Abstract
Objectives
We investigated the temporal pattern and predictive value (alone and in combination) of four urinary biomarkers [neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18), liver fatty-acid binding protein (L-FABP) and kidney injury molecule-1 (KIM-1)] for cardiac surgery-associated acute kidney injury (AKI).
Background
Serum creatinine (SCr) is a delayed marker for AKI after cardiopulmonary bypass (CPB). Rapidly detectable AKI biomarkers could allow early intervention and improve outcomes.
Methods
Data from 220 pediatric patients were analyzed. Urine samples were obtained before and at intervals after CPB initiation. AKI was defined as a ≥50% increase in SCr from baseline within 48h after CPB. The temporal pattern of biomarker elevation was established and biomarker elevations were correlated with AKI severity and clinical outcomes. Biomarker predictive abilities were evaluated by AUC, net reclassification improvement (NRI) and integrated discrimination improvement (IDI).
Results
AKI occurred in 27% of patients. Urine NGAL significantly increased in AKI patients at 2h after CPB initiation. IL-18 and L-FABP increased at 6h and KIM-1 increased at 12h. Biomarker elevations correlated with AKI severity and clinical outcomes, and improved AKI prediction above a clinical model. At 2h, addition of NGAL increased the AUC from 0.74 to 0.85 (p<0.0001). At 6h, NGAL, IL-18 and L-FABP each improved the AUC from 0.72 to 0.91, 0.84 and 0.77, respectively (all p<0.05). The added predictive ability of the biomarkers was supported by NRI and IDI. Biomarker combinations further improved AKI prediction.
Conclusion
Urine NGAL, IL-18, L-FABP, and KIM-1 are sequential predictive biomarkers for AKI and correlate with disease severity and clinical outcomes after pediatric CPB. These biomarkers, particularly in combination, may help establish the timing of injury and allow earlier intervention in AKI.
Keywords: Acute Kidney Injury, Cardiopulmonary Bypass, Ischemia, Biomarkers
INTRODUCTION
Acute kidney injury (AKI) after cardiac surgery is associated with adverse outcomes, including prolonged intensive care and hospital stays, diminished quality of life, and increased long-term mortality (1). AKI occurs frequently, complicating 30–40% of adult and pediatric cardiac surgeries (2, 3). Even mild degrees of post-operative AKI portend a significant increase in mortality (4) and morbidity (5). Episodes of AKI may also lead to the development of chronic kidney disease (6).
AKI diagnosis has relied on a rise in serum creatinine (SCr) concentration, a delayed and unreliable measure in the acute setting (7). The failure of interventional trials to attenuate AKI after cardiac surgery has been attributed in part to delays in diagnosis. Recent studies have focused on the discovery and validation of early biomarkers of AKI. Initial studies have demonstrated that neutrophil gelatinase-associated lipocalin (NGAL), interleukin 18 (IL-18), liver fatty-acid binding protein (L-FABP), and kidney injury molecule-1 (KIM-1) are individually elevated in the urine early after ischemic AKI (2, 8–10). Most studies have focused on the performance of only one of these biomarkers to detect AKI prior to a SCr rise. The aims of this study were to 1) evaluate the temporal pattern of elevation in these four biomarkers following cardiac surgery and 2) determine their predictive values, individually and in combination, when added to a clinical predictive model.
METHODS
Study Design
This study was approved by the Institutional Review Board of Cincinnati Children’s Hospital Medical Center. All patients <18 years of age undergoing cardiac surgery with cardiopulmonary bypass (CPB) at our center between January 2004 and July 2007 were approached for study inclusion. Patients with severe pre-existing renal insufficiency (SCr >2 times age-adjusted normal range) were excluded. Written informed consent was obtained before enrollment from the legal guardian of each patient with assent from the patient when appropriate.
Urine samples for biomarker analysis were obtained immediately before and at 2, 6, 12, and 24 hours after initiation of CPB, and stored in aliquots at −80°C. SCr was routinely measured at baseline (within 72 hours before surgery), immediately after surgery and at least daily in the post-operative period.
The primary outcome was AKI development, defined as a ≥50% increase in SCr from pre-operative baseline within 48 hours of surgery Complexity of surgery was categorized according to the Risk Adjustment for Congenital Heart Surgery 1 (RACHS-1) consensus-based scoring system (11). Secondary outcomes included severity of AKI based on the pediatric modified RIFLE (pRIFLE) criteria (12), duration of AKI, duration of mechanical ventilation, hospital length of stay and hospital mortality. We determined pRIFLE by calculation of estimated creatinine clearance (eCCl) using the modified Schwartz formula (13), with “Risk” defined as eCCL decrease of 25% from baseline, “Injury” defined as eCCl decrease of 50%, and “Failure” defined as eCCl decrease of 75% or absolute value <35 ml/min/1.73m2. Duration of AKI was defined as the number of days SCr was ≥50% above baseline. We utilized dosages of inotropic infusions to calculate an inotrope score at 24h after CPB to provide a quantified index of the post-operative hemodynamic state (14).
Biomarker Measurements
Laboratory investigators were blinded to clinical outcomes. Urine NGAL was assayed using a human-specific commercially available ELISA (AntibodyShop, Grusbakken, Denmark) (15). Urine IL-18 and L-FABP were measured using commercially available ELISA kits (Medical & Biological Laboratories Co., Nagoya, Japan and CMIC Co., Tokyo, Japan, respectively) per manufacturer’s instructions. The urine KIM-1 ELISA was constructed using commercially available reagents (R & D Systems, Inc., Minneapolis, MN) as described previously (16).
Statistical Methods
The analysis subset included patients who had measurement of all four biomarkers at baseline and at a minimum of one post-operative time-point (n=220) to permit comparisons of biomarkers. Statistical analysis was performed using SAS version 9.2 (SAS Institute, Cary, NC) and R 2.12.1 (R Development Core Team, 2010). Demographics, baseline measurements and clinical outcomes were compared between AKI and non-AKI patients, using the nonparametic Wilcoxon rank sum test (continuous variables) or χ2 or Fisher’s exact tests (categorical variables) as appropriate.
At each time-point, the median biomarker measurements were compared between AKI and non-AKI patients and among patients in different pRIFLE strata using the rank transformation approach. Tukey-Kramer adjustment was utilized to adjust for multiple comparisons at each time-point and adjusted p-values were reported. Spearman correlation coefficients were calculated to assess the association between biomarker concentrations at each time-point and the clinical parameters.
Univariable logistic regression was used to assess the discriminative ability of biomarkers to predict AKI. Receiver operating characteristic (ROC) curves were generated for each biomarker at each time-point. The areas under the curve (AUC) were compared between biomarkers using the methods developed by DeLong (17). In a secondary analysis, patients with pRIFLE “F” or “I” were assigned as “severe AKI” and we examined the ability of the biomarkers to predict severe AKI.
Multivariable logistic regression analyses were conducted to assess predictors of AKI as well as the performance of the predictive models while combining biomarkers with clinical factors. In addition to biomarkers, potential predictor variables included age, sex, race, CPB duration, prior CPB, RACHS-1 score and post-operative inotrope score. A parsimonious clinical model (containing clinical factors only) was first determined using backward elimination. The four urinary biomarkers were added individually and in combination to the clinical model. Improvements in the model performance were evaluated by AUC, net reclassification improvement (NRI) and integrated discrimination improvement (IDI). No risk categories were selected for the calculation of NRI. R package Hmisc was used for the calculation of NRI and IDI (18, 19).
RESULTS
Of 391 patients enrolled, 220 (56%) were retained for analysis. The 171 excluded patients were among the first 174 patients enrolled. Because their samples were also used for assay optimization, insufficient volume remained for KIM-1 or L-FABP testing. Retained and excluded patients were similar in incidence of AKI (27% vs. 35%, p=0.10) and mortality (0.4% vs. 2.3%, p=0.05). No patient in either group needed dialysis. AKI occurred in 60 of the 220 patients. Retained patient characteristics are shown in Table 1. SCr increased by a median of 78% in AKI patients. 98% of AKI patients were diagnosed one day after surgery. Among AKI patients, 45% developed pRIFLE-R, 35% developed pRIFLE-I and 20% developed pRIFLE-F.
Table 1.
Patient characteristics
| Characteristic | No AKI (N=160) | AKI (N=60) | P-value** |
|---|---|---|---|
| Age, years | 3.3 (0.5, 6.0) | 0.6 (0.4, 1.8) | < 0.0001 |
| Male, n (%) | 84 (53) | 26 (43) | 0.23 |
| White, n (%) | 142 (89) | 52 (87) | 0.67 |
| Prior surgery, n (%) | 76 (48) | 24 (40) | 0.32 |
| Bypass time, min | 92 (67, 127) | 113 (84, 172) | 0.003 |
| Baseline Scr, mg/dl | 0.4 (0.4, 0.6) | 0.3 (0.2, 0.4) | < 0.0001 |
| Baseline eCCL, ml/min/1.73m2 | 81.8 (65.0, 99.1) | 110.6 (84.0, 144.6) | < 0.0001 |
| Baseline urine NGAL, ng/ml | 8.5 (4.0, 12.5) | 16.0 (10.0, 24.0) | < 0.0001 |
| Baseline urine IL-18, pg/ml | 1.2 (0.0, 8.1) | 0.9 (0.0, 10.0) | 0.65 |
| Baseline urine KIM-1, pg/ml | 193.9 (83.8, 301.4) | 211.5 (96.2, 377.9) | 0.43 |
| Baseline urine FABP, ng/ml | 7.3 (2.3, 27.4) | 10.6 (4.2, 31.2) | 0.34 |
| % Scr change | 0.0 (0.0, 25.0) | 77.5 (64.6, 108.3) | < 0.0001 |
| Hospital stay, days | 5 (3, 7) | 7 (5, 14) | < 0.0001 |
| Ventilator, days | 1 (1, 2) | 2 (1, 2) | < 0.0001 |
| Inotrope score | 0.0 (0.0, 0.0) | 0.0 (0.0, 7.1) | 0.19 |
| Death, n (%) | 0 (0) | 1 (2) | 0.27 |
| RACHS score, n (%) | |||
| 1 | 20 (13) | 4 (7) | |
| 2 | 68 (43) | 29 (48) | |
| 3 | 60 (38) | 24 (40) | 0.14 |
| 4 | 9 (6) | 0 (0) | |
| 5 | 2 (1) | 1 (2) | |
| 6 | 1 (1) | 2 (3) | |
| pRIFLE, n (%) | |||
| R | --- | 27 (45) | --- |
| I | --- | 21 (35) | |
| F | --- | 12 (20) | |
| Duration of AKI, days | --- | 2 (2, 3) | --- |
| Dialysis (%) | 0 (0) | 0 (0) | --- |
Median (interquartile range, IQR) reported for continuous variables, p-values from Wilcoxon rank sum test. Frequency (proportion) reported for categorical variables, with p-values from χ2 test or Fisher’s exact test. pRIFLE and Duration of AKI reported for AKI patients only.
Patients who developed AKI were younger and had lower baseline SCr. CPB times were longer in AKI patients and they had longer duration of mechanical ventilation and hospital length of stay. Baseline urinary biomarker concentrations were similar in AKI and non-AKI groups, with the exception of urine NGAL.
Temporal Elevation of Biomarkers
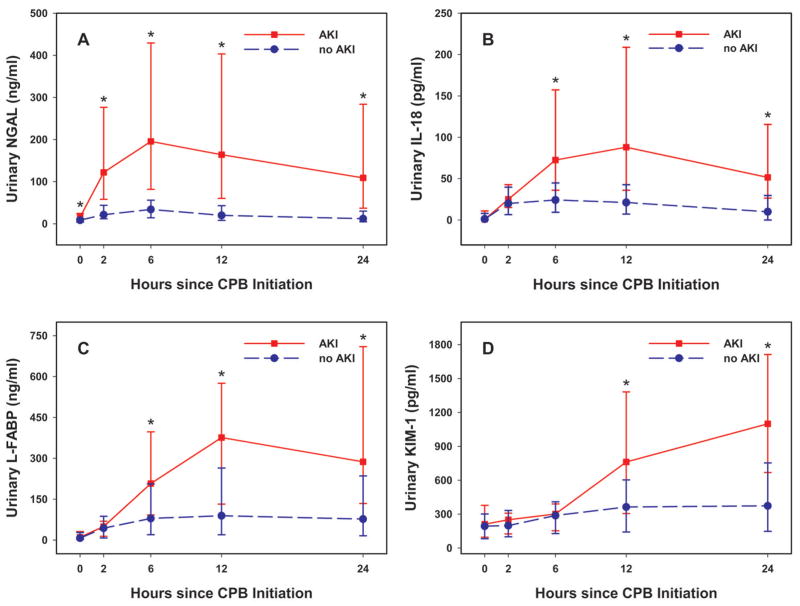
As shown in Figure 1, the temporal trends for all four urine biomarkers in non-AKI patients were consistently low. In AKI patients, each of the four urine biomarkers rose significantly at some time-point after CPB initiation. Significant differences between AKI and non-AKI patients were first seen at 2h for NGAL, at 6h for IL-18 and L-FABP, and at 12h for KIM-1 (Figure 1A–D). Once biomarkers were elevated, they each remained elevated in AKI patients at subsequent time points (all p<0.0001).
Figure 1. Urine biomarker concentrations by AKI status.
Median and interquartile range (IQR) are presented. Statistically significant differences (p<0.0001) in medians between AKI and non-AKI patients are denoted by *. A: urine NGAL; B: urine IL-18; C: urine L-FABP; D: urine KIM-1.
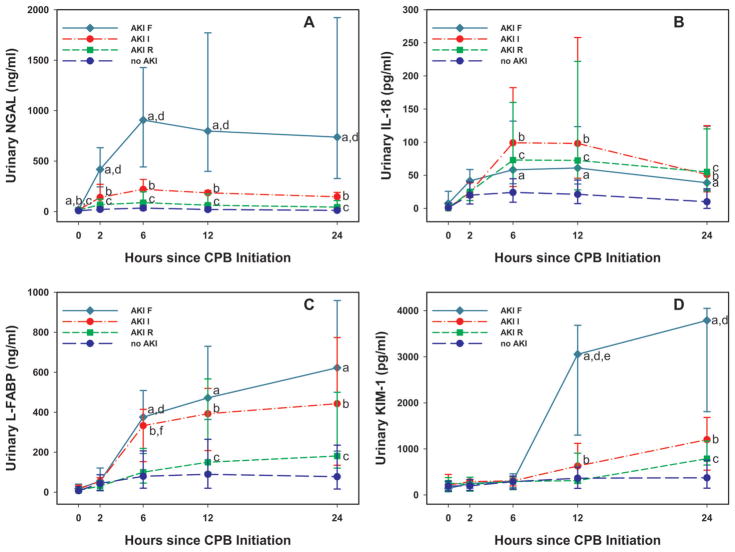
Figure 2 demonstrates the temporal trend in each urinary biomarker according to pRIFLE. With the exception of IL-18 in pRIFLE-F patients, a step-wise increase in each biomarker was seen with worsening AKI severity.
Figure 2. Urine biomarker concentrations by pRIFLE categories.
Median and interquartile range (IQR) are presented. Medians were compared between pRIFLE categories. Statistically significant differences (p<0.05) in medians are denoted by letters: a (F vs. no AKI), b (I vs. no AKI), c (R vs. no AKI), d (F vs. R), e (F vs. I), f (I vs. R). A: urine NGAL; B: urine IL-18; C: urine L-FABP; D: urine KIM-1.
Associations between Urine Biomarkers and Clinical Characteristics
Spearman correlation coefficients for each biomarker at its earliest elevation (2h NGAL, 6h IL-18, 6h L-FABP, and 12h KIM-1) with clinical parameters are summarized in Table 2. Urine NGAL at 2h correlated with younger age, greater SCr change, longer CPB time, higher RACHS-1 scores, longer hospital stay, longer ventilation time and longer duration of AKI. Urine IL-18 at 6h correlated with younger age, greater SCr change, longer hospital stay and longer ventilation time, but not CPB time, RACH-1 score or duration of AKI. Urine L-FABP at 6h and KIM-1 at 12h correlated with younger age, greater SCr change, longer CPB time, higher RACHS-1 scores, longer hospital stay, longer ventilation time and longer duration of AKI.
Table 2.
Spearman correlation coefficients between urinary biomarker concentrations and clinical characteristics and outcomes
| T=2h | T=6h | T=12h | ||
|---|---|---|---|---|
| NGAL | IL-18 | L-FABP | KIM-1 | |
| Age | −0.21 | −0.18 | −0.24 | −0.29 |
| % SCr change | 0.55 | 0.34 | 0.33 | 0.26 |
| CPB time | 0.38 | 0.11* | 0.33 | 0.14 |
| Hospital LOS | 0.44 | 0.18 | 0.37 | 0.28 |
| Ventilator days | 0.37 | 0.22 | 0.28 | 0.31 |
| RACHS-1 score | 0.24 | 0.003* | 0.21 | 0.13 |
| Duration of AKI† | 0.50 | −0.09* | 0.31 | 0.48 |
Insignificant correlation coefficients. All other correlation coefficients are significant (p< 0.0
For AKI patients only
To determine if biomarkers were independently correlated with hospital LOS and ventilator days, we performed Spearman partial correlation, adjusting for the effect of age, CPB time and RACHS-1 score. At 2h, NGAL independently correlated with hospital LOS and ventilator days. At 6h, L-FABP independently correlated with hospital LOS and at 12h, KIM-1 independently correlated with ventilator days. IL-18 did not show significant independent correlation with these outcomes.
Predictive Ability of Biomarkers
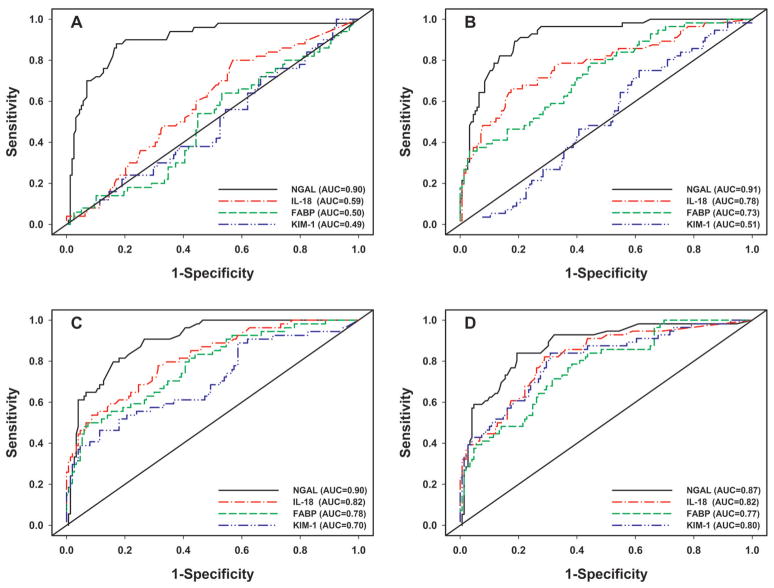
Figure 3 demonstrates the progression of ROC curves for each biomarker at each time-point, from univariable logistic regression analysis. At 2h after CPB initiation, urine NGAL was the only predictive biomarker of AKI with an AUC of 0.90, significantly higher than the AUC for any of the other three biomarkers (all p<0.0001, Figure 3A). At 6h, urine NGAL, IL-18 and L-FABP were significant predictors of AKI, but NGAL still had the highest discrimination (all p≤0.001, Figure 3B). At 12 hours, all four biomarkers performed well, with AUC in the good to outstanding range, but urine. NGAL exhibited the highest AUC (all p≤0.04, Figure 3C). At 24 hours, the predictive value of all four biomarkers remained very good, with improvement in the AUC of KIM-1 to 0.80. At 24 hours, NGAL remained significantly better than L-FABP (p=0.0004) but was not significantly superior to either IL-18 (p=0.17) or KIM-1 (p=0.11) (Figure 3D).
Figure 3. Receiver operating characteristic (ROC) curves for prediction of AKI using urine biomarkers at time intervals following cardiopulmonary bypass (CPB).
A: T=2h; B: T=6h; C: T=12h; D: T=24h.
We then performed multivariable logistic regression analyses for the prediction of AKI. Evaluating clinical factors alone, younger age and longer CPB time were independent predictors for AKI. Each biomarker was then added to the clinical model to assess improvement in the predictive ability of the model (Table 3). At 2h after CPB initiation, adding urine NGAL to the clinical model increased the AUC from 0.74 to 0.85 (p < 0.0001). At 6h, urine NGAL, IL-18 and L-FABP each improved the AUC from 0.72 to 0.91, 0.84 and 0.77, respectively (all p<0.05). At 12h, all the four biomarkers improved the AUC with urine IL-18 having the most improvement which was maintained at 24h after CPB initiation. The added predictive ability of individual biomarker to the clinical model was also supported by the results of NRI and IDI (Table 3).
Table 3.
Evaluation of the performance of predictive models after adding a urinary biomarker to the clinical model
| Model | T=2h (N=208, 50 AKI) | T=6h (N=211, 56 AKI) | T=12h (N=204, 54 AKI) | T=24h (N=205, 56 AKI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC | NRI | IDI | AUC | NRI | IDI | AUC | NRI | IDI | AUC | NRI | IDI | |
| Clinical model | 0.74 | --- | --- | 0.72 | --- | --- | 0.72 | --- | --- | 0.72 | --- | --- |
| + NGAL | 0.85* | 0.87† | 0.12† | 0.91* | 1.24† | 0.27† | 0.85* | 1.03† | 0.12† | 0.82* | 0.99† | 0.09† |
| + IL-18 | 0.74 | 0.14 | 0.007 | 0.84* | 0.93† | 0.18† | 0.86* | 0.86† | 0.25† | 0.86* | 0.84† | 0.24† |
| + L-FABP | 0.73 | 0.17 | 0.004 | 0.77* | 0.61† | 0.11† | 0.81* | 0.69† | 0.15† | 0.79* | 0.60† | 0.12† |
| + KIM-1 | 0.74 | −0.03 | 0.004 | 0.73 | 0.33† | 0.006 | 0.79* | 0.44† | 0.11† | 0.84* | 0.83† | 0.19† |
Compared with the clinical model, age + CPB time), statistically significant (p < 0.05) improvement in AUC.
AUC=Area under the Curve, NRI=Net Reclassification Improvement; IDI=Integrated Discrimination Improvement
For NRI, results correspond to % improvement in the performance of the predictive model. For example, NRI=0.87 means 87% improvement over the reference model (i.e. clinical model: age + CPB time).
p < 0.05.
Next, in order to assess whether the combination of NGAL with other biomarkers would further improve the predictive ability, we evaluated the performance of the predictive models by adding one, two or three other biomarkers (IL-18, L-FABP, KIM-1) to the reference model including age, CPB time and NGAL as predictors. At 2 hours after CPB initiation, only urine NGAL predicted AKI even after other biomarkers were included in the model. At other time points, the significance (p < 0.05) of each biomarker in the model, AUC improvement, NRI and IDI are summarized in Table 4. At the 6h time-point, the combination of NGAL+IL-18 provided the best results in terms of AUC improvement, NRI, and IDI. At 12h, the best predictive ability was obtained when all four biomarkers were included. However, the more parsimonious combination of NGAL+IL-18+L-FABP provided comparable results. Similarly, at the 24-hour mark, the best combination was all four biomarkers but the combination of NGAL + IL-18 + KIM-1 provided comparable results and could be used as the best combination.
Table 4.
Summary of biomarker combinations in the model: significance, AUC improvement, NRI and IDI
| Combination | Biomarker | T=6h | T=12h | T=24h | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p<0.05 | Δ AUC | NRI | IDI | p<0.05 | Δ AUC | NRI | IDI | p<0.05 | Δ AUC | NRI | IDI | ||
| I | NGAL | Yes | 0.013 | 0.64* | 0.10* | Yes | 0.056* | 0.77* | 0.21* | Yes | 0.069* | 0.77* | 0.21* |
| IL-18 | Yes | Yes | Yes | ||||||||||
|
| |||||||||||||
| NGAL | Yes | −0.019 | 0.22 | 0.02 | Yes | −0.002 | 0.41* | 0.09* | Yes | 0.005 | 0.34* | 0.08* | |
| L-FABP | Yes | Yes | Yes | ||||||||||
|
| |||||||||||||
| NGAL | Yes | −0.007 | 0.48* | 0.01 | Yes | −0.012 | 0.41* | 0.05* | Yes | 0.026 | 0.66* | 0.12* | |
| KIM-1 | No | Yes | Yes | ||||||||||
|
| |||||||||||||
| II | NGAL | Yes | 0.009 | 0.60* | 0.11* | Yes | 0.060* | 0.95* | 0.27* | Yes | 0.073* | 0.93* | 0.25* |
| IL-18 | Yes | Yes | Yes | ||||||||||
| L-FABP | No | Yes | Yes | ||||||||||
|
| |||||||||||||
| NGAL | Yes | 0.013 | 0.56* | 0.10* | Yes | 0.058* | 0.79* | 0.24* | No | 0.084* | 1.00* | 0.27* | |
| IL-18 | Yes | Yes | Yes | ||||||||||
| KIM-1 | No | Yes | Yes | ||||||||||
|
| |||||||||||||
| NGAL | Yes | −0.003 | 0.21 | 0.03 | Yes | 0.010 | 0.63* | 0.10* | No | 0.045 | 0.84* | 0.15* | |
| L-FABP | Yes | Yes | Yes | ||||||||||
| KIM-1 | No | Yes | Yes | ||||||||||
|
| |||||||||||||
| III | NGAL | Yes | 0.012 | 0.61* | 0.12* | Yes | 0.064* | 0.98* | 0.28* | No | 0.089* | 1.01* | 0.29* |
| IL-18 | Yes | Yes | Yes | ||||||||||
| L-FABP | No | Yes | No | ||||||||||
| KIM-1 | No | No | Yes | ||||||||||
At 2h, only NGAL was significant
AUC=Area under the Curve, NRI=Net Reclassification Improvement; IDI=Integrated Discrimination Improvement
ΔAUC = Improvement in AUC when compared with the reference model (age + CPB time + NGAL). For NRI, results correspond to % improvement in the performance of the predictive model. For example, NRI=0.64 means 64% improvement over the reference model (i.e. age + CPB time ± NGAL).
p< 0.05
Lastly, we evaluated the patients with “severe AKI” [RIFLE “I” and “F”] to determine the ability of the biomarkers to predict severe AKI. 33 patients were in this group. Our results were very similar to the full AKI cohort (Table 5, online), with similar timing of the biomarker elevations.
DISCUSSION
Our results confirm that urine NGAL, IL-18, L-FABP and KIM-1 are predictors of post-cardiac surgery AKI. To our knowledge, the current study is the first to 1) establish a temporal pattern of biomarker elevation after CPB and 2) demonstrate the utility of biomarker combinations for the improved prediction of AKI beyond clinical models. These findings could enhance the potential for appropriately timed application of therapeutic interventions. Additionally, these biomarkers offer severity and prognostic information at early time-points.
The etiology of AKI following CPB is multi-factorial and incompletely understood. Since many pathways are involved, it is not surprising that combinations of biomarkers with different properties may prove most predictive. In this study, we found that a clinical model of age and CPB time could predict AKI with reasonable certainty. The addition of biomarkers, however, particularly in combination, significantly increased the predictive value of the model. Thus, a panel of strategically selected and rapidly testable biomarkers may prove optimal for early diagnosis and institution of treatment.
We studied four biomarkers as candidates for a sequential AKI panel. Urine IL-18, NGAL, L-FABP and KIM-1 are all up-regulated and released by the kidney tubules during injury and are biological intermediates in the causal mechanisms of ischemia-reperfusion injury to the kidney. These biomarkers are present at low concentrations pre-operatively, and their levels increase by several fold in patients who develop kidney injury. IL-18, a mediator of inflammation, is produced by proximal tubules, and is activated by caspase-1 following AKI (20). It is more specific to ischemic AKI and is not significantly affected by nephrotoxins or chronic kidney disease. The gene for NGAL is significantly up-regulated in the kidney after ischemic and nephrotoxic injury, and the protein is over-expressed in distal tubule cells (21). NGAL may play a primary role in renal tubular survival and recovery, and has been used therapeutically in ischemia-reperfusion injury in animal models (22). Recent studies using an NGAL reporter mouse have established that the kidney is the primary source of urinary NGAL in AKI (23). L-FABP is an inflammatory proximal tubular protein that is upregulated following a variety of acute kidney injuries (9, 24, 25). KIM-1 is a trans-membrane protein that is over-expressed in de-differentiated proximal tubule cells after ischemic or toxic injury (10, 21–23)
We found urine NGAL concentrations increased at the earliest time-point after CPB and were associated with the highest predictive value. We do note that, in some studies, NGAL has not performed as well (26). We hypothesize that this may be related to the confounding disease states (such as diabetes, lung disease, inflammation) that may be more prevalent in adults and may affect NGAL concentrations. This further highlights the need for a comprehensive “panel” of biomarkers, optimizing both sensitivity and specificity. Notably, our results demonstrate that combinations of biomarkers provide additional predictive value. Interestingly, we found small (but significantly) higher pre-operative NGAL levels in patients who developed post-CPB AKI. It is possible that some of our patients had subclinical pre-operative AKI, which may have predisposed them to development of AKI.
With the exception of IL-18 in the RIFLE “F” patients, all biomarker concentrations increased in a step-wise fashion with worsening kidney injury. It is unclear why IL-18 did not increase in the same pattern in RIFLE “F” patients, particularly since it has performed well in other studies (8). It is possible that this is due to the small number of “F” patients analyzed but further evaluation in larger studies is warranted.
The importance of determining the temporal sequence of the biomarkers is underscored by the fact that the course of experimental AKI proceeds in four phases: initiation, extension, maintenance, and recovery (27). The initiation phase is the period during which initial exposure to the ischemic insult occurs, intracellular ATP depletion is profound, and generation of reactive oxygen molecules and labile iron is initiated. Vasodilator, ATP-donor, anti-oxidant, and iron chelation therapies may be especially effective during this phase, and the appearance of the earliest non-invasive biomarkers such as NGAL may be used to trigger such therapies. Prolongation of ischemia followed by reperfusion ushers in the extension phase. Tubules undergo reperfusion-mediated cell death, and the injured endothelial and epithelial cells amplify the inflammatory cascades. This phase probably represents a window of opportunity for early diagnosis with intermediate biomarkers such as L-FABP and IL-18, and active therapeutic intervention with anti-apoptotic and anti-inflammatory strategies. During the maintenance phase, both cell injury and regeneration occur simultaneously. Measures such as growth factors and stem cells that accelerate the endogenous regeneration processes, initiated by later biomarkers with high specificity such as KIM-1, may be most effective during this phase.
Our study has several strengths. First, we utilized a prospective cohort design and employed a rigorous protocol to collect specimens, followed by blinded measurements of biomarkers. Second, we enrolled a large, relatively homogenous cohort of subjects in whom the most proximate etiology for AKI would be CPB. The study design also allowed for the precise determination of the temporal rise in biomarkers following CPB.
This study does have important limitations. First, this remains a single center study which needs validation at the multi-center level. Second, our results may not be generalizable to adults undergoing CPB, or to the myriad other clinical scenarios that commonly lead to AKI in hospitalized patients. Third, SCr levels were not measured at the same frequency as the urinary biomarkers examined, and the exact timing of SCr rise in our study population is uncertain. Fourth, the definition of AKI was based on elevations in SCr, making it very likely that we captured only those with greater than mild injury. Additionally, since patients with congenital heart disease often have decreased muscle mass, elevations in SCr may reflect more injury than in a healthy population. Indeed, in a recent multicenter pooled analysis of 2322 critically ill children and adults with cardio-renal syndrome, 20% of patients had early elevations in NGAL but never developed increases in SCr (28). Importantly, this sub-group of “NGAL-positive, SCr -negative” subjects encountered a substantial increase in adverse outcomes, including mortality, dialysis requirement, ICU stay, and prolonged hospital stay. Thus, early biomarker measurements may identify patients with sub-clinical AKI who have an increased risk of adverse outcomes, even in the absence of SCr rise.
This study is the first to demonstrate the temporal elevation and progression of clinically relevant and predictive biomarkers following AKI, and the first to demonstrate the enhanced prediction of AKI using biomarker combinations. The application of biomarker technology to create a bedside AKI “panel” could allow clinicians the ability to pinpoint timing of insult to the kidney and perhaps direct therapeutic interventions. Results of this study support the inclusion of urine NGAL, IL-18, L-FABP and KIM-1 in such a panel.
Supplementary Material
Acknowledgments
Funding Sources: NIH (R01-HL08676, R01-HL085757, R01-DK069749)
We are indebted to our nurse coordinators: Tracey VanVliet, Rachel Griffiths, Lauren Hoctor, and Julie Ciambarella for their assistance, and to our patients and their families for their participation.
ABBREVIATIONS
- AKI
acute kidney injury
- CPB
cardiopulmonary bypass
- SCr
serum creatinine
- NGAL
neutrophil gelatinase-associated lipocalin
- IL-18
interleukin-18
- L-FABP
liver fatty acid-binding protein
- KIM-1
kidney injury molecule-1
- eCCl
estimated creatinine clearance
- RACHS-1
risk adjustment for congenital heart surgery score version 1
Footnotes
Disclosures: Dr. Devarajan is a co-inventor on patents related to NGAL and is a consultant to Abbott Diagnostics and Biosite, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lok CE, Austin PC, Wang H, Tu JV. Impact of renal insufficiency on short- and long-term outcomes after cardiac surgery. Am Heart J. 2004;148(3):430–8. doi: 10.1016/j.ahj.2003.12.042. [DOI] [PubMed] [Google Scholar]
- 2.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 3.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104(4):343–8. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 4.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15(6):1597–605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 5.Zappitelli M, Bernier PL, Saczkowski RS, Tchervenkov CI, Gottesman R, Dancea A, et al. A small post-operative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney Int. 2009;76(8):885–92. doi: 10.1038/ki.2009.270. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein SL, Devarajan P. Acute kidney injury in childhood: should we be worried about progression to CKD? Pediatr Nephrol. 2011;26(4):509–22. doi: 10.1007/s00467-010-1653-4. [DOI] [PubMed] [Google Scholar]
- 7.Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: physiological principles. Intensive Care Med. 2004;30(1):33–7. doi: 10.1007/s00134-003-2078-3. [DOI] [PubMed] [Google Scholar]
- 8.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16(10):3046–52. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 9.Portilla D, Dent C, Sugaya T, Nagothu KK, Kundi I, Moore P, et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73(4):465–72. doi: 10.1038/sj.ki.5002721. [DOI] [PubMed] [Google Scholar]
- 10.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–44. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins KJ. Risk adjustment for congenital heart surgery: the RACHS-1 method. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:180–4. doi: 10.1053/j.pcsu.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028–35. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–37. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wernovsky G, Giglia TM, Jonas RA, Mone SM, Colan SD, Wessel DL. Course in the intensive care unit after ‘preparatory’ pulmonary artery banding and aortopulmonary shunt placement for transposition of the great arteries with low left ventricular pressure. Circulation. 1992;86(5 Suppl):II133–9. [PubMed] [Google Scholar]
- 15.Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3(3):665–73. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaturvedi S, Farmer T, Kapke GF. Assay validation for KIM-1: human urinary renal dysfunction biomarker. Int J Biol Sci. 2009;5(2):128–34. doi: 10.7150/ijbs.5.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 19.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melnikov VY, Faubel S, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest. 2002;110(8):1083–91. doi: 10.1172/JCI15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14(10):2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 22.Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24(3):307–15. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 23.Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med. 2011;17(2):216–22. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson MA, Vaidya VS, Waikar SS, Collings FB, Sunderland KE, Gioules CJ, et al. Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int. 2010;77(8):708–14. doi: 10.1038/ki.2009.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Negishi K, Noiri E, Doi K, Maeda-Mamiya R, Sugaya T, Portilla D, et al. Monitoring of urinary L-type fatty acid-binding protein predicts histological severity of acute kidney injury. Am J Pathol. 2009;174(4):1154–9. doi: 10.2353/ajpath.2009.080644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54(6):1012–24. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17(6):1503–20. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 28.Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57(17):1752–61. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.