Life expectancy has been increasing in the united States for well over a century. While some analysts, including the Social Security administration, have projected a continued rise over the next seven decades (Board of Trustees 2009), others have argued that the substantial rise in obesity could cause life expectancy to level off or decline within the first half of this century (Olshansky et al. 2005). Recent research on the joint effects of obesity and smoking on US life expectancy forecasts that the negative effects of rising obesity will potentially overwhelm the positive effects gained from declining smoking rates, retarding increases in life expectancy (Stewart, Cutler, and Rosen 2009). The US has witnessed substantial increases in the prevalence of obesity. Adult obesity (body mass index [BMI] ≥30.0 kg/m2) has more than doubled over the past three decades, and obesity among school-aged children (BMI-for-age ≥95th percentile) has tripled (Flegal et al. 2010; Ogden et al. 2002). Projections of the effect of obesity on life expectancy, however, generally assume that the risk of death imposed by obesity has been and will remain stable (Stewart, Cutler, and Rosen 2009; Olshansky et al. 2005).
A large number of studies have shown that class I obesity (BMI 30.0–34.9) and class II/III obesity (BMI ≥35.0) have strong associations with mortality (Prospective Studies Collaboration 2009; Hu et al. 2004; Mokdad et al. 2004; Fontaine et al. 2003; Peeters et al. 2003; Allison et al. 1999; Calle et al. 1999; Manson et al. 1995). All of these studies, however, rely on mortality data collected before 1990. In contrast, research using more recent data has found that more moderate levels (class I) of obesity are not strongly associated with mortality (Mehta and Chang 2009; Reuser, Bonneux, and Willekens 2009; Flegal et al. 2007a; Flegal et al. 2005) and that only a small proportion of excess deaths in the US is attributable to obesity (Mehta and Chang 2009). For example, recent research on middle-aged adults estimates that less than 5 percent of deaths in 1999 were attributable to obesity (BMI ≥30.0) (Mehta and Chang 2009). Although numerous methodological differences likely contribute to prior divergent estimates, discrepancies may be partly explained by the fact that this relationship has weakened over time (Mehta and Chang, in press, reviews additional methodological differences).
A decrease in the association between obesity and mortality may be promoted by changes in health behaviors and improvements in medical care, particularly for cardiovascular disease (CVD). Obese persons have experienced substantial declines in high blood pressure, smoking, and total cholesterol since the 1960s (Gregg et al. 2005). For example, between 1960–62 and 1999–2000, the prevalence of hypertension has decreased by 49 percent among the obese, and high cholesterol has decreased by 54 percent. In fact, physicians may even be more aggressive with risk-factor modification among obese persons. Reductions in cholesterol have been proportionately greater for obese patients than for patients of normal weight (ibid.), and recent research finds that obese patients are more likely to receive recommended diabetes care than normal-weight patients (Chang, Asch, and Werner 2010).
In addition to its importance in forecasting life expectancy, understanding change over time in the magnitude of the association between obesity and mortality is also critical for estimating obesity's contribution to current variations in national mortality patterns. The United States has a considerably higher level of obesity and a lower life expectancy than most other high-income countries (Preston and Stokes in press). Findings from the recent National Research Council (NRC) report on the causes of longevity differences among high-income countries indicate that obesity accounts for approximately 41 percent and 67 percent of the shortfall in US longevity among women and men, respectively (compared to the average of 12 other high-income countries) (National Research Council 2011). These estimates, however, are based on a set of relative risks for obesity derived from a study in which the mean year of death was 1986. When relative risks of obesity are derived from more recent data, the NRC report indicates that obesity accounts for approximately 20–30 percent of the US shortfall in longevity.
Our objective is to investigate secular trends in the association between obesity and mortality in the United States. We rely on three long-standing US data sources on health and mortality: (1) the Framingham Heart Study, (2) the National Health and Nutrition Examination Survey (NHANES), and (3) the National Health Interview Survey (NHIS). We compare periods of mortality that are non-overlapping and of similar duration within each data source and cover a time period extending from 1948 to 2006. We investigate trends for both all-cause mortality and cause-specific (CVD, cancer, and non-CVD/non-cancer) mortality.
Methods
Data
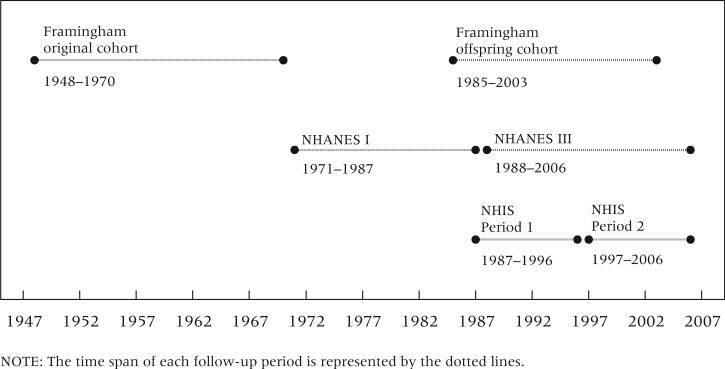
Table 1 presents characteristics of the data. In order to examine trends, we sought data sources that followed independent cohorts over distinct time periods and were linked to mortality. We defined an “earlier” and “later” mortality period within each of our three data sources (Framingham, NHANES, and NHIS) to examine change over time in the association between obesity and mortality. Mortality follow-up for the two periods within each data source is non-overlapping in time, of comparable duration, and based on independent samples. The timelines are shown in Figure 1. We included individuals between ages 50 and 74 at study entry.
TABLE 1.
Data characteristics of participants in three US mortality studies, ages 50–74 at baseline
| Framingham |
NHANES |
NHIS |
||||
|---|---|---|---|---|---|---|
| Original cohort | Offspring cohort | NHANES I | NHANES III | Period 1 | Period 2 | |
| Entry years | 1948–1962 | 1985–1992 | 1971–1975 | 1988–1994 | 1987–1991a | 1997–2000 |
| Final year of mortality follow-up | 1970 | 2003 | 1987 | 2006 | 1996 | 2006 |
| Sample size | 3,135 | 2,744 | 3,269 | 5,257 | 48,698 | 33,817 |
| Mean years of follow-upb | 13.7 | 14.8 | 12.3 | 13.0 | 7.5 | 7.6 |
| Person-years of follow-up | 42,803 | 40,756 | 38,903 | 65,788 | 346,085 | 258,401 |
| Mean age at entry, yearsb | 53.8 | 55.4 | 60.5 | 61.8 | 61.7 | 60.9 |
| Total number of deaths | 714 | 470 | 1,165 | 2,007 | 7,318 | 4,479 |
| Cause-specific deaths, no. (%)c | ||||||
| CVD | 404 (59.5%) | 95 (24.2%) | 587 (51.7%) | 813 (41.1%) | 3,015 (41.4%) | 1,505 (33.8%) |
| Cancer | 176 (25.9%) | 175 (44.5%) | 302 (26.6%) | 541 (27.4%) | 2,372 (32.5%) | 1,523 (34.2%) |
| All other causes | 99 (14.6%) | 123 (31.3%) | 247 (21.7%) | 624 (31.5%) | 1,903 (26.1%) | 1,419 (31.9%) |
Excludes 1989 survey.
Results for NHANES and NHIS reflect sample weighting.
Deaths from specific causes do not sum to total deaths because of missing cause-of-death information for some subjects. Percentages are based on deaths with known causes.
FIGURE 1.
Timeline of follow-up periods in three US mortality studies
Framingham Heart Study
The Framingham Heart Study is a multi-cohort study conducted by the National Heart, Lung, and Blood Institute (Kannel et al. 1979; Drawber, Meadors, and Moore 1951). The study began in 1948 with a sample of adults in Framingham, Massachusetts. Beginning in 1971, the children of the original cohort and their spouses were enrolled. We used the original cohort for the earlier period and the offspring cohort for the later period. We included persons who reached age 50 in any of the first seven exams (1948–1962) of the original cohort and who reached age 50 in exams three to five (1985–1992) of the offspring cohort. We restrict entry ages in both Framingham datasets to ages 50–69 because no respondent in the original cohort was above age 70 in the first exam. We also excluded earlier waves of the offspring cohort because body weight was only available in 5-pound intervals, precluding a precise calculation of BMI. We followed deaths in the original cohort through 1970 and deaths in the offspring cohort through 2003. Thus, the mortality periods covered were 1948–1970 (original cohort) and 1985–2003 (offspring cohort). While the Framingham study is not nationally representative like the other data used in this analysis, it allows for the estimation of obesity-related mortality risks during a relatively early period.
National Health and Nutrition Examination Survey
NHANES is a nationally representative cross-sectional survey of the US population conducted by the National Center for Health Statistics (NCHS). We used data from NHANES I (1971–1975) for the earlier period and data from NHANES III (1988–1994) for the later period. For NHANES I, we restricted our sample to a subset that was surveyed about smoking, which is a key confounder in the association between obesity and mortality. The mortality periods were 1971–1987 for NHANES I and 1988–2006 for NHANES III.
National Health Interview Survey
NHIS is a nationally representative annual cross-sectional survey of the US population conducted by NCHS. For the earlier period, which we denote as Period 1, we pooled the 1987–1991 annual surveys and measured deaths through 1996 (the 1989 survey was excluded because data on smoking were unavailable). For the later period (Period 2), we pooled the 1997–2000 surveys and assessed mortality through 2006. For both periods, we restricted the analyses to subsamples that were administered a supplementary questionnaire on smoking.
Measures
The NHANES III and NHIS data are linked to the National Death Index by NCHS. We use the 1992 NHANES I Epidemiologic Follow-Up Study to obtain data on deaths in NHANES I. Deaths in the Framingham study are ascertained by a panel of Framingham investigators and are available in the data. Approximately 1 percent of respondents had insufficient data with which to ascertain mortality status.
In cause-specific analyses, we examined three categories of deaths: CVD, cancer, and non-CVD/non-cancer. CVD and cancer are leading causes of death in the United States (Jemal et al. 2005), and both are associated with obesity (Calle et al. 1999; Calle et al. 2003). For NHIS and NHANES, deaths were classified according to the NCHS 113 Selected Causes of Death recodes following Flegal et al. (2007a): cardiovascular disease (codes: 53–74), cancer (codes: 19–43), and non-CVD/non-cancer (all other codes). We grouped deaths in the Framingham study into the same three categories using available data on causes of death.
Weight status was modeled using standard categories: underweight (BMI <18.5), normal (BMI 18.5–24.9), overweight (BMI 25.0–29.9), class I obese (BMI 30.0–34.9), and class II/III obese (BMI ≥35.0) (world Health Organization 2000; National Heart, Lung, and Blood Institute 1998). We combined class II (BMI 35.0–39.9) and class III (BMI ≥40.0) obese categories because of the small number of individuals in the samples who are class III obese. The percentage of class III obesity within the class II/III category remained relatively stable over the NHANES periods (approximately 30 percent), increased in the Framingham study (from 23 percent to 34 percent), and declined in the NHIS (from 27 percent to 21 percent). Weight and height were clinically measured in the Framingham study and NHANES and were self-reported in the NHIS. Our previous work suggests that the estimated association between obesity and mortality is not highly sensitive to potential bias from self-reported height and weight (Mehta and Chang 2009).
Socioeconomic status is associated with BMI and mortality (Lantz et al. 2010; Chang and Lauderdale 2005; Mujahid et al. 2005). We included both family income (quartiles) and education (<12 years, 12 years, 13–15 years, ≥16 years). Family income quartiles were estimated using the distributions of family income in the baseline data. Family income was unavailable for the Framingham study, and the two highest education categories (13–15 and ≥16 years) were combined owing to data availability. Other covariates included sex, cigarette smoking (never, former, current <1 pack daily, 1 to <2 packs daily, and ≥2 packs daily), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, non-Hispanic other), and marital status (never married, married, widowed/divorced/separated). For the Framingham data, we excluded marital status because it was not consistently available, and race/ethnicity was excluded because the cohorts are predominantly white. In the NHIS and NHANES analyses, we additionally adjusted for region of residence in the US (Northeast, South, Midwest, and West).
Statistical analysis
We estimated hazard ratios (HRs) for the BMI categories from Cox proportional hazard models using age as the time scale (Korn, Graubard, and Midthune 1997). Each of the three data sources was analyzed separately. Within each source, we pooled data from the two mortality periods and constructed a 0/1 indicator variable for period, with the later period coded as 1. We estimated a model that included the covariates noted above and two-way interactions between each covariate and the period variable. The p values of the interaction terms for the BMI categories indicate whether there was a significant change between periods in their hazard ratios for mortality. Survivors were censored at the end of the follow-up period. For cause-specific mortality analyses, individuals who died from causes not under investigation were censored at the time of death.
With exceptions noted below, we used list-wise deletion for missing covariates. The number of individuals with missing data on at least one covariate in each data source was less than 5 percent, with the exception of education in the offspring cohort (8 percent) and income in NHANES III (8 percent) and NHIS (18 percent). Persons with missing education data in the Framingham study or missing income data in NHANES or NHIS were included in the analyses using an indicator variable for missing data. Within each data source, we estimated two additional models to check for potential bias from missing data on these variables. We assigned persons with missing data first to the highest category for that variable and then to the lowest. Results from these sensitivity analyses were highly similar to those we present, and our conclusions remained unchanged. In additional sensitivity analyses, we included interaction terms between BMI categories and age at baseline, and alternatively between BMI categories and attained age (age at exposure). These models also did not result in any meaningful changes to our findings.
Estimates for NHIS and NHANES reflect sample weighting and account for the complex survey designs. For NHIS and NHANES I, we used sample weights applicable to the subsamples of individuals who were administered the supplementary questionnaire that included questions about smoking. For NHANES III, all adults were asked about smoking. STATA 11.0 was used for all analyses. This research was approved by the Institutional Review Boards of the University of Michigan and the University of Pennsylvania.
Results
Table 2 shows the unadjusted distributions of BMI. For all data sources, both class i obesity and class II/III obesity increased significantly over time. For example, the prevalence of class I obesity increased from 14.4 percent in NHANES I to 19.4 percent in NHANES III, and the prevalence of class II/III obesity nearly doubled from 5.2 percent to 9.7 percent. The lower prevalence of class II/III obesity in NHIS Period 1 (1987–1991) than in NHANES III (1988–1994)—studies that cover a similar period—may reflect under-reporting of weight among persons who were moderately and severely obese in NHIS. The prevalence of overweight remained relatively stable, although there was a modest and significant increase in the NHIS from 38.9 percent to 41.3 percent.
TABLE 2.
Prevalence of weight status categories in three US mortality studies, ages 50–74 at baseline (percent distributions)
| Framingham |
NHANES |
NHIS |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Original cohort | Offspring cohort | p for trend | NHANES I | NHANES III | p for trend | Period 1 | Period 2 | p for trend | |
| Entry years | 1948–1962 | 1985–1992 | — | 1971–1975 | 1988–1994 | — | 1987–1991 | 1997–2000 | — |
| Sample size | 3,135 | 2,744 | — | 3,269 | 5,257 | — | 48,698 | 33,817 | — |
| Weight status | |||||||||
| Underweight | 0.9 [0.5–1.2] | 0.7 [0.1–1.0] | .36 | 2.8 [2.1–3.5] | 1.6 [1.2–2.1] | .01 | 2.3 [2.1–2.5] | 0.8 [0.7–0.9] | <.001 |
| Normal weight | 40.8 [39.1–42.6] | 34.7 [32.9–36.5] | <.001 | 40.7 [38.4–42.9] | 31.5 [29.4–33.5] | <.001 | 42.8 [42.3–43.4] | 34.3 [33.7–34.9] | <.001 |
| Overweight | 42.4 [40.7–44.2] | 41.6 [39.7–43.4] | .51 | 36.9 [34.6–39.3] | 37.8 [35.8–39.9] | .57 | 38.9 [38.2–39.4] | 41.3 [40.7–41.9] | <.001 |
| Class I obese | 12.7 [11.5–13.8] | 17.2 [15.8–18.6] | <.001 | 14.4 [13.0–15.7] | 19.4 [17.8–20.9] | <.001 | 11.9 [11.5–12.3] | 17.8 [17.3–18.2] | <.001 |
| Class II/III obese | 3.2 [2.6–3.8] | 5.8 [5.0–6.7] | <.001 | 5.2 [4.0–6.3] | 9.7 [8.5–10.9] | <.001 | 4.2 [4.0–4.4] | 5.9 [5.6–6.2] | <.001 |
NOTE: Percentage [95% confidence interval] unless otherwise noted. Results for NHANES and NHIS reflect sample weighting. Sample sizes are unweighted in all data. Weight status categories: underweight (BMI <18.5), normal weight (BMI 18.5–24.9), overweight (BMI 25.0–29.9), class I obese (BMI 30.0–34.9), class II/III obese (BMI ≥35.0).
Table 3 shows unadjusted death rates overall and by BMI category. Consistent with population trends, overall mortality declined across time in all three data sources. With the exception of the underweight and overweight categories in NHANES, declines in mortality are observed for most BMI categories across time in the three data sources. The trend of declining mortality for class I obesity was statistically significant in all three data sources. For class II/III obesity, declines were significant in NHIS but not in the Framingham study. No declines were observed in NHANES.
TABLE 3.
Death rates (per 1,000 person-years) by BMI category in three US mortality studies, ages 50–74 at baseline
| Framingham |
NHANES |
NHIS |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Original cohort | Offspring cohort | p for trend | NHANES I | NHANES III | p for trend | Period 1 | Period 2 | p for trend | |
| Overall | 16.7 [15.5–17.9] | 11.3 [10.3–12.3] | <.001 | 26.0 [24.4–27.8] | 24.7 [23.0–26.5] | .29 | 20.1 [19.5–20.7] | 15.7 [15.1–16.2] | <.001 |
| BMI category | |||||||||
| Underweight | 33.3 [13.2–53.4] | 30.6 [5.8–55.5] | .87 | 61.1 [43.4–78.7] | 61.8 [37.0–86.6] | .96 | 41.4 [36.3–46.6] | 31.7 [23.3–40.0] | .05 |
| Normal weight | 16.6 [14.7–18.4] | 10.1 [8.5–11.7] | <.001 | 25.7 [23.0–28.4] | 24.0 [21.4–26.5] | .35 | 19.8 [19.0–20.7] | 17.0 [16.1–17.9] | <.001 |
| Overweight | 15.0 [13.3–16.8] | 11.3 [9.8–12.9] | .002 | 21.1 [18.6–23.8] | 24.0 [21.1–26.9] | .15 | 18.3 [17.4–19.1] | 13.8 [13.0–14.6] | <.001 |
| Class I obese | 20.4 [16.8–23.9] | 11.9 [9.4–14.4] | <.001 | 33.5 [26.6–40.4] | 22.6 [19.7–25.4] | .01 | 21.2 [19.5–22.9] | 15.5 [14.1–16.8] | <.001 |
| Class II/III obese | 21.3 [14.2–28.3] | 15.7 [10.6–20.8] | .21 | 29.1 [21.6–36.6] | 29.8 [24.5–35.2] | .87 | 26.5 [23.0–30.1] | 19.2 [16.7–21.7] | <.001 |
NOTE: Rate per 1,000 person-years [95% confidence interval] unless otherwise noted. Results for NHANES and NHIS reflect sample weighting. Sample sizes are unweighted in all data. Weight status categories: underweight (BMI <18.5), normal weight (BMI 18.5–24.9), overweight (BMI 25.0–29.9), class I obese (BMI 30.0–34.9), class II/III obese (BMI ≥35.0).
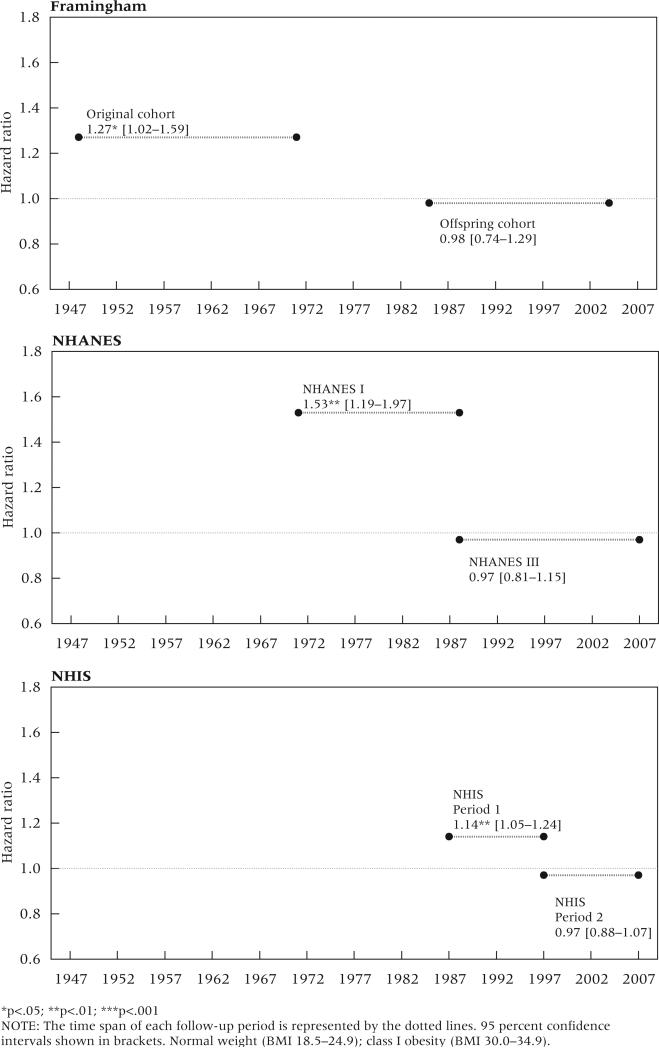
Table 4 presents results from the multivariate all-cause mortality models. The top panel shows main effects for the weight status categories, which denote hazard ratios for the earlier periods. The middle panel shows hazard ratios for the weight status/time period interactions, and the bottom panel shows hazard ratios for weight status in the later periods, which were obtained by multiplying the main and interaction hazard ratios. Across all three data sources, the interaction effects for class I obesity were less than 1.0, indicating a decline over time in its association with mortality. In the earlier periods, class I obesity was associated with significantly higher mortality relative to normal weight, with hazard ratios of 1.27, 1.53, and 1.14 in the Framingham, NHANES, and NHIS data, respectively. In the later period of each data source, however, the hazard ratios for class I obesity declined to approximately 1.0 and were not statistically significant. Figure 2 shows the declining association between mortality and class I obesity over time. Despite a significant positive association with mortality in the earlier period for all three data sources, no association is observed in the later period for any of the data. These declines were statistically significant in NHANES and the NHIS. Also noteworthy, the hazard ratios associated with underweight (BMI <18.5) are higher than those associated with the two obese classifications.
TABLE 4.
Hazard ratios and 95 percent confidence intervals for all-cause mortality in three US mortality studies
| Framingham | NHANES | NHIS | |
|---|---|---|---|
| Main effects (earlier period) | Original cohort 1948–1970 | NHANES I 1971–1987 | Period 1 1987–1996 |
| Underweight (BMI<18.5) | 2.20* [1.17–4.14] | 2.15*** [1.57–2.95] | 1.72*** [1.50–1.97] |
| Normal weight (18.5–24.9) | 1.00 [Ref.] | 1.00 [Ref.] | 1.00 [Ref.] |
| Overweight (25.0–29.9) | 0.88 [0.75–1.05] | 0.83* [0.70–0.98] | 0.88*** [0.83–0.94] |
| Class I obese (30.0–34.9) | 1.27* [1.02–1.59] | 1.53** [1.19–1.97] | 1.14** [1.05–1.24] |
| Class II/III obese (35.0+) | 1.46 [1.00–2.13] | 1.60** [1.16–2.21] | 1.56*** [1.34–1.84] |
| Interaction effects | |||
| Underweight × period | 1.26 [0.47–3.42] | 0.90 [0.54–1.48] | 0.94 [0.68–1.30] |
| Overweight × period | 0.99 [0.75–1.31] | 1.20 [0.97–1.50] | 0.91 [0.83–1.01] |
| Class I obese × period | 0.77 [0.54–1.19] | 0.63** [0.47–0.85] | 0.85* [0.75–0.97] |
| Class II/III obese × period | 1.27 [0.74–2.16] | 0.97 [0.65–1.36] | 0.81* [0.65–1.00] |
| Main × interaction effects (later period) | Offspring cohort 1985–2003 | NHANES III 1988–2006 | Period 2 1997–2006 |
| Underweight (BMI<18.5) | 2.77* [1.28–6.00] | 1.92** [1.30–2.84] | 1.61** [1.21–2.16] |
| Normal weight (18.5–24.9) | 1.00 [Ref.] | 1.00 [Ref.] | 1.00 [Ref.] |
| Overweight (25.0–29.9) | 0.87 [0.70–1.09] | 0.99 [0.87–1.14] | 0.81*** [0.74–0.88] |
| Class I obese (30.0–34.9) | 0.98 [0.74–1.29] | 0.97 [0.81–1.15] | 0.97 [0.88–1.07] |
| Class II/III obese (35.0+) | 1.85** [1.27–2.69] | 1.56*** [1.22–1.99] | 1.26** [1.09–1.45] |
p<.05
p<.01
p<.001
NOTE: All models adjust for sex, cigarette smoking, and education. The NHANES and NHIS analyses are further adjusted for family income, race/ethnicity, marital status, and US region of residence. Dates refer to time span of the mortality period. Main effects indicate hazard ratios for the earlier period of each data source. Hazard ratios for later period were obtained by multiplying the main effects by the interaction effects (may not be exact because of rounding).
FIGURE 2.
Trends in all-cause mortality hazard ratios between earlier and later periods for class I obesity relative to normal BMI in three US mortality studies
We additionally found a significant decline in the association between mortality and class II/III obesity in NHIS. In Period 1, the hazard ratio for class II/III obesity was 1.56, which declined to 1.26 in Period 2. Unlike class I obesity, class II/III obesity was associated with significant excess mortality in the later periods for all three data sources. Finally, we found no evidence of trends in the hazard ratios for the underweight and overweight categories. Overweight was generally associated with lower mortality, a finding consistent with prior studies (Lantz et al. 2010; Mehta and Chang 2009; Kulminski et al. 2008; Flegal et al. 2005; McGee 2005).
Table 5 presents results for cardiovascular disease mortality. Similar to results for all-cause mortality, class I obesity was associated with significantly higher CVD mortality in the earlier periods, but not in the later periods. The strongest proportionate decline in excess risk was observed in NHANES: class I obesity was associated with a hazard ratio of 1.82 in NHANES I and 1.18 in NHANES III. This change represents an 89 percent decline in excess risk. The excess risks for class I obesity declined by 63 percent in the NHIS and by 47 percent in the Framingham study. Overweight was not associated with excess CVD mortality in any period of investigation, and there were no significant trends for this category. Our assessment of cancer and non-CVD/non-cancer mortality detected no significant time trends in the hazard ratios for the overweight or obese groups (results not shown).
TABLE 5.
Hazard ratios and 95 percent confidence intervals for cardiovascular disease mortality in three US mortality studies
| Framingham | NHANES | NHIS | |
|---|---|---|---|
| Main effects (earlier period) | Original cohort 1948–1970 | NHANES I 1971–1987 | Period 1 1987–1996 |
| Underweight (BMI<18.5) | 0.54 [0.08–3.87] | 1.79* [1.08–2.98] | 1.25 [0.98–1.59] |
| Normal weight (18.5–24.9) | 1.00 [Ref.] | 1.00 [Ref.] | 1.00 [Ref.] |
| Overweight (25.0–29.9) | 1.00 [0.80–1.25] | 0.83* [0.65–1.05] | 0.97 [0.88–1.08] |
| Class I obese (30.0–34.9) | 1.53** [1.14–2.04] | 1.82*** [1.30–2.55] | 1.40*** [1.23–1.59] |
| Class II/III obese (35.0+) | 2.13** [1.34–3.39] | 1.68* [1.05–2.69] | 1.80*** [1.47–2.21] |
| Interaction effects | |||
| Underweight × period | 5.53 [0.33–92.97] | 0.74 [0.30–1.79] | 0.80 [0.46–1.38] |
| Overweight × period | 1.17 [0.67–2.07] | 1.19 [0.86–1.65] | 0.94 [0.79–1.12] |
| Class I obese × period | 0.83 [0.42–1.66] | 0.65* [0.42–1.00] | 0.83 [0.68–1.01] |
| Class II/III obese × period | 1.17 [0.46–2.99] | 0.88 [0.48–1.61] | 0.96 [0.69–1.32] |
| Main × interaction effects (later period) | Offspring cohort 1985–2003 | NHANES III 1988–2006 | Period 2 1997–2006 |
| Underweight (BMI<18.5) | 2.99 [0.40–22.52] | 1.32** [0.64–2.73] | 0.99 [0.61–1.63] |
| Normal weight (18.5–24.9) | 1.00 [Ref.] | 1.00 [Ref.] | 1.00 [Ref.] |
| Overweight (25.0–29.9) | 1.18 [0.70–1.98] | 0.98 [0.79–1.22] | 0.91 [0.79–1.05] |
| Class I obese (30.0–34.9) | 1.28 [0.69–2.38] | 1.18 [0.89–1.55] | 1.15 [0.99–1.35] |
| Class II/III obese (35.0+) | 2.48* [1.10–5.61] | 1.49 [1.02–2.16] | 1.73*** [1.35–2.22] |
p<.05
p<.01
p<.001
NOTE: All models adjust for sex, cigarette smoking, and education. The NHANES and NHIS analyses are further adjusted for family income, race/ethnicity, marital status, and US region of residence. Dates refer to time span of the mortality period. Main effects indicate hazard ratios for the earlier period of each data source. Hazard ratios for later period were obtained by multiplying the main effects by the interaction effects (may not be exact because of rounding).
Discussion
Our objective was to investigate whether the association between obesity and mortality in the United States has changed over time by comparing estimates across non-overlapping time periods of similar length within three well-known data sources. For class I obesity, which represents by far the largest proportion of obesity, we found substantial weakening of the association over time in all three data sources examined. While class I obesity was significantly associated with higher all-cause mortality relative to normal weight status in the earlier periods, this excess risk was eliminated by the later periods. As expected, these changes may be attributable to declines in the association between class I obesity and cardiovascular mortality. In contrast to class I obesity, class II/III obesity remained significantly associated with mortality in the later periods in all data three sources, and the existence of a trend is less clear. While the NHIS showed a 54 percent decline in excess mortality associated with class II/III obesity relative to normal weight, the NHANES and Framingham data showed no evidence of a trend.
These reductions in the magnitude of the association between obesity and mortality have occurred during a period when obesity levels in the United States have risen substantially (Flegal et al. 2010; Ogden et al. 2002). Our findings suggest that obesity would be having a much larger influence on present-day US mortality had the association between obesity and mortality not been lowered. We can use attributable-risk calculations to broadly assess the extent to which a declining trend may have benefited US mortality patterns.1 Calculations show that approximately one-quarter of all deaths to middle-aged adults in 2003–2004 are attributable to obesity when the risks from NHANES I (1971–1987) are used. In contrast, if risks from the more recent NHANES III period (1988–2006) are used, only about one-tenth of all deaths are attributable to obesity in 2003–2004. Furthermore, mortality attributable to obesity drops to 5 percent if we use risks from the most recent period (nHiS Period 2, 1997–2006).
The research cited at the beginning of this article suggested that rising obesity levels may threaten future gains in US life expectancy. Recent projections by both Olshansky et al. (2005) and Stewart, Cutler, and Rosen (2009) used earlier NHANES data to estimate the association between obesity and mortality, estimates that those authors then applied to life expectancy projections. Thus, prior projections did not account for possible secular changes in the obesity/mortality association and assumed that estimates from earlier periods are directly applicable to current conditions. Similarly, estimates of the contribution of changing risk factors (e.g., cholesterol, smoking, blood pressure, and obesity) to declines in deaths from coronary heart disease (CHD) from 1980 to 2000 relied on data from earlier periods to estimate the association between obesity and CHD mortality, potentially overestimating the countervailing effects of obesity (Ford et al. 2007).
Since the 1980s, the United States has experienced substantial declines in cardiovascular disease mortality (Jemal et al. 2005). Our findings suggest that obese persons have benefited from these mortality improvements, perhaps more so than persons of normal weight. Overall reductions in CVD mortality have been attributed to pharmaceutical innovations, the increased effectiveness of invasive medical treatments, and behavioral changes (Ford et al. 2007; Cutler and Kadiyala 2003). We accounted for changes in smoking behaviors, so the reductions we observed are likely driven by other factors. Along with advances in the treatment of cardiovascular disease, improved control of its risk factors may be a contributing explanation. As previously noted, obese persons have experienced declines in high blood pressure and total cholesterol over the past few decades (Gregg et al. 2005). In fact, the prevalence of high cholesterol has dropped further for the obese compared to those with a BMI<25.0 (ibid.). Moreover, recent research suggests that physicians may be more aggressive in risk-factor modification for obese patients with diabetes relative to normal-weight patients with diabetes (Chang, Asch, and Werner 2010). Changes in social norms and obesity-related stigma could also play a role. Large increases in the prevalence of obesity over time may lead to improvements in the relative status of persons who are mildly obese, potentially attenuating social isolation and discrimination in employment and health care as their body type becomes more commonplace.
While we report favorable trends with respect to obesity's association with mortality, increased survival among obese persons may have come at a cost of increasing levels of disability (Alley and Chang 2007). Recent work suggests that obesity's association with disability increased among older adults between 1988–1994 and 1999–2004, potentially because people are now living longer with obesity (ibid.). Indeed, declining mortality among the obese may be contributing to the increase in prevalence of obesity observed at older ages (Doshi, Polsky, and Chang 2007). Furthermore, recent studies examining the simultaneous association of obesity with disability and mortality report that obesity is more likely to shorten disability-free life expectancy than overall life expectancy at middle and older ages (Reuser, Bonneux, and Willekens 2009; Al Snih et al. 2007; Reynolds, Saito, and Crimmins 2005). Thus, efforts to improve the health of the obese population may have been more successful at increasing life span than at reducing obesity-related disability. Obesity continues to have important public health and economic consequences.
The trends in the association between obesity and mortality we observed between NHANES I and III are consistent with estimates by Flegal et al. (2007a; 2005), who also showed that estimates of relative risk of death from NHANES II (1976–1980 baseline) were lower than those from NHANES I. In a letter to The New England Journal of Medicine, Calle, Teras, and Thun (2005) reported no decline in the obesity/mortality association for nonsmokers across three periods between 1982 and 1998 using the Cancer Prevention Study II. This study followed a single cohort with BMI measured in 1982. Thus, comparisons of estimates across periods were based on the same set of individuals. In contrast, our study measured trends across independent samples that had a similar mean age at baseline and a comparable length of mortality follow-up. Confidence in our findings is further increased by testing for statistical significance in the observed trends, by the use of non-overlapping follow-up periods, and by the finding of a consistent set of results from three independent data sources.
Nevertheless, uncertainty remains about the extent to which obesity currently increases the risk of dying. An analysis of an international sample of white adults by Berrington de Gonzalez et al. (2010), which incorporates data covering 1976–2002, suggests that mild levels of obesity are associated with excess mortality compared to a BMI within the normal range. This analysis combined multiple prospective studies primarily from the United States, though none were nationally representative. Further research examining whether a decline has occurred in the association between obesity and mortality in other large-scale datasets would be enlightening.
Our study has limitations. First, sample size considerations precluded our examining narrower time periods in the Framingham study and NHANES. Second, our ability to detect significant changes in the hazard ratios of persons with class II/III obesity may have been limited by the very low prevalence of this category in earlier periods. Nonetheless, we detected significant declines for all-cause mortality in the NHIS, which covered more recent periods and offers the largest sample size. Finally, debate continues over the extent to which the obesity/mortality association is confounded by preexisting diseases (Mehta and Chang in press; Flegal et al. 2011; Hu 2008; Flegal et al. 2007b; Manson et al. 2007). We did not adjust for prevailing diseases because they may lie on the causal pathway between obesity and death. For NHANES and NHIS, however, sensitivity analyses that adjusted for overall self-rated health, and sensitivity analyses limited to persons reporting excellent, very good, or good overall health, yielded results that are highly similar to those we reported above and that are consistent with prior studies indicating that preexisting illnesses do not substantially bias the association between obesity and mortality (Flegal et al. 2011; Mehta and Chang 2009; Al Snih et al. 2007; Flegal et al. 2007b). Self-rated health was unavailable in the Framingham study.
In sum, the association between obesity and mortality, as well as the long-term influence of obesity on population health in the united States, remains highly controversial. While numerous methodological differences likely contribute to divergent estimates of the association between obesity and mortality (Mehta and Chang in press), our findings suggest that the period of mortality follow-up is an important source of variation, and that findings based on data from earlier periods may lead to an over-estimation of the current association. Projections of the future influence of obesity on population health and longevity are critical to assessing future health care expenditures and public costs, including the solvency of age-based entitlement programs such as Social Security and Medicare. The increased prevalence of obesity among children and adolescents, along with an increase in severe obesity among adults, will likely have a substantial impact on future associations between obesity and mortality.
Footnotes
References
- Al Snih Soham, et al. The effect of obesity on disability vs. mortality in older Americans. Archives of Internal Medicine. 2007;167(8):774–780. doi: 10.1001/archinte.167.8.774. [DOI] [PubMed] [Google Scholar]
- Alley Dawn E., Chang Virginia W. The changing relationship of obesity and disability, 1988–2004. Journal of the American Medical Association. 2007;298(17):2020–2027. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- Allison David B., Fontaine Kevin R., Manson JoAnn E., Stevens June, Vanitallie Theodore B. Annual deaths attributable to obesity in the United States. Journal of the American Medical Association. 1999;282(16):1530–1538. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- Berrington de Gonzalez Amy, et al. Body-mass index and mortality among 1.46 million white adults. New England Journal of Medicine. 2010;363(23):2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board of Trustees The 2009 Annual Report of the Board of Trustees of the Federal Old-Age and Survivors Insurance and Federal Disability Insurance Trust Funds. 2009.
- Calle Eugenia E., Rodriguez Carmen, Walker-Thurmond Kimberly, Thun Michael J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. The New England Journal of Medicine. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Calle Eugenia E., Teras Lauren R., Thun Michael J. Obesity and mortality. The New England Journal of Medicine. 2005;353(20):2197–2199. doi: 10.1056/NEJM200511173532020. [DOI] [PubMed] [Google Scholar]
- Calle Eugenia E., Thun Michael J., Petrelli Jennifer M., Rodriguez Carmen, Heath Clark W., Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. New England Journal of Medicine. 1999;341(15):1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- Chang Virginia W., Asch David A., Werner Rachel M. Quality of care among obese patients. Journal of the American Medical Association. 2010;303(13):1274–1281. doi: 10.1001/jama.2010.339. [DOI] [PubMed] [Google Scholar]
- Chang Virginia W., Lauderdale Diane S. Income disparities in body mass index and obesity in the United States, 1971–2002. Archives of Internal Medicine. 2005;165(18):2122–2128. doi: 10.1001/archinte.165.18.2122. [DOI] [PubMed] [Google Scholar]
- Cutler David M., Kadiyala Srikanth. The return to biomedical research: Treatment and behavioral effects. In: Murphy Kevin H., Topel Robert H., editors. Measuring the Gains from Medical Research: An Economic Approach. The University of Chicago Press; Chicago: 2003. pp. 110–162. [Google Scholar]
- Doshi Jalpa A., Polsky Daniel, Chang Virginia W. Prevalence and trends in obesity among aged and disabled U.S. Medicare beneficiaries, 1997–2002. Health Affairs. 2007;26(4):1111–1117. doi: 10.1377/hlthaff.26.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawber Thomas R., Meadors Gilcin F., Moore Felix E., Jr. Epidemiological approaches to heart disease: The Framingham Study. American Journal of Public Health. 1951;41(3):279–286. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal Katherine M., Carroll Margaret D., Ogden Cynthia L., Curtin Lester R. Prevalence and trends in obesity among US adults, 1999–2008. Journal of the American Medical Association. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Flegal Katherine M., Graubard Barry I., Williamson David F., Cooper Richard S. Reverse causation and illness-related weight loss in observational studies of body weight and mortality. American Journal of Epidemiology. 2011;173(1):1–9. doi: 10.1093/aje/kwq341. [DOI] [PubMed] [Google Scholar]
- Flegal Katherine M., Graubard Barry I., Williamson David F., Gail Mitchell H. Excess deaths associated with underweight, overweight, and obesity. Journal of the American Medical Association. 2005;293(15):1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- Flegal Katherine M., Graubard Barry I., Williamson David F., Gail Mitchell H. Cause-specific excess deaths associated with underweight, overweight, and obesity. Journal of the American Medical Association. 2007a;298(17):2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- Flegal Katherine M., Graubard Barry I., Williamson David F., Gail Mitchell H. Impact of smoking and preexisting illness on estimates of the fractions of deaths associated with underweight, overweight, and obesity in the US population. American Journal of Epidemiology. 2007b;166(8):975–982. doi: 10.1093/aje/kwm152. [DOI] [PubMed] [Google Scholar]
- Fontaine Kevin R., Redden David T., Wang Chenxi, Westfall Andrew O., Allison David B. Years of life lost due to obesity. Journal of the American Medical Association. 2003;289(2):187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- Ford Earl S., et al. Explaining the decrease in US deaths from coronary disease, 1980–2000. New England Journal of Medicine. 2007;356(23):2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- Gregg Edward W., et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. Journal of the American Medical Association. 2005;293(15):1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- Hu Frank B. Obesity and mortality. In: Hu Frank B., editor. Obesity Epidemiology. Oxford University Press; New York: 2008. [Google Scholar]
- Hu Frank B., et al. Adiposity as compared with physical activity in predicting mortality among women. New England Journal of Medicine. 2004;351(26):2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- Jemal Ahmedin, Ward Elizabeth, Hao Yongping, Thun Michael. Trends in the leading causes of death in the United States, 1970–2002. Journal of the American Medical Association. 2005;294(10):1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- Kannel William B., Feinleib Manning, McNamara Patricia M., Garrison Robert J., Castelli William P. An investigation of coronary heart disease in families: The Framingham Offspring Study. American Journal of Epidemiology. 1979;110(3):281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- Kom Edward L., Graubard Barry I., Midthune Douglas. Time-to-event analysis of longitudinal follow-up of a survey: Choice of the time-scale. American Journal of Epidemiology. 1997;145(1):72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- Kulminski Alexander M., et al. Body mass index and nine-year mortality in disabled and nondisabled older U.S. individuals. Journal of the American Geriatrics Society. 2008;56(1):105–110. doi: 10.1111/j.1532-5415.2007.01494.x. [DOI] [PubMed] [Google Scholar]
- Lantz Paula M., Golberstein Ezra, House James S., Morenoff Jeffrey. Socioeconomic and behavioral risk factors for mortality in a national 19-year prospective study of U.S. adults. Social Science and Medicine. 2010;70(10):1558–1566. doi: 10.1016/j.socscimed.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JoAnn E., et al. Body weight and mortality among women. New England Journal of Medicine. 1995;333(11):677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- Manson JoAnn E., et al. Estimating the number of deaths due to obesity: Can the divergent findings be reconciled? Journal of Women's Health. 2007;16(2):168–176. doi: 10.1089/jwh.2006.0080. [DOI] [PubMed] [Google Scholar]
- McGee Daniel L. Body mass index and mortality: A meta-analysis based on person-level data from twenty-six observational studies. Annals of Epidemiology. 2005;15(2):87–97. doi: 10.1016/j.annepidem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Mehta Neil K., Chang Virginia W. Mortality attributable to obesity among middle-aged adults in the United States. Demography. 2009;46(4):851–872. doi: 10.1353/dem.0.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta Neil K., Chang Virginia W. Obesity and mortality. In: Cawley John., editor. Handbook of the Social Science of Obesity. Oxford University Press; In press. [Google Scholar]
- Mokdad Ali H., Marks James S., Stroup Donna F., Gerberding Julie L. Actual causes of death in the United States, 2000. Journal of the American Medical Association. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Mujahid Mahasin S., Diez Roux Ana V., Borrell Luisa N., Nieto F. Javier. Cross-sectional and longitudinal associations of BMI with socioeconomic characteristics. Obesity Research. 2005;13(8):1412–1421. doi: 10.1038/oby.2005.171. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute . Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. U.S. Public Health Service; Washington, DC: 1998. [Google Scholar]
- National Research Council . In: Explaining Divergent Levels of Longevity in High-Income Countries. Crimmins Eileen M., Preston Samuel H., Cohen Barney., editors. The National Academies Press. Panel on Understanding Divergent Trends in Longevity in High-Income Countries, Division of Behavioral and Social Sciences and Education; Washington, DC: 2011. [PubMed] [Google Scholar]
- Ogden Cynthia L., Flegal Katherine M., Carroll Margaret D., Johnson Clifford L. Prevalence and trends in overweight among US children and adolescents, 1999–2000. Journal of the American Medical Association. 2002;288(14):1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- Olshansky S. Jay, et al. A potential decline in life expectancy in the United States in the 21st century. New England Journal of Medicine. 2005;352(11):1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- Preston Samuel H., Stokes Andrew. Is the high level of obesity in the United States related to its low life expectancy? American Journal of Public Health. In press. [Google Scholar]
- Peeters Anna, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Annals of Internal Medicine. 2003;138(1):24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- Prospective Studies Collaboration Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuser Mieke, Bonneux Luc G., Willekens Frans J. Smoking kills, obesity disables: A multistate approach of the US Health and Retirement Survey. Obesity. 2009;17(4):783–789. doi: 10.1038/oby.2008.640. [DOI] [PubMed] [Google Scholar]
- Reynolds Sandra L., Saito Yasuhiko, Crimmins Eileen M. The impact of obesity on active life expectancy in older American men and women. Gerontologist. 2005;45(4):438–444. doi: 10.1093/geront/45.4.438. [DOI] [PubMed] [Google Scholar]
- Stewart Susan T., Cutler David M., Rosen Allison B. Forecasting the effects of obesity and smoking on U.S. life expectancy. New England Journal of Medicine. 2009;361(23):2252–2260. doi: 10.1056/NEJMsa0900459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Obesity: Preventing and managing the global epidemic. Report of a WHO Consultation. 2000. [PubMed]