional lipid profile analysis identifies the atherogenic pattern seen with NMR analysis. (20)
Table 1.
Acceptable, Borderline High, and High Plasma Lipid and Lipoprotein Concentrations (mg/dL) for Children and Adolescents*
| Category | Acceptable | Borderline High | High† |
|---|---|---|---|
| Total Cholesterol | <170 | 170–199 | ≥200 |
| LDL–Cholesterol | <110 | 110–129 | ≥130 |
| Triglyceride: | |||
| 0–9 years | <75 | 75–99 | ≥100 |
| 10–19 years | <90 | 90–129 | ≥130 |
| Category | Acceptable | Borderline High | Low† |
|---|---|---|---|
| HDL–Cholesterol | >45 | 40–45 | <40 |
NOTE: Values given are in mg/dL. To convert to SI units, divide the results for total cholesterol (TC), low-density lipoprotein cholesterol (LDL–C), high-density lipoprotein cholesterol (HDL–C), and non-HDL–C by 38.6; for triglycerides (TG), divide by 88.6.
Values for plasma lipid and lipoprotein levels are from the National Cholesterol Education Program (NCEP) Expert Panel on Cholesterol Levels in Children.(7) Non-HDL–C values are from the Bogalusa Heart Study and are equivalent to the NCEP Pediatric Panel cut points for LDL–C.
The cut points for high and borderline high represent approximately the 95th and 75th percentiles, respectively. Low cut points for HDL–C represent approximately the 10th percentile.
The root cause of atherogenesis remains subendothelial retention of LDL-containing lipoproteins.(23) The combined dyslipidemia of obesity is particularly atherogenic for multiple reasons: small dense LDL particles are inefficiently cleared by LDL receptors elevated total circulating LDL particles heighten the risk of entrapment in the subendothelial matrix, and decreased large HDL particles limit reverse cholesterol transport. (24,25) The atherogenicity of the combined dyslipidemia seen with childhood obesity manifests in structural and functional vascular changes assessed non-invasively as increased carotid intima-media thickness (cIMT) and increased arterial stiffness(26,27). In adults, combined dyslipidemia is the most prevalent pattern seen in individuals presenting with early clinical cardiovascular events.(21,28–30) In children, a recent report from the longitudinal Young Finns study revealed that, at 21-year follow-up, subjects with the combined dyslipidemia pattern beginning in childhood had significantly increased cIMT compared with normolipidemic controls, even after adjustment for other risk factors; cIMT was further increased when the dyslipidemia occurred in the context of the metabolic syndrome.(31) A paper from the CDC evaluated multiple CVD risk factors in US children, specifically, systolic blood pressure, fasting glucose and components of the lipid profile (32). The mean and median values of TC, LDL-C and glucose remained unchanged over multiple cohorts of US children and adolescents. However, over successive cohorts, there was a significant increase in mean and median values of TG and SBP and a decrease in HDL-C, all components of the metabolic syndrome cluster. So, whether you believe in the metabolic syndrome as a separate entity or not, pediatric lipid profiles are qualitatively worse, even if TC and LDL-C levels have not increased. Thus, the combined dyslipidemia pattern seen with obesity in childhood is increasing in prevalence and predicts vascular dysfunction in young adulthood and early clinical events in adult life.
Lifestyle Management of Combined Dyslipidemia
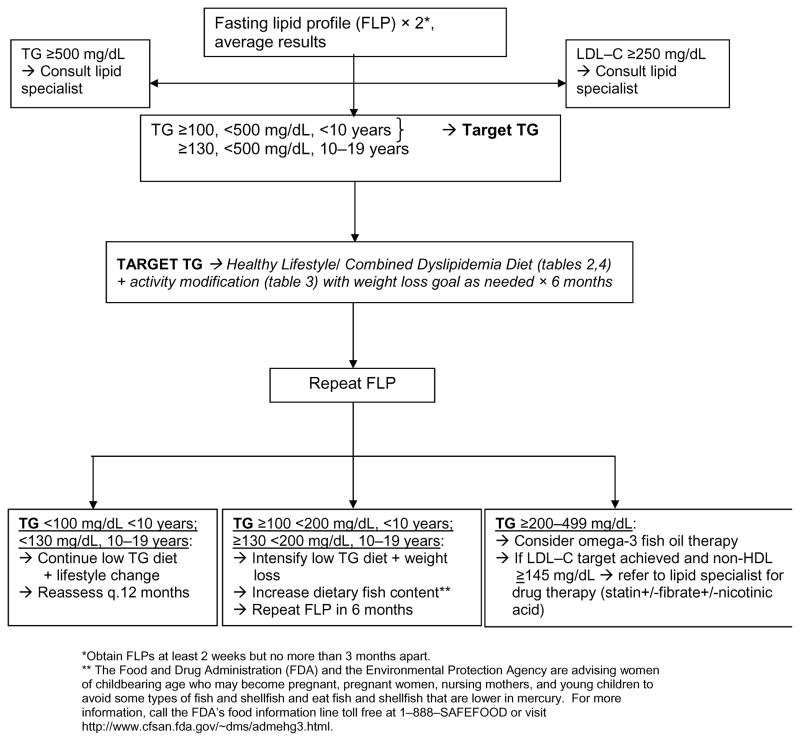
Treatment for combined dyslipidemia of obesity is primarily lifestyle change and this is often highly effective. Combined dyslipidemia has been shown to be very responsive to even small changes in weight status, diet composition, and activity. Most importantly, in obese children, adolescents and adults, even small amounts of weight loss are associated with significant decreases in TG levels and increases in HDL–C levels.(33–37) Even without weight loss, exercise training is associated with a significant decrease in TG levels and an increase in HDL-C, with reversion to baseline when subjects became less active.(35,38–40) A diet that limits both simple carbohydrate intake and calories addresses both combined dyslipidemia and obesity. In adults with hypertriglyceridemia, diet patterns like this significantly decreased TG by a mean of 63 percent and increased HDL–C by 8 percent.(41,42) In adolescents, a low-carbohydrate diet associated with minimal weight loss significantly reduced TG levels.(43) A 12-month follow-up study of 21-month-old children with elevated TG levels treated with a carbohydrate-restricted diet showed that a decrease in sugar and carbohydrate intake was associated with a very significant decrease in TG levels from a mean of 274.1 +/− 13.1 mg/dL before treatment to 88.8 +/− 13.3 mg/dL, within normal limits.(44) The concept of glycemic load has also been evaluated in the setting of obesity and combined dyslipidemia in adolescents and adults. The glycemic index is a measure of the blood glucose response to a 50 g portion of a selected carbohydrate; the glycemic load is the mathematic product of the glycemic index and the carbohydrate amount. In adolescents and young adults, there is evidence that low glycemic-load diets are at least as effective as low-fat diets in achieving weight loss, with decreased TG and increased HDL in subjects on the low glycemic-load diet.(45–48) For all dietary change in children and adolescents, family-based training with a registered dietitian has been shown to be the most effective way to both begin and sustain change. A straightforward approach using a diet low in simple carbohydrates and sugar with controlled calories and a regular exercise schedule is shown in Tables 2, 3 and 4 and in Figure 1; the latter was derived from forthcoming NHLBI guidelines for management of cardiovascular risk factors in childhood. (49) This is usually all that is necessary to address combined dyslipidemia in most obese children and adolescents.
Table 2.
DIET COMPOSITION: Healthy Lifestyle / Combined Dyslipidemia Diet
These diet recommendations are those recommended for all healthy children over age 2 with specific differences focused on appropriate portion size and limitation of simple carbohydrate intake.
|
**The Food and Drug Administration (FDA) and the Environmental Protection Agency are advising women of childbearing age who may become pregnant, pregnant women, nursing mothers, and young children to avoid some types of fish and shellfish and eat fish and shellfish that are lower in mercury. For more information, call the FDA’s food information line toll free at 1–888–SAFEFOOD or visit http://www.cfsan.fda.gov/~dms/admehg3.html.
Table 3.
|
Examples of moderate to vigorous physical activities are walking briskly or jogging.
Examples of vigorous physical activities are running, playing singles tennis or soccer.
Table 4. Estimated Calorie Requirements (in Kilocalories [kcal]) for Gender and Age Groups at Three Levels of Physical Activitya (58).
Estimates are rounded to nearest 200 calories and were determined using the Institute of Medicine (IOM) equation.
| Activity Level b,c,d | ||||
|---|---|---|---|---|
| Gender | Age (years) | Sedentaryb | Moderately Activec | Actived |
| Child | 2–3 | 1,000 | 1,000–1,400e | 1,000–1,400e |
| Female | 4–8 9–13 14–18 19–30 |
1,200 1,600 1,800 2,000 |
1,400–1,600 1,600–2,000 2,000 2,000–2,200 |
1,400–1,800 1,800–2,200 2,400 2,400 |
| Male | 4–8 9–13 14–18 19–30 |
1,400 1,800 2,200 2,400 |
1,400–1,600 1,800–2,200 2,400–2,800 2,600–2,800 |
1,600–2,000 2,000–2,600 2,800–3,200 3,000 |
These levels are based on Estimated Energy Requirements from the IOM Dietary Reference Intakes macronutrients report (2002), calculated by gender, age, and activity level for reference-size individuals. "Reference size," as determined by the IOM, is based on median height and weight for ages up to age 18 years and median height and weight for that height to give a body mass index of 21.5 for adult females and 22.5 for adult males.
A sedentary activity level in childhood, as in adults, means a lifestyle that includes only the light physical activity associated with typical day-to-day life.
Moderately active in childhood means a lifestyle that includes some physical activity, equivalent to an adult walking about 1.5 to 3 miles per day at 3 to 4 miles per hour, in addition to the light physical activity associated with typical day-to-day life.
Active means a lifestyle that includes more physical activity, equivalent to an adult walking more than 3 miles per day at 3 to 4 miles per hour, in addition to the light physical activity associated with typical day-to-day life.
The calorie ranges shown recognize the needs of different ages within the group. For growing children and adolescents, more calories are needed at older ages.
Figure 1.
ALGORITHM FOR MANAGEMENT OF COMBINED DYSLIPIDEMIA/ HIGH TG (49)
Drug Therapy for Combined Dyslipidemia
In the rare child with combined dyslipidemia and severe hypertriglyceridemia for whom diet and exercise interventions are insufficient, there are nutriceutical and medication options that can be considered. The TG level and the timing for consideration of more advanced therapy are outlined in the algorithm (Figure 1). A recent systematic review demonstrated that omega-3 fish oil capsules are both safe and effective in adults, reducing TG by 30–45 percent, with significant associated increases in HDL–C.(50) For children, the safety of omega-3 fish oil was observed in their use in children with immunoglobulin A nephropathy and in a small series of children with dyslipidemia.(51) Because fish oil preparations are marketed directly to the public, pediatric care providers can expect to encounter children who are taking these supplements and should be prepared to offer guidance about their use. There are many generic forms of fish oil capsules that are commercially available. The University of Wisconsin maintains a preventive cardiology patient education Web site http://www.heartdecision.org. The “fish oil” section includes information about the content of various preparations. The Web site is updated every 6 months (https://www.heartdecision.org/chdrisk/v_hd/patient_edu_docs/Fish_Oil_11-2007.pdf). There is one FDA approved omega-3 fish oil preparation called Lovaza. Each capsule contains 1 gram of fish oil with a recommended dose of 4 grams/d, given either all at once or as 2 grams bid. At the recommended dosage, there are almost no reported side effects for fish oil preparations except a fishy after taste and increased burping. In some studies, there has been a 5 – 10% dose-related increase in LDL-C levels. Allergic reactions can occur in individuals who have allergies to fish. The statin medications have been studied in adults with combined dyslipidemia and have been shown to reduce total LDL particle numbers and to selectively decrease small, dense LDL.(52) One trial in children with familial hypercholesterolemia showed similar results with a significant decrease in total LDL particle number and in small, dense LDL concentration.(53) In adults, fibrates have been used to lower TG levels, and a small series in children demonstrated effective reductions in TG levels and an associated increase in HDL–C levels.(54) Finally, niacin has been used extensively in adults, but there is limited experience in children, with a single series demonstrating a high rate of side effects.(55) The use of any medication except omega-3 fish oil in youths with combined dyslipidemia should be undertaken only with the assistance of a lipid specialist.
Conclusion
The prevalence of obesity has continued to rise over the past 3 decades. The obesity epidemic is already impacting the cardiovascular health of today’s adults in their 30s and 40s. In children, obesity - especially abdominal obesity - is strongly associated with insulin resistance and with a high prevalence of the atherogenic combined dyslipidemia described here. While these cardiometabolic abnormalities have not resulted in measurable increases in total or LDL cholesterol, adult and pediatric data have revealed qualitative changes in LDL and HDL cholesterol associated with elevated measures of atherosclerosis among adolescents and with clinical disease in adults. A focused, family-based approach to management of these lipid abnormalities is critical and will require randomized trials of enhanced lifestyle approaches that will result in evidence-based treatment strategies.
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- CVD
cardiovascular disease
- TC
Total Cholesterol
- HDL-C
high density lipoprotein
- LDL-C
low density lipoprotein
- TG
Triglycerides
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–98. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50:2128–32. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the Future of Cardiovascular Disease in the United States: A Policy Statement From the American Heart Association. Circulation. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 4.Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. Adolescent overweight and future adult coronary heart disease. N Engl J Med. 2007;357:2371–9. doi: 10.1056/NEJMsa073166. [DOI] [PubMed] [Google Scholar]
- 5.McGill HC, Jr, McMahan CA, Zieske AW, Sloop GD, Walcott JV, Troxclair DA, Malcom GT, Tracy RE, Oalmann MC, Strong JP. Associations of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth. The Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb Vasc Biol. 2000;20(8):1998–2004. doi: 10.1161/01.atv.20.8.1998. [DOI] [PubMed] [Google Scholar]
- 6.Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. New England Journal of Medicine. 1998 June 4;338(23):1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 7.NCEP Expert Panel of Blood Cholesterol Levels in Children and Adolescents. National Cholesterol Education Program (NCEP) Highlights of the Report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 1992;89:495–501. [PubMed] [Google Scholar]
- 8.Kwiterovich PO., Jr Recognition and Management of dyslipidemia in children and adolescents. J Clin Endocrinol Metab. 2008;93(11):4200–4209. doi: 10.1210/jc.2008-1270. [DOI] [PubMed] [Google Scholar]
- 9.Ford ES, Li C, Zhao G, Mokdad AH. Concentrations of low-density lipoprotein cholesterol and total cholesterol among children and adolescents in the United States. Circulation. 2009;119:1108 –1115. doi: 10.1161/CIRCULATIONAHA.108.816769. [DOI] [PubMed] [Google Scholar]
- 10.Daniels SR, Greer FR for the AAP Committee on Nutrition. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 11.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 12.Skelton J, Cook S, Auinger P, Klein JD, Barlow SE. Prevalence and Trends of Severe Obesity among US Children and Adolescents. Academic Pediatrics. 2009 September–October;9(5):322–329. doi: 10.1016/j.acap.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: An American Heart Association/ National Heart, Lung and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 14.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Archives of Pediatrics & Adolescent Medicine. 2003;157:821–7. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Ford E, Mokdad A, Cook S. Recent Waist Circumference Trends and Waist-to-Height Ratio among US Children and Adolescents. Pediatrics. 2006 November;118(5):e1390–1398. doi: 10.1542/peds.2006-1062. [DOI] [PubMed] [Google Scholar]
- 16.Cook S, Auinger P, Li C, Ford E. Metabolic Syndrome Rates in U.S. Adolescents, from the National Health and Nutrition and Examination Survey 1999-2002. Journal of Pediatrics. 2008 February;152(2):165–170. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150(1):12–17. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 18.McGill HC, McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, et al. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. 2002;105:2712–2718. doi: 10.1161/01.cir.0000018121.67607.ce. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Prevalence of abnormal lipid levels among youths --- United States, 1999-2006. MMWR Morb Mortal Wkly Rep. 59:29–33. [PubMed] [Google Scholar]
- 20.Laakso M, Sarlund H, Mykkanen L. Insulin resistance is associated with lipid and lipoprotein abnormalities in subjects with varying degrees of glucose tolerance. Arteriosclerosis. 1990;10:223–31. doi: 10.1161/01.atv.10.2.223. [DOI] [PubMed] [Google Scholar]
- 21.Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106:1930–7. doi: 10.1161/01.cir.0000033222.75187.b9. [DOI] [PubMed] [Google Scholar]
- 22.Kuller L, Arnold A, Tracy R, et al. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arterioscler Thromb Vasc Biol. 2002;22:1175–80. doi: 10.1161/01.atv.0000022015.97341.3a. [DOI] [PubMed] [Google Scholar]
- 23.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–44. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 24.Campos H, Arnold KS, Balestra ME, Innerarity TL, Krauss RM. Differences in receptor binding of LDL subfractions. Arterioscler Thromb Vasc Biol. 1996;16:794–801. doi: 10.1161/01.atv.16.6.794. [DOI] [PubMed] [Google Scholar]
- 25.Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–10. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 26.Paramsothy P, Knopp RH, Bertoni AG, Blumenthal RS, Wasserman BA, Tsai MY, Rue T, Wong ND, Heckbert SR. Association of combinations of lipid parameters with carotid intima-media thickness and coronary artery calcium in the MESA (Multi-Ethnic Study of Atherosclerosis) J Amer Coll Cardiol. 2010;56(13):1034–1041. doi: 10.1016/j.jacc.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 27.Koivistoinen T, Hutri-Kahonen N, Juonala M, et al. Apolipoprotein B is related to arterial pulse wave velocity in young adults: the Cardiovascular Risk in Young Finns Study. Atherosclerosis. 214:220–4. doi: 10.1016/j.atherosclerosis.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 28.Kathiresan S, Otvos JD, Sullivan LM, et al. Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation. 2006;113:20–9. doi: 10.1161/CIRCULATIONAHA.105.567107. [DOI] [PubMed] [Google Scholar]
- 29.Cromwell WC, Otvos JD, Keyes MJ, et al. LDL Particle Number and Risk of Future Cardiovascular Disease in the Framingham Offspring Study - Implications for LDL Management. J Clin Lipidol. 2007;1:583–92. doi: 10.1016/j.jacl.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuller L, Arnold A, Tracy R, et al. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arterioscler Thromb Vasc Biol. 2002;22:1175–80. doi: 10.1161/01.atv.0000022015.97341.3a. [DOI] [PubMed] [Google Scholar]
- 31.Juonala M, Viikari JS, Ronnemaa T, Marniemi J, Jula A, Loo BM, Raitakari OT. Associations of dyslipidemias from childhood to adulthood with carotid intima-media thickness, elasticity, and brachial flow-mediated dilatation in adulthood: the Cardiovascular Risk in Young Finns Study. Arteriosclerosis, Thrombosis & Vascular Biology. 2008;28(5):1012–7. doi: 10.1161/ATVBAHA.108.163329. [DOI] [PubMed] [Google Scholar]
- 32.Ford ES, Mokdad AH, Ajani UA. Trends in risk factors for cardiovascular disease among children and adolescents in the United States. Pediatrics. 2004;114:1534 –1544. doi: 10.1542/peds.2004-0674. [DOI] [PubMed] [Google Scholar]
- 33.Epstein LH, Kuller LH, Wing RR, Valoski A, McCurley J. The effect of weight control on lipid changes in obese children. Am J Dis Child. 1989;143(4):454–457. doi: 10.1001/archpedi.1989.02150160080016. [DOI] [PubMed] [Google Scholar]
- 34.Nemet D, Barkan S, Epstein Y, Friedland O, Kowen G, Eliakim A. Short- and long-term beneficial effects of a combined dietary-behavioral-physical activity intervention for the treatment of childhood obesity. Pediatrics. 2005;115(4):e443–e449. doi: 10.1542/peds.2004-2172. [DOI] [PubMed] [Google Scholar]
- 35.Kang HS, Gutin B, Barbeau P, Owens S, Lemmon CR, Allison J, Litaker MS, Le NA. Physical training improves insulin resistance syndrome markers in obese adolescents. Med Sci Sports Exerc. 2002;34(12):1920–1927. doi: 10.1097/00005768-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Becque MD, Katch VL, Rocchini AP, Marks CR, Moorehead C. Coronary risk incidence of obese adolescents: reduction by exercise plus diet intervention. Pediatrics. 1988;81(5):605–612. [PubMed] [Google Scholar]
- 37.Siri-Tarino PW, Williams PT, Fernstrom HS, Rawlings RS, Krauss RM. Reversal of small, dense LDL subclass phenotype by normalization of adiposity. Obesity. 2009;17:1768–1775. doi: 10.1038/oby.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferguson MA, Gutin B, Le NA, Karp W, Litaker M, Humphries M, Okuyama T, Riggs S, Owens S. Effects of exercise training and its cessation on components of the insulin resistance syndrome in obese children. Int J Obes Relat Metab Disord. 1999;23(8):889–895. doi: 10.1038/sj.ijo.0800968. [DOI] [PubMed] [Google Scholar]
- 39.Watts K, Beye P, Siafarikas A, O'Driscoll G, Jones TW, Davis EA, Green DJ. Effects of exercise training on vascular function in obese children. J Pediatr. 2004;144(5):620–625. doi: 10.1016/j.jpeds.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 40.Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, Lam CW, Metreweli C, Celermajer DS. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation. 2004;109(16):1981–1986. doi: 10.1161/01.CIR.0000126599.47470.BE. [DOI] [PubMed] [Google Scholar]
- 41.Pieke B, von Eckardstein A, Gulbahce E, Chirazi A, Schulte H, Assmann G, Wahrburg U. Treatment of hypertriglyceridemia by two diets rich in unsaturated fatty acids or in carbohydrates: effects on lipoprotein subclasses, lipolytic enzymes, lipid transfer proteins, insulin and leptin. Int J of Obesity. 2000;24:1286–1296. doi: 10.1038/sj.ijo.0801440. [DOI] [PubMed] [Google Scholar]
- 42.Musunuru K. Atherogenic dyslipidemia: Cardiovascular risk and dietary intervention. Lipids. 2010;45:907–914. doi: 10.1007/s11745-010-3408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sondike SB, Copperman N, Jacobson MS. Effects of a low-carbohydrate diet on weight loss and cardiovascular risk factors in overweight adolescents. J Pediatr. 2003;142(3):253–258. doi: 10.1067/mpd.2003.4. [DOI] [PubMed] [Google Scholar]
- 44.Ohta T, Nakamura R, Ikeda Y, Hattori S, Matsuda I. Follow up study on children with dyslipidaemia detected by mass screening at 18 months of age: effect of 12 months dietary treatment. Eur J Pediatr. 1993;152(11):939–943. doi: 10.1007/BF01957537. [DOI] [PubMed] [Google Scholar]
- 45.Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. 2004 Nov 24;292(20):2482–90. doi: 10.1001/jama.292.20.2482. [DOI] [PubMed] [Google Scholar]
- 46.Ebbeling CB, Leidig MM, Sinclair KB, Seger-Shippee LG, Feldman HA, Ludwig DS. Effects of an ad libitum low-glycemic load diet on cardiovascular disease risk factors in obese young adults. Am J Clin Nutr. 2005 May;81(5):976–82. doi: 10.1093/ajcn/81.5.976. [DOI] [PubMed] [Google Scholar]
- 47.Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low glycemic-load vs low fat diet in obese young adults. JAMA. 2007;297:2092–2102. doi: 10.1001/jama.297.19.2092. [DOI] [PubMed] [Google Scholar]
- 48.Ebbeling CB, Leidig MM, Sinclair KB, Hangen JP, Ludwig DS. A reduced glycemic load diet in the treatment of adolescent obesity. Arch Pediatr Adolesc med. 2003;157:773–779. doi: 10.1001/archpedi.157.8.773. [DOI] [PubMed] [Google Scholar]
- 49.National Institutes of Health. National Heart, Lung and Blood Institute. [Accessed August 3, 2011];NHLBI Pediatric Guidelines for Cardiovascular Health and Risk Reduction. http://www.nhlbi.nih.gov/guidelines/cvd_ped.
- 50.Goldberg RB, Sabharal AK. Fish oil in the treatment of dyslipidemia. Current Opin Endocrinol Diabetes and Obes. 2008;15:167–174. doi: 10.1097/MED.0b013e3282f76728. [DOI] [PubMed] [Google Scholar]
- 51.Engler MM, Engler MB, Malloy MJ, Paul SM, Kulkarni KR, Mietus-Snyder ML. Effect of docosahexaenoic acid on lipoprotein subclasses in hyperlipidemic children (the EARLY study) Am J Cardiol. 2005;95(7):869–871. doi: 10.1016/j.amjcard.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 52.Guerib M, Egger P, Soudant C, LeGoff W, van Tol A, Dupuid R, Chapman MJ. Dose dependent action of atorvastatin in type IIB hyperlipidemia: preferential and progressive reduction of atherogenic apoB-containing lipoprotein subclasses (VLDL-2, IDL, small dense LDL) and stimulation of cellular cholesterol efflux. Atherosclerosis. 2002;163:287–296. doi: 10.1016/s0021-9150(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 53.van der Graaf A, Rodenburg J, Vissers MN, Hutten BA, Wiegman A, Trip MD, Stroes ES, Wijburg FA, Otvos JD, Kastelein JJ. Atherogenic lipoprotein particle size and concentrations and the effect of pravastatin in children with familial hypercholesterolemia. Journal of Pediatrics. 2008;152(6):873–8. doi: 10.1016/j.jpeds.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 54.Wheeler KA, West RJ, Lloyd JK, Barley J. Double blind trial of bezafibrate in familial hypercholesterolaemia. Arch Dis Child. 1985;60(1):34–37. doi: 10.1136/adc.60.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colletti RB, Neufeld EJ, Roff NK, McAuliffe TL, Baker AL, Newburger JW. Niacin treatment of hypercholesterolemia in children. Pediatrics. 1993;92:78–82. [PubMed] [Google Scholar]
- 56.Strong WB, Malina RM, Blimkie CJ, Daniels SR, Dishman RK, Gutin B, Hergenroeder AC, Must A, Nixon PA, Pivarnik JM, Rowland T, Trost S, Trudeau F. Evidence based physical activity for school-age youth. J Pediatr. 2005;146(6):732–737. doi: 10.1016/j.jpeds.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 57.Physical Activity Guidelines for Americans. 2008 www.health.gov/paguidelines.
- 58.Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) Institute of Medicine. National Academies Press; Washington, DC: 2005. [Google Scholar]