Abstract
The field of biomimicry is embracing the construction of complex assemblies that imitate both biological structure and function. Advancements in the design of these mimetics have generated a growing vision for creating an artificial or proto- cell. Polymersomes are vesicles that can be made from synthetic, biological or hybrid polymers and can be used as a model template to build cell-like structures. In this perspective, we discuss various areas where polymersomes have been used to mimic cell functions as well as areas in which the synthetic flexibility of polymersomes would make them ideal candidates for a biomembrane mimetic. Designing a polymersome that comprehensively displays the behaviors discussed herein has the potential to lead to the development of an autonomous, responsive particle that resembles the intelligence of a biological cell.
Keywords: Polymersomes, artificial cell, protocell, cell mimicry
To demonstrate our understanding of biological processes, scientists often strive to synthetically recreate them.1–4 Biomimicry has progressed from simple replication of lipid vesicles to compartmentalized gene expression, a process that is essential for the construction of an artificial, self-replicating cell. The complexity of creating an autonomous, artificially intelligent particle has prompted many researchers to parse the goal of cellular biomimicry into smaller, readily achievable goals. One approach has been to create a minimal cell,3, 5 in which genes in a living cell are systematically knocked out in order to determine the minimal number of genes, and their corresponding functions, that are necessary to maintain a cell’s metabolic processes and allow for its replication. Another approach has been to engineer a ‘living’ system from the bottom-up that can mimic biological functions or create new ones. This latter model, often called an engineered protocell, does not necessarily start with living material, but rather attempts to create an artificial cell by synthesizing individual components with both biotic and abiotic materials and assembling them to create a particle with artificially generated metabolic processes. Rasmussen et al.6 describe a protocell as a particle that has been “built from scratch.” While its internal functions and phenotype may display aspects of cellular metabolism, the protocell can potentially be endowed with new behaviors emerging from unique structures. A general consensus exists that work constructing an autonomous particle from the ground-up should have the ‘living’ properties of self-maintenance and self-reproduction. Attempts to construct a proto-cell will serve not only to enhance our understanding of the complex network of genetic and physical processes that must coordinate to maintain life in a biological cell, but also enable the development of future technologies, where creating ‘smart’, autonomously-functioning particles can serve anywhere from biomedical devices to self-regulating bioreactors.
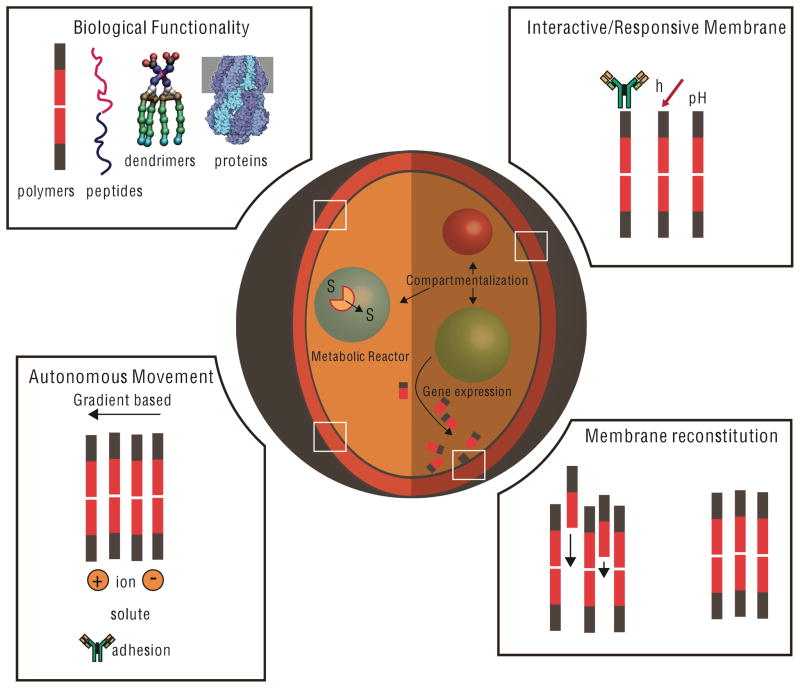
To this end, much work in creating artificial cells from the bottom-up has used cell-sized, phospholipid vesicles as an initial platform in which different cellular processes can be recreated. The use of lipids to serve as the frame for artificial cells is reasonable and obvious, since lipids are the principal component of most biological cell membranes. In order to expand the range of possible responses beyond that programmed by Nature and to improve the stability of artificial cells, it would be ideal to build membranes from materials that enable more synthetic control over their chemical and physical properties. A recent advance with potential to expand the properties of membranes are polymersomes – vesicles whose membranes are assembled from synthetic block co-polymers.7 Related structures derived from polymersomes – in which one of the blocks is made from biological components such as peptides – are also now available.8 In this perspective, we specifically focus on advances in polymersome science that have been used to mimic cell structures or processes. These areas of focus are schematically outlined in Figure 1. Progress on the road to creating a true artificial cell, in which critical subordinate processes such as coordinated movement to signaling are required, will also be included.
Figure 1. A Polymersome Protocell.
Polymersomes can be used as a template to create cell-like structures. Creating an autonomous particle that mimics the diverse behaviors of a cell will require incorporation of biological functionality in the membrane, the ability to trigger responses, autonomous movement, and morphological changes. Many of these functions will require organized metabolic reactions and gene expression to be housed within distinct spatial environments in the polymersome.
Illustration of the mechanosensitive protein channel courtesy of David S. Goodsell and the RCSB PBD (doi:10.2210/rcsb_pdb/mom_2008_11). Dendrimer structure taken from Percec, V. and Co-authors, 2011: Self-Assembly of Janus Dendrimers into Uniform Dendrimersomes and Other Complex Architectures. Science, 328, 5981. Reprinted with permission from AAAS.
Defining the Boundary
Cells are, in their simplest description, self-enclosed containers of biological material. Vital to their existence is the presence of a membrane—a perm-selective boundary—separating that which is enclosed within the cell from the cell’s surroundings. The membrane is responsible for maintaining an asymmetry between the interior and exterior contents, as well as maintaining a local concentration of components within the cytoplasm necessary for cellular responses. We begin this perspective with an overview of the variety of synthetic components that can be used to make membranes for an artificial cell.
The functional building block of a cellular membrane is the amphiphilic phospholipid, which self-assembles into a bilayer structure that encloses aqueous media. Synthetic lipid vesicles (liposomes) have been used for a variety of applications including drug encapsulation and studies of membrane behavior.9, 10 Many recent studies, however, suggest that synthetic analogues to the phospholipid may be advantageous for the development of membranes of superior and tunable material properties. Such materials include block copolymers, dendrimers, and amphiphilic peptides and proteins.
Polymersomes were originally conceived as vesicles self-assembled from amphiphilic block copolymers.7 Similar to liposomes, polymersomes comprise an aqueous core protected from the outside aqueous environment by a hydrophobic membrane. Consequently, as with liposomes, polymersomes are capable of encapsulating hydrophilic molecules within the aqueous core of the vesicle.11 In comparison to liposomes, however, polymersome membranes are often several times thicker, owing to the larger molecular weight of the polymer amphiphile. This additional thickness lends polymersomes several advantages compared to liposomes that make them more suitable as artificial cell membranes. Even in the liquid state, polymersomes are significantly tougher than liposomes, and can support far greater membrane elongation.7, 12. Polymersomes also have significantly lower critical aggregation concentrations; once assembled they generally stay assembled, barring perturbation. Phospholipids are subject to oxidation, but polymersomes may be assembled from relatively stable materials and can have significantly longer shelf lives13, 14. Finally, since the membranes of polymersomes are thicker than those of liposomes, the membranes are able to solvate large quantities of hydrophobic molecules while still supporting encapsulation of hydrophilic molecules.11, 15
Polymersomes may be assembled from multiblock polymers as well. When the membrane element is a diblock, the assembled membranes are bilayers, whereas multiblock polymers lead to novel architectures. Membranes assembled with triblock copolymers are often monolayers, with the hydrophobic middle block of the polymer stabilized on either side by the hydrophilic end blocks 13. To date, polymersomes have been assembled from a host of di and triblock copolymers. The original polymersomes reported were synthesized from a diblock copolymer of poly(ethylene oxide) (PEO) and poly(ethyl ethylene).7 Most typically, the hydrophilic block has been PEO, though other hydrophilic blocks have included poly(acrylate),16 poly(2-(methacryloyloxy)ethyl phosphorylcholine),17 and polymeric sugars (i.e. dextran and hyaluronan).18, 19 Many different hydrophobic blocks have been reported, including poly(1,2-butadiene),12 poly(isoprene),20 poly(propylene oxide),21 poly(propylene sulfide),22 poly(caprolactone),11 poly(benzyl glutamate),23 poly(styrene),24 poly(lactide),25 poly(γ-methylcaprolactone),26 poly(2-diisopropylaminoethylmethacrylate),27 and poly(trimethylene carbamate).28 For biomedical applications, polymersomes from polymers such as poly(caprolactone) and poly(lactide) are desirable as they are bioresorbable and have regulatory approval for use in implantable biomedical materials for humans 13, 29. These materials, however, are semi-crystalline, leading to solid membranes that are unable to mimic the fluidity of cellular membranes.30
While there are many advantages to having hyper-thick membranes significantly thicker than lipid membranes (such as the storage of hydrophobic solutes), there may be applications for which thinner membranes are desired to better facilitate inclusion of other biological functionalities within the membrane. To address this concern, Percec and coworkers recently reported a novel series of dendrimeric amphiphiles that assemble into ordered architectures including vesicles (dendrimersomes).31 In addition to having membranes with thickness more similar to lipid membranes, these dendrimers are also advantageous over block copolymers because they, like lipids, are entirely monodisperse. Interestingly, the assembled vesicles were also far more monodisperse than typical liposome or polymersome dispersions. Dendrimersome membranes had mechanical properties spanning over an order of magnitude, some being significantly softer than polymersomes and liposomes while others were as tough as SOPC (1-stearoyl-2-oleoylphosphatidylcholine) liposomes containing over 50% cholesterol. Dendrimersomes, however, did not support significant areal strains, and failed at significantly lower membrane tensions than polymersomes.
Another emerging area of is the creation of synthetic membranes from bio-inspired building blocks. The first example of protein materials assembling into vesicles was reported by the Deming group.8 Bridging the gap between block copolymers and proteins, they synthesized a series of block co-polypeptides of poly(leucine) and poly(lysine, arginine, or aspartic acid) that readily assemble into vesicles.8, 32, 33 Membrane formation is driven by hydrophobic collapse of leucine α-helices and stabilization by the highly charged lysine, arginine, or aspartic acid block. Interestingly, membranes of block-co-peptides can be assembled when the amphiphiles contains much larger hydrophilic block fractions than synthetic polymers 13. Versluis et al.34 reported a pH-responsive vesicle system in which an amphiphilic β-cyclodextrin is stabilized by an adamantane-terminated alternating octapeptide of valine and glutamic acid. Li and coworkers35 templated the assembly of block copolypeptides using relatively unstable PEO-poly(propylene oxide) vesicles. Unlike the peptides synthesized by Deming, these peptides were obtained through yeast expression. The peptides partitioned into the membrane of the vesicles with loading as high as 70 percent. Since polypeptides are derived entirely from natural materials, their continued development into membrane-forming formulations promises to be an exciting addition to the current library of materials for assembling vesicles. Research in this area this far, however, has been limited, and the use of biological materials for assembling synthetic membranes has tremendous potential.36
Biological Functionality in Synthetic Membranes
Adhesion
Various routes have been taken to incorporate biological functionalities in synthetic membranes. The most basic functionalization of a membrane is the addition of small peptides or proteins to the surface of the vesicle in order to facilitate specific adhesion. Numerous groups have reported the attachment of short peptides to the surface of polymersomes to improve their binding to target cells and tissues. For example, the PEO surface of poly(butadiene)-based vesicles was modified with a short peptide that recognizes a prostate cancer marker to enhance the vesicles’ binding to tumor tissue.37 Pang and coworkers modified the synthesis of PCL-PEO to facilitate attachment of a brain tissue-specific peptide to enable polymersomes to accumulate in brain tissue.38 Christian and coworkers labeled highly-fluorescent polymersomes with the HIV-derived TAT peptide and used the resulting vesicles to target and label dendritic cells.39, 40 Similar to the TAT peptide, it has been found that highly charged arginine segments in their peptide block copolymers drive uptake into cells.32 In this case, the hydrophilic block of the polymer does not require modification; rather it is the block itself that facilitates cell binding and endocytosis. Consequently, by using this system, researchers can avoid the potential problems of membrane functionalization altering the assembly behavior of the macromolecules. A concern is that the functionalization of polymers can affect the architecture of their assembly, as the peptides used can significantly alter the hydrophilic/hydrophobic balance that enables self-assembly into membranes.41 For example, the addition of a 1 kDa peptide can significantly alter the hydrophilic/hydrophobic balance of a 4 kDa polymer, driving a transition from vesicles to other structures.
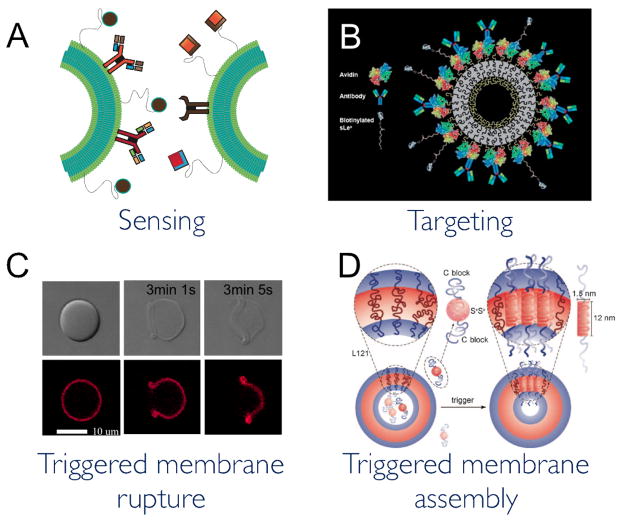
Higher order manipulations of bioadhesion in polymersomes have been achieved. Targeting and segregating undesired antigens, the immune response remains one of the most powerful and prevailing responses in many biological organisms. Mimicking immune cells has been envisioned as a possible synthetic route to treating inflammation, cardiovascular disease, and cancers.42 Replicating immunological cell targeting, adhesion, and signaling with polymersomes could provide biomimetic cells with the ability to respond to antigen-specific stimuli, by either labeling the antigen for subsequent engulfment by macrophages, releasing signals to recruit immunological cells, or by endocytosing or destroying the antigen, themselves. Hammer has focused on designing polymersomes that have similar adhesive properties as immune cells, an initial step in targeting antigens. Polymersomes incorporating surface ligands in the two major adhesion pathways could mimic the rolling and adhesive behavior of leukocytes under physiological flow and shear rates.42, 43 Through conjugation of a common selectin ligand (sialyl Lewisx), that mediates leukocyte rolling, and an anti-integrin antibody (anti-ICAM-1), that mediates firm cell arrest and adhesion, the adhesive properties of polymersome-based leukocyte mimetics (leukopolymersomes) were fine tuned and demonstrated targeted and selective adherence to inflamed endothelium (Fig. 2A, B).44
Figure 2. Polymersome membranes have been designed to interact with other polymersomes or cells and to respond to stimuli.
(A, B) Modification of polymersome surfaces with antibodies and ligands can allow polymersomes to mimic some adhesive and targeting abilities of immunological cells. . (C) Composite polymersomes loaded with a porphyrin based fluorophore and dextran rupture in response to light. (D) Block copolypeptides can be induced to assemble into a membrane in response to a pH shift. (B) Adapted from reference 42. Reproduced by permission of The Royal Society of Chemistry. (C), (D) Adapted from references 63 and 35, respectively. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with Permission
The ability for polymersomes to signal other immune cells or leukopolymersomes would diversify and enhance the responsive functions of immunological mimetics. Vaccination methods rely on T cell stimulation to effectively target an antigen, yet often require viral vectors to elicit T cell and antibody responses against a particular antigen. Recently, Moon et al. developed interbilayer-crosslinked, multilamellar lipid vesicles loaded with antigen and adjuvant that were able to effectively trigger T cell and antibody responses comparable to those achieved by viral vectors.45 Using a synthetic carrier prevented antivector immunity and polymerized cross-linking allowed for carrier stability in the presence of serum proteins. Another possible route to signal T cells is through formation of an immunological synapse, where polymersomes mimicking an antigen-presenting cell bind the T cell receptor. The ability to signal and recruit immunological cells by the presentation of antigen would advance communication and cooperative behavior amongst mimetic immunological particles.
Permeability
Like lipid membranes, polymer membranes are generally permselective; however there have been limited tests of the permeability of polymer membranes. Discher and coworkers showed that the permeability of vesicles to water was limited, owing to the hyper thickness of the polymersome membrane.46 Due to its expected limited permeability, a strategy would be the addition of channels and carriers into the membrane. In general, polymersome membranes are too thick to accommodate many naturally occurring transmembrane proteins. Several channel proteins and enzymes, such as FhuA47 and complex I,48 have been incorporated in polymersome membranes.47-49 Percec and Hammer and coworkers assembled helical pore-forming dendrimers in polymersome membranes.50 The pores facilitated proton transport while otherwise not significantly altering the structure of the polymersomes. Molecules too large for the pores, such as dyes that were stably encapsulated in the vesicles, did not leak. In another route to facilitate more rapid transmembrane flux, a small amount of lipid was blended into crosslinkable polymersome membranes.51 Following crosslinking of the polymer, the lipid was extracted from the polymersome, yielding porous polymersomes that allowed for the transport of water across the membrane. These vesicles have shown promise for the use of magnetic resonance imaging agents since encapsulated gadolinium enhances water relaxivity as water flux is increased through the vesicles.52
Sensory and Interactive membranes
The ability to incorporate functional proteins, polymers and targeting moieties into polymersome membranes has generated polymersomes with responsive behaviors. Responsiveness can be coded into the amphiphile, or can be provided by solutes that are co-encapsulated within the membrane. Initial work in responsive polymersomes and diblock copolymers focused on pH amphiphiles that would degrade or undergo conformational changes and subsequently release the vesicles’ contents in response to a pH shift, such as is seen in the endosome during internalization.53, 54 These responsive vesicles generally utilized the expedited degradation of the polymersome carbonyl or amide bonds in the presence of a lower or higher pH, respectively, to create a site-sensitive reaction. Responsive polymersomes have typically been designed to react to stimuli that include pH, temperature, light, and magnetic fields and have been described in detail by several reviews.53, 55–58
A strategy for making responsive membranes is to build an asymmetrical response in the membrane to the desired stimulus; the stimulus then shifts the membrane’s hydrophilic-hydrophobic balance, ultimately leading to a morphological change or membrane failure.54, 55,59, 60 These stimuli-responsive vesicles can respond to intrinsic stimuli, such as chemical concentrations at specific locations within cells55, or external stimuli, such as light, in which the vesicles can be externally controlled at a specific time and location dictated by the user. This latter type of temporal control over the vesicle response is expected to increase therapeutic capabilities of responsive membranes, allowing for limited release in undesired locations and a potentially increased payload at the desired site. In biomimetic polymersomes, the control offered by light stimuli could be used to trigger a response from specific vesicle compartments, or facilitate communication with neighboring vesicles through cues, while not affecting biological components. With the incorporation of light sensitive proteins, like rhodopsin,61 or electromagnetic field-sensitive DNA sequences, like those in the heat shock 70 promoter,62 into polymersomes, light or magnetic field-triggered responses have potential to be engineered into biomimetic polymersomes to provide stimuli-specific responses. For example, we recently reported a system of zinc porphyrin-polymersome composites that undergo membrane disruption upon exposure to light (Fig 2C).63 Mabrouk et al.64 incorporated a UV responsive polymer into one leaflet of a polymersome bilayer, which led to polymer coiling and, ultimately, membrane bursting in response to UV light. Polymersomes responsive to internal stimuli, however, may prove advantageous for responding to local chemical changes in order to maintain equilibrium.
While polymersomes have typically been designed to deform or disassemble in response to stimuli, recent studies have also demonstrated that polymersomes can be triggered to assemble in response to a stimulus.35, 65 Li et al.35 recently designed protein polymersomes using triblock copolymers in which the middle block consists of a peptide with multiple chargeable amino acids (Fig 2D). In response to a pH shift, the middle protein block became hydrophobic and inserted itself into a surrounding Pluronic matrix, quickly assembling into a unilamellar membrane. These protein polymersomes could also be induced to assemble by the addition of negatively charged siRNA, thereby demonstrating how this vesicle assembly method could be triggered by– and subsequently encapsulate— certain biological molecules. This type of assembly response could ultimately be used to quickly segregate undesired compounds into a lysosome-type compartment or even simply maintain osmotic balance within a mimetic cell by removing products of a chemical reaction.
Shape Changes
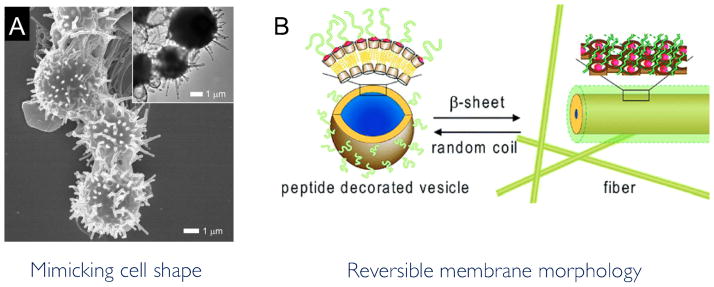
The interconnection between cell shape and function has been long been known.66, 67 On a molecular level, the geometry of a cell has been shown to influence cell differentiation, growth, and apoptosis. Here, we focus on a larger scale, where cell shape and more importantly, deformability, can influence function by way of its macroscopic structure. For example, the concave shape and flexibility of red blood cells increase cell surface area available for gas exchange and reduce flow resistance within blood vessels.68 Immunological cells depend on this same elasticity to extravasate out of the blood and in the case of dendritic cells, contain long processes that extend and contract in order to continuously probe their environment. Figure 3A is an example of a synthetic membrane that has been created to contain dendritic-like extenstions.69 Polymersomes with unique shapes, from concave to hemispherical, have been designed and demonstrate how control over polymersome shape can be achieved through manipulating polymer phase behavior and vesicle preparation methods.70–72 An early review by Antonietti et al. explains the thermodynamic and biophysical parameters that control vesicle shape and size.73 Yet, more important than the static vesicle shape, the ability to change polymersome morphology is vital for mimicking the dynamic nature of cell structure. The tunability of polymersome material properties allows us to make vesicles with a variety of mechanical properties and phase behaviors.12 Here, we focus on the deformation of the polymersome membrane in a variety of ways as a method to mimic cellular deformation and remodeling.
Figure 3. The static and dynamic shape of a polymersome can be controlled through vesicle preparation methods and triggered stimulus.
(a) The morphology of particles made from PS-PEO diblock copolymers can be changed to mimic the protrusions seen on dendritic cells by blending in a PS homopolymer with the copolymers during particle preparation. (b) The morphology of a peptide-decorated vesicle can be reversibly switched to that of a fiber for repeated cycles in response to a PH shift. (a) Adapted from reference 69. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with Permission. (b) Adapted from reference 34.
Dynamic membrane instability is an important process that cells use to mediate membrane trafficking, motility, and division.74, 75 Deformation of polymersome membranes has been studied with both thermodynamic and biophysical approaches to better understand the membrane shape changes that occur biologically and to understand more basic physical parameters that guide and influence shape changes. Sens et al. reviewed biophysical approaches to ‘curvactant’-induced membrane deformation, discussing that to deform a membrane, particles need only be sufficiently adhesive and rigid.60 Control of these two parameters simplifies the deformation process and allows it to be recreated in synthetic vesicles. Synthetic vesicles have been induced to bud and deform in response to particle binding, mimicking protein-induced membrane deformation in cells.76, 77 In addition to budding, other forms of deformation including membrane fusion, invagination, pearling instabilities, and complete membrane rupture have all been demonstrated with polymersomes and other synthetic vesicles.73 While these types of deformation provide useful information on how to physically alter membrane structure, they render the vesicle permanently changed.
Vesicles that can reversibly change structure can better mimic the continuously remodeling cell membrane. A polymersome comprised of triblock copolymers was designed to reversibly change membrane thickness and size in response to changes in pH.78 A recent study by Versluis et al.34 designed a peptide-decorated vesicle, made of self-assembled β-cyclodextrins, that changed to a fiber morphology upon pH-triggered transition of the secondary structure of the peptides (Fig. 3B). These fibers could be triggered to assemble back into a vesicular morphology upon changing the pH back to the original condition. Thermally and photo-inducible morphological changes have also been demonstrated between micelle and vesicular structures.79–81 This type of reversibility in morphology could allow vesicles to undergo multiple cycles of structural membrane change. It could also allow for selective presentation of a molecule or peptide based on environmental conditions. In this way, the vesicle could behave as a buffer, exposing reactive groups in order to shift microenvironments back to a chemical or physical equilibrium and burying the reactive group when equilibrium has been established.
Compartmentalization
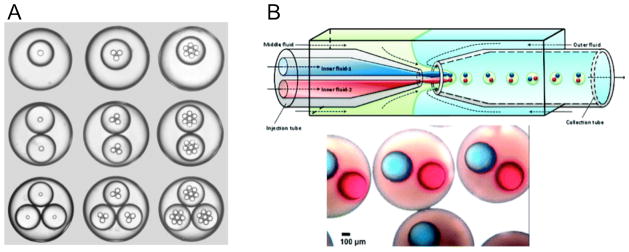
Compartmentalization is increasingly becoming recognized as an essential feature for recreating the diverse microenvironments and functions of cell organelles.82 The creation of distinct compartment vesicles within a larger one can be achieved through first forming the compartment vesicles and subsequently forming the larger polymersome in the presence of the compartment vesicles.83, 84 Self assembly of the larger vesicle will inevitably encapsulate compartment vesicles that are present in solution; however, controlling the number of compartment vesicles or the stoichiometry of one compartment-type to another within the newly formed outer membrane is impossible with this approach. Recent developments with microfluidic techniques have made encapsulating controlled numbers of vesicles with unique encapsulates possible. Chu et al. demonstrated that double emulsions could be generated with controlled numbers of internal aqueous droplets and that by essentially cascading the devices used to form these emulsions, internal droplets within a compartment droplet, could be created as well (Fig. 4A).85 This higher level of control in the design of compartmentalized double emulsions, where compartment droplets can even further encapsulate subsequent compartment droplets, expands the architectural options available for designing hierarchally-encapsulated vesicles and dramatically increases the complexity of compartment vesicles. Another recent major advance in the development of compartmentalized vesicles was the production of a vesicle with two distinct interior compartments (Fig. 4B).86, 87 The novelty in this design lies in the ability to encapsulate two distinct internal compartments within a capsule at controlled sizes of both the compartment and encapsulating capsule and controlled stoichiometry of the compartment capsules. Progression of the aforementioned microfluidic techniques will enable polymersomes and other synthetic bilayer vesicles to be created with similar structures.
Figure 4. Compartmentalization in polymer particles has been improved through the development of microfluidic techniques.
(A) Double emulsions with a highly controllable number and size of internal droplets can be produced with capillary microfluidic devices. (B) By using multiple injection tubes in a microcapillary device, distinct internal compartments with controlled stoichiometry can now be encapsulated within a single particle. (a) Adapted from reference 85. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with Permission. (b) Adapted from reference 86. Copyright American Chemical Society.
Nano/Microreactors
The higher level of organization imparted by compartmentalization facilitates one of the most important processes defining a cell: metabolic function. Possession of a metabolic system has long defined ‘living’ organisms from ‘non-living’ ones (i.e. viruses), where the autonomous and intelligent behavior of a cell is regulated through a series of chemical reactions. These reaction networks continuously promote and inhibit one another through signaling cascades and feedback loops, maintaining chemical equilibrium and enabling dynamic physical changes like motility and division. By confining metabolic processes to separate, internal compartments, a cell can protect itself from degradative byproducts, position enzymes for cascade reactions, and maintain multiple, chemically distinct environments that enable intracellular communication and biomolecule synthesis.
To replicate a metabolic process on the most basic level, a single enzyme reaction can be performed in a single compartment. The concept of spatially confining a metabolic process to a polymersome compartment was first demonstrated by the Meier group.49 By incorporating a channel-forming protein, OmpF, into the polymersome membrane, they created a size selective filter to enable entrance of a substrate into the vesicle interior for subsequent hydrolysis by an internally encapsulated B-lactamase enzyme. Since then, the engineering of polymersome-based nanoreactors has quickly progressed from single enzyme to multiple enzyme nanoreactors. Touted for their increased chemical and physical stability as well as the ease of chemical modification, polymersomes have proven to be an ideal container to house chemical reactions. We refer the interested reader to several outstanding reviews that have recently discussed advances and work in the field of synthetically-based nanoreactors.88, 89
In the past several years, work has progressed towards increasing the communication between enzymatic processes. Fig 5 illustrates ways that the encapsulation of enzymes in polymersomes has progressed to mimic increasingly complex metabolic systems. One approach to extend the single enzyme in a single compartment system is through cellular integration, where an enzymatic polymersome is encapsulated into a larger cell. By conjugating TAT peptides to the surface of a horseradish peroxidase (HRP) enzyme-filled polymersome, van Dongen et al.90 demonstrated that the vesicles were taken up by cells and retained enzyme activity even after cellular uptake.91 A second approach to extending the complexity of the enzyme-encapsulated polymersome is to introduce a second enzymatically-active polymersome that can communicate and interact with the first, creating a signaling cascade between two individual compartments.92 Finally, the complexity of the enzyme-containing polymersome can be enhanced by increasing the number of enzymes in a single polymersome. By localizing three separate enzymes to the membrane surface, membrane and aqueous core of polymersomes, respectively, the van Hest group enabled site-specific positioning of enzymes in a single vesicle.90 These enzymes were able to cooperate in a cascade reaction and set the stage for creating colonies of communicating polymersomes, each containing multiple enzymes and signaling cascades in their interior.
Figure 5. Approaches to increasing the complexity of polymersome bioreactors.
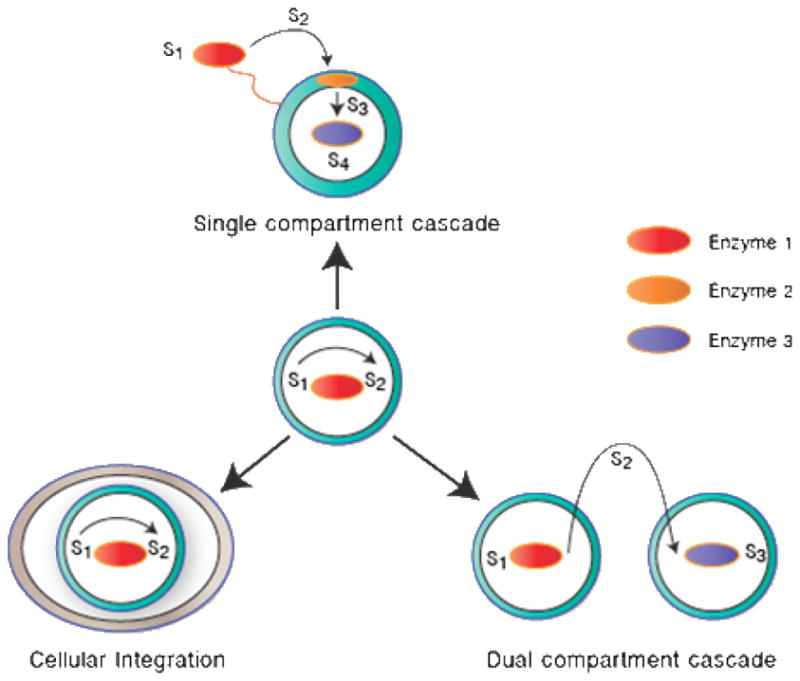
(Top) Multiple enzymes can be positioned within a single polymersome to create a signaling cascade. (Left) An enzyme-containing polymersome can be encapsulated into an alternate environment like a cell or an organelle. (Right) A signaling cascade can be carried out between multiple polymersomes by requiring an intermediate reactant to diffuse from one vesicle to another.
Metabolic processes related to energy metabolism are vital for the development of a self-sustaining synthetic cell. Energy-producing processes ultimately depend on redox reactions where electron transport drives processes like photosynthesis or phosphorylation. The incorporation of enzymes, like complex I, into a polymersome membrane that mediate electron transfer48 or incorporation of ATP synthase to produce high energy phosphates like ATP,93 mimics some of the oldest and fundamental cellular metabolic reactions. In the latter example, ATP synthase was driven via protons produced by another membrane-incorporated protein, bacteriorhodopsin, that generated protons in response to light. By coupling ATP synthesis to an intermediary protein, this study illustrates how to increase the complexity and sensitivity of enzymatic systems, requiring intermediate molecules to mediate reactions. Intermediate molecules that enhance or diminish the incoming supply of reactants, or simply inhibit a reaction from proceeding until triggered, will provide higher levels of temporal control over metabolic processes.
The development of compartmentalized reactors within polymersomes can lead to artificial organelles. Martin and coworkers recreated the process of enzymatically modifying the glycosaminoglycan, heparan sulfate (HS) - a reaction that is commonly completed in the Golgi.94 By using a microfluidic device, the group was able to sequentially control enzymatic reactions with HS as the glycoaminoglycan moved through the device. With the results achieved in positioning enzymes in a single vesicle90, 95 or spatially patterning several enzymatically active polymersomes,96 progress has been made in achieving the type of spatial, and sequential control of enzyme reactions in a cell.
Gene expression
Ultimately, sustaining the metabolic processes within an artificial cell will require production and replication of the cell’s internal components. Polynucleotide replication and protein expression are vital enzymatic reactions for replicating the self-maintenance and reproductive capabilities of a cell. Recent reviews have discussed advances in recreating these processes, which include RNA replication, polymerase chain reaction (PCR), DNA elongation, DNA transcription, and mRNA translation, within the confines of synthetic membranes.82, 97–99 In addition, much work has been dedicated to understanding the minimal genes required to sustain a cell, as well as recreating division and replication in lipid vesicles. Because of the breadth of studies and topics that have been used to relate synthetic biology to a minimal cell, we limit our discussion here to concepts that can most easily and ideally be recreated within polymersomes.
Propitious combination of individual components can lead to the recreation of enzymatic pathways. For example, components required to produce a specific enzyme can be encapsulated into the membrane compartment. The synthesized enzyme then initiates the desired enzymatic reaction, yet the addition of a progenitorial step provides a higher level of control over the final reaction. Protein production in lipid membranes has generally been demonstrated through synthesis of green fluorescent protein, which is visually easy to detect. For example, Ota et al.100 recently showed that GFP was expressed in lipid vesicles upon encapsulation of a plasmid carrying the GFP gene, phosphate, ribosomes, tRNA, translation initiation, elongation, and termination factors, RNA polymerase, 20 amino acids, salts, and an ATP regenerating system. Kits that include these components for in vitro protein synthesis can be commercially obtained and easily incorporated into synthetic membranes. This system can quickly increase in complexity by requiring the expression of multiple genes in a single compartment. Recent advances in polymersome production through microfluidic techniques, that enable controlled compartmentalization, controlled vesicle size, and high encapsulation efficiencies, should aid in the simultaneous expression of multiple proteins.
The process of protein production can be entered earlier, replicating the DNA templates used to produce the mRNA transcripts. By encapsulating nucleotides and DNA polymerase or by producing the basic building blocks of amino acids, nucleic acids, sugars, and lipids, PCR and protein expression can be performed in a liposome with higher levels of complexity and control. Then, recombinant DNA technology can be used for the expression of novel proteins and proteinaceous -based assemblies.98 As the relationship between DNA sequence and the subsequent polypeptide structure continues to be revealed through more basic biological and computational studies, we may create artificial cells with truly novel functions.
Maintaining these processes for extended periods of time will require exchange of nutrients and reaction byproducts through the membrane as well as positive and negative feedback where synthesized proteins can, in turn, initiate transcription and translation or degrade mRNA transcripts and proteins. In the former case, the incorporation of proteins, like alpha-hemolysin, that increases membrane permeability, was shown to extend the duration of protein production in a lipid vesicles from hours to days by allowing for influx of nutrients and components required for gene expression.99 In the latter case, Ichihashi et al. created a ‘self-encoded’ system within a liposome.101 The group used an mRNA that encodes for a Qβ replicase, which once produced, replicates the mRNA. In this way, transcription of this mRNA promotes continued transcription of the same species of mRNA.
While several processes associated with gene expression have been recreated in lipid vesicles, to the best of our knowledge, many of these have not be demonstrated in polymersomes. The chemical and physical flexibility that can be designed into polymersome membranes may enhance control in triggering gene expression, maintaining structural integrity during reactive processes, and even enable controlled deformation to allow for vesicle reproduction in ways that have yet to be achieved in any synthetic vesicle.97
Autonomous movement
As cells continuously sample their constantly changing environment, they are often required to leave their current location and migrate away or towards chemical or physical cues and to recruit or signal to other cells. The concept of mimicking this self-propelled movement with an inanimate particle has become a popular means of both studying intracellular and cellular motility and engineering micro-and nano-motors that do not require external stimuli to demonstrate directed motion. Creating synthetic polymersomes with the ability to move in response to their environment will be an important step towards creating an autonomous, synthetic cell.
A large number of studies have tried to understand cellular mechanics by reconstituting biological molecules, like cytoskeletal filaments and motor proteins, within in vitro systems.2 These studies have advanced our understanding of how motor proteins work in conjunction with the dynamic assembly and disassembly of actin filaments and microtubules to facilitate intracellular transport, organelle positioning, cell polarity, and motility. Actin polymerization, in particular, is vital for cell locomotion.102 Demonstration that actin polymerization can induce movement was established over a decade ago by creating self-propelled polystyrene beads.103 More recently, actin polymerization was demonstrated within a lipid vesicle,104 an important step towards creating cytoskeletal networks within the confines of synthetic membranes.
Generating directed movement in artificial systems has also been investigated with a physical science approach. These studies have developed methods to generate directed movement without the use of a polymerizable cytoskeleton. Instead, the manipulation of Brownian motion in asymmetric environments can be used to drive particle movement and mimic macroscopic cell motion. While control of particle movement has been demonstrated with external field-induced stimuli, including magnetic fields, electric fields, and thermal gradients, it is often impractical to apply an external field in biological systems. Recent work has focused on creating localized gradients in the form of ions, chemical reactants, and adhesivity to direct particle motion. Electrophoretic motors have been created in which an oxidation reaction produces protons on one side of a particle and electrons on the other.105, 106 The ion flux across the particle induces its motion. Conversely, if the solvent consists of charged species, differential diffusion of the cations and anions in solution can set up a local electric field within which a charged particle will move. Ion transport has been used to direct lipid vesicle movement as well. In this case, a negatively charged lipid vesicle was placed on positively charged supported lipid bilayers.107 Lipid exchange between the supported bilayer and the lipid vesicle leads to a charge gradient that caused vesicle motion. Neutrally charged particles can also be induced to move through the generation of chemical gradients that are hypothesized to create osmotically-propelled particles.108 In this case, a chemical reaction generates a particle concentration gradient across a semi-impermeable membrane. The osmotic pressure that develops in response to this gradient creates potential energy in the membrane system that is relieved through motion. The speed of the reaction as well as the permeability of the membrane determines the osmotic velocity of the particle.
Finally, theoretical work from the Balazs group has proposed that microcapsules could be induced to move haptotactically on adhesion gradients.109 This work employs the idea of communicating capsules in which two capsules are originally adherent to a surface. When a signaling capsule releases nanoparticles, these particles deposit onto an adhesive surface and physically restrict interaction between the capsule and the surface, reducing capsule adherence. Due to diffusion, an adhesion gradient is created as more nanoparticles are deposited close to the releasing signaling capsule and less particles are deposited farther away from the capsule. A nearby targeting capsule that senses this adhesion gradient will move away towards an area of increased adhesion. This latter approach of creating particles that can induce the movement and behavior of another particle would help mimic the migratory behavior of cells that are recruited through chemical cues from distant cells. These types of communicating capsules can potentially be reconstructed in polymersome systems.
Future directions
While many advances towards developing artificial cells from the bottom up have been achieved in recent years, the field as a whole is still in its infancy. Through this perspective we have discussed many facets of this research, highlighting the accomplishments in mimicking the autonomous and responsive behaviors of polymersomes and many of the remaining challenges. Polymersomes offer a particularly attractive platform on which to base synthetic biology; their robustness coupled with their structural similarity to the liposome and broad diversity in polymer functionality suggest they should be able to support multiple facets of synthetic life. Moving forward, we believe that gene expression, interparticle communication, and compartmentalization will be the primary foci of continued research. The ability to induce gene expression via trigger is one particular challenge that remains. Coupling triggered gene expression with cascade systems will lead to the development of synthetic gene circuits. In addition, reconstitution of a membrane component through gene expression continues to be goal of many research groups, and will likely be achieved soon. Advances relating to synthetic communication will benefit a diverse array of potential technological applications ranging from medicine to energy. Combining both responsive, communicative behaviors and self-sustaining internal metabolic processes could be useful for developing colonies of artificial cells, with the ability to interact and influence one another’s’ actions. As the cell biology community continues “top-down” research toward the minimalist cell—the minimal functions required for life—further insights will become exposed to guide what is necessary from the bottom up synthetic approach currently harnessed by the materials community. While synthetically coupling many of these functions within a single unit will remain a primary challenge, the progression towards creating a synthetic form of life and the continued evolution of this field is exciting.
Quotes to Highlight.
Attempts to construct a proto-cell will serve not only to enhance our understanding of the complex network of genetic and physical processes that must coordinate to maintain life in a biological cell, but also enable the development of future technologies, where creating ‘smart’, autonomously-functioning particles can serve anywhere from biomedical devices to self-regulating bioreactors.”
In biomimetic polymersomes, the control offered by light stimuli could be used to trigger a response from specific vesicle compartments, or facilitate communication with neighboring vesicles through cues, while not affecting biological components.”
Vesicles that can reversibly change structure can better mimic the continuously remodeling cell membrane.”
Ultimately, sustaining the metabolic processes within an artificial cell will require production and replication of the cell’s internal components.”
Acknowledgments
This work was supported by the National Institutes of Health Grant R01CA115229, the NSF MRSEC program under award number DMR05-20020, and by the National Science Foundation Grant CHE-0548188. N.P.K and J.S.K thank the NSF for Graduate Fellowships.
Biography
Neha P. Kamat received her B.S. from Rice University in Bioengineering and is a graduate student at the University of Pennsylvania pursuing a Ph.D. in Bioengineering within Daniel A. Hammer’s laboratory. Her research is in designing photoresponsive membranes. Joshua S. Katz received an S.B. in Chemistry from the Massachusettes Institute of Technology and recently received his Ph.D. in Bioengineering from the University of Pennsylvania in Daniel A. Hammer’s and Jason A. Burdick’s laboratories. His research has involved synthesizing and studying responsive polymers for polymersome applications. Both Neha and Joshua were recipients of graduate research fellowships from the National Science Foundation (NSF). Daniel A. Hammer received his B.S.E from Princeton University (1982) and his Ph.D. (1987) in Douglas Lauffenburger’s laboratory at the University of Pennsylvania. His present research focuses on quantitative immunology and the development of cellular mimetics for drug delivery and imaging. More information can be found on his lab website: http://www.seas.upenn.edu/~hammer.
References
- 1.Hawking SW. The Universe in a Nutshell. Bantam Books; New York: 2001. [Google Scholar]
- 2.Schwille P, Diez S. Synthetic Biology of Minimal Systems. Crit Rev Biochem Mol Biol. 2009;44:223–242. doi: 10.1080/10409230903074549. [DOI] [PubMed] [Google Scholar]
- 3.Walde P. Building Artificial Cells and Protocell Models: Experimental Approaches with Lipid Vesicles. Bioessays. 2010;32:296–303. doi: 10.1002/bies.200900141. [DOI] [PubMed] [Google Scholar]
- 4.Simpson ML. Cell–Free Synthetic Biology: A Bottom–up Approach to Discovery by Design. Mol Syst Biol. 2006;2 doi: 10.1038/msb4100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moya A, Gil R, Latorre A, Pereto J, Garcillan-Barcia MP, de la Cruz F. Toward Minimal Bacterial Cells: Evolution Vs. Design. FEMS Microbiol Rev. 2009;33:225–235. doi: 10.1111/j.1574-6976.2008.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasmussen S, Chen LH, Nilsson M, Abe S. Bridging Nonliving and Living Matter. Artif Life. 2003;9:269–316. doi: 10.1162/106454603322392479. [DOI] [PubMed] [Google Scholar]
- 7.Discher BM, Won YY, Ege DS, Lee JCM, Bates FS, Discher DE, Hammer DA. Polymersomes: Tough Vesicles Made from Diblock Copolymers. Science. 1999;284:1143–1146. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- 8.Bellomo EG, Wyrsta MD, Pakstis L, Pochan DJ, Deming TJ. Stimuli-Responsive Polypeptide Vesicles by Conformation-Specific Assembly. Nature Materials. 2004;3:244–248. doi: 10.1038/nmat1093. [DOI] [PubMed] [Google Scholar]
- 9.Torchilin VP. Recent Advances with Liposomes as Pharmaceutical Carriers. Nature Rev Drug Disc. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 10.Evans E, Needham D. Physical-Properties of Surfactant Bilayer-Membranes -Thermal Transitions, Elasticity, Rigidity, Cohesion, and Colloidal Interactions. Journal of Physical Chemistry. 1987;91:4219–4228. [Google Scholar]
- 11.Ghoroghchian PP, Li GZ, Levine DH, Davis KP, Bates FS, Hammer DA, Therien MJ. Bioresorbable Vesicles Formed through Spontaneous Self-Assembly of Amphiphilic Poly(Ethylene Oxide)-Block-Polycaprolactone. Macromol. 2006;39:1673–1675. doi: 10.1021/ma0519009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bermudez H, Brannan AK, Hammer DA, Bates FS, Discher DE. Molecular Weight Dependence of Polymersome Membrane Structure, Elasticity, and Stability. Macromol. 2002;35:8203–8208. [Google Scholar]
- 13.Delcea M, Yashchenok A, Videnova K, Kreft O, Mohwald H, Skirtach AG. Multicompartmental Micro- and Nanocapsules: Hierarchy and Applications in Biosciences. Macromolecular Bioscience. 2010;10:465–474. doi: 10.1002/mabi.200900359. [DOI] [PubMed] [Google Scholar]
- 14.Discher DE, Ortiz V, Srinivas G, Klein ML, Kim Y, David CA, Cai SS, Photos P, Ahmed F. Emerging Applications of Polymersomes in Delivery: From Molecular Dynamics to Shrinkage of Tumors. Prog Polym Sci. 2007;32:838–857. doi: 10.1016/j.progpolymsci.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghoroghchian PP, Frail PR, Susumu K, Blessington D, Brannan AK, Bates FS, Chance B, Hammer DA, Therien MJ. Near-Infrared-Emissive Polymersomes: Self-Assembled Soft Matter for in Vivo Optical Imaging. Proc Natl Acad Sci US A. 2005;102:2922–2927. doi: 10.1073/pnas.0409394102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabane E, Malinova V, Meier W. Synthesis of Photocleavable Amphiphilic Block Copolymers: Toward the Design of Photosensitive Nanocarriers. Macromolecular Chemistry and Physics. 2010;211:1847–1856. [Google Scholar]
- 17.Lomas H, Massignani M, Abdullah KA, Canton I, Lo Presti C, MacNeil S, Du JZ, Blanazs A, Madsen J, Armes SP, et al. Non-Cytotoxic Polymer Vesicles for Rapid and Efficient Intracellular Delivery. Faraday Discussions. 2008;139:143–159. doi: 10.1039/b717431d. [DOI] [PubMed] [Google Scholar]
- 18.Zhang YL, Wu F, Yuan WE, Jin T. Polymersomes of Asymmetric Bilayer Membrane Formed by Phase-Guided Assembly. J Cont Rel. 2010;147:413–419. doi: 10.1016/j.jconrel.2010.07.121. [DOI] [PubMed] [Google Scholar]
- 19.Upadhyay KK, Le Meins JF, Misra A, Voisin P, Bouchaud V, Ibarboure E, Schatz C, Lecommandoux S. Biomimetic Doxorubicin Loaded Polymersomes from Hyaluronan-Block-Poly(Gamma-Benzyl Glutamate) Copolymers. Biomacromolecules. 2009;10:2802–2808. doi: 10.1021/bm9006419. [DOI] [PubMed] [Google Scholar]
- 20.Sundararaman A, Stephan T, Grubbs RB. Reversible Restructuring of Aqueous Block Copolymer Assemblies through Stimulus-Induced Changes in Amphiphilicity. J Am Chem Soc. 2008;130:12264–12265. doi: 10.1021/ja8052688. [DOI] [PubMed] [Google Scholar]
- 21.Li F, Ketelaar T, Marcelis ATM, Leermakers FAM, Stuart MAC, Sudholter EJR. Stabilization of Polymersome Vesicles by an Interpenetrating Polymer Network. Macromol. 2007;40:329–333. [Google Scholar]
- 22.Cerritelli S, Velluto D, Hubbell JA. Peg-Ss-Pps: Reduction-Sensitive Disulfide Block Copolymer Vesicles for Intracellular Drug Delivery. Biomacromolecules. 2007;8:1966–1972. doi: 10.1021/bm070085x. [DOI] [PubMed] [Google Scholar]
- 23.Marsden HR, Gabrielli L, Kros A. Rapid Preparation of Polymersomes by a Water Addition/Solvent Evaporation Method. Polymer Chemistry. 2010;1:1512–1518. [Google Scholar]
- 24.Yu SY, Azzam T, Rouiller I, Eisenberg A. “Breathing” Vesicles. J Am Chem Soc. 2009;131:10557–10566. doi: 10.1021/ja902869q. [DOI] [PubMed] [Google Scholar]
- 25.Meng FH, Hiemstra C, Engbers GHM, Feijen J. Biodegradable Polymersomes. Macromol. 2003;36:3004–3006. [Google Scholar]
- 26.Zupancich JA, Bates FS, Hillmyer MA. Aqueous Dispersions of Poly(Ethylene Oxide)-B-Poly(Gamma-Methyl-Epsilon-Caprolactone) Block Copolymers. Macromol. 2006;39:4286–4288. [Google Scholar]
- 27.Lomas H, Canton I, MacNeil S, Du J, Armes SP, Ryan AJ, Lewis AL, Battaglia G. Biomimetic Ph Sensitive Polymersomes for Efficient DNA Encapsulation and Delivery. Advanced Materials. 2007;19:4238–4243. [Google Scholar]
- 28.Sanson C, Schatz C, Le Meins JF, Brulet A, Soum A, Lecommandoux S. Biocompatible and Biodegradable Poly(Trimethylene Carbonate)-B-Poly (L-Glutamic Acid) Polymersomes: Size Control and Stability. Langmuir. 2010;26:2751–2760. doi: 10.1021/la902786t. [DOI] [PubMed] [Google Scholar]
- 29.Nair LS, Laurencin CT. Biodegradable Polymers as Biomaterials. Prog Polym Sci. 2007;32:762–798. [Google Scholar]
- 30.Robbins GP, Lee D, Katz JS, Frail PR, Therien MJ, Crocker JC, Hammer DA. Effects of Membrane Rheology on Leuko-Polymersome Adhesion to Inflammatory Ligands. Soft Matter. 2011;7:769–779. doi: 10.1039/C0SM00554A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Percec V, Wilson DA, Leowanawat P, Wilson CJ, Hughes AD, Kaucher MS, Hammer DA, Levine DH, Kim AJ, Bates FS, et al. Self-Assembly of Janus Dendrimers into Uniform Dendrimersomes and Other Complex Architectures. Science. 328:1009–1014. doi: 10.1126/science.1185547. [DOI] [PubMed] [Google Scholar]
- 32.Holowka EP, Sun VZ, Kamei DT, Deming TJ. Polyarginine Segments in Block Copolypeptides Drive Both Vesicular Assembly and Intracellular Delivery. Nature Materials. 2007;6:52–57. doi: 10.1038/nmat1794. [DOI] [PubMed] [Google Scholar]
- 33.Holowka EP, Pochan DJ, Deming TJ. Charged Polypeptide Vesicles with Controllable Diameter. J Am Chem Soc. 2005;127:12423–12428. doi: 10.1021/ja053557t. [DOI] [PubMed] [Google Scholar]
- 34.Versluis F, Tomatsu I, Kehr S, Fregonese C, Tepper A, Stuart MCA, Ravoo BJ, Koning RI, Kros A. Shape and Release Control of a Peptide Decorated Vesicle through Ph Sensitive Orthogonal Supramolecular Interactions. J Am Chem Soc. 2009;131:13186–13187. doi: 10.1021/ja9026264. [DOI] [PubMed] [Google Scholar]
- 35.Li F, de Wolf FA, Marcelis ATM, Sudholter EJR, Stuart MAC, Leermakers FAM. Triggered Templated Assembly of Protein Polymersomes. Angewandte Chemie-International Edition. 2010;49:9947–9950. doi: 10.1002/anie.201004003. [DOI] [PubMed] [Google Scholar]
- 36.Cornelissen J, Fischer M, Sommerdijk N, Nolte RJM. Helical Superstructures from Charged Poly(Styrene)-Poly(Isocyanodipeptide) Block Copolymers. Science (New York, NY. 1998;280:1427–30. doi: 10.1126/science.280.5368.1427. [DOI] [PubMed] [Google Scholar]
- 37.Demirgoz D, Pangburn TO, Davis KP, Lee S, Bates FS, Kokkoli E. Pr_B-Targeted Delivery of Tumor Necrosis Factor-Alpha by Polymersomes for the Treatment of Prostate Cancer. Soft Matter. 2009;5:2011–2019. [Google Scholar]
- 38.Pang Z, Lu W, Gao H, Hu K, Chen J, Zhang C, Gao X, Jiang X, Zhu C. Preparation and Brain Delivery Property of Biodegradable Polymersomes Conjugates with Ox26. J Cont Rel. 2008;128:120–127. doi: 10.1016/j.jconrel.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Christian NA, Benencia F, Milone MC, Li G, Frail PR, Therien MJ, Coukos G, Hammer DA. In Vivo Dendritic Cell Tracking Using Fluorescence Lifetime Imaging and near-Infrared-Emissive Polymersomes. Molecular Imaging and Biology. 2009;11:167–177. doi: 10.1007/s11307-008-0184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christian NA, Milone MC, Ranka SS, Li GZ, Frail PR, Davis KP, Bates FS, Therien MJ, Ghoroghchian PP, June CH, et al. Tat-Functionalized near-Infrared Emissive Polymersomes for Dendritic Cell Labeling. Bioconj Chem. 2007;18:31–40. doi: 10.1021/bc0601267. [DOI] [PubMed] [Google Scholar]
- 41.Zupancich JA, Bates FS, Hillmyer MA. Synthesis and Self-Assembly of Rgd-Functionalized Peo-Pb Amphiphiles. Biomacromolecules. 2009;10:1554–1563. doi: 10.1021/bm900149b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hammer DA, Robbins GP, Haun JB, Lin JJ, Qi W, Smith LA, Ghoroghchian PP, Therien MJ, Bates Fs. Leuko-Polymersomes. Faraday Discuss. 2008;139:129–141. doi: 10.1039/b717821b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin JJ, Ghoroghchian P, Zhang Y, Hammer DA. Adhesion of Antibody-Functionalized Polymersomes. Langmuir. 2006;22:3975–3979. doi: 10.1021/la052445c. [DOI] [PubMed] [Google Scholar]
- 44.Robbins GP, Saunders RL, Haun JB, Rawson J, Therien MJ, Hammer DA. Tunable Leuko-Polymersomes That Adhere Specifically to Inflammatory Markers. Langmuir. 2010;26:14089–14096. doi: 10.1021/la1017032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu HP, Huang B, Sohail M, Luo S, Um SH, Khant H, et al. Interbilayer-Crosslinked Multilamellar Vesicles as Synthetic Vaccines for Potent Humoral and Cellular Immune Responses. Nature Mater. 2011;10:243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Discher BM, Won YY, Ege DS, Lee JC, Bates FS, Discher DE, Hammer DA. Polymersomes: Tough Vesicles Made from Diblock Copolymers. Science (New York, NY. 1999;284:1143–6. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- 47.Nallani M, Benito S, Onaca O, Graff A, Lindemann M, Winterhalter M, Meier W, Schwaneberg U. A Nanocompartment System (Synthosome) Designed for Biotechnological Applications. Journal of Biotechnology. 2006;123:50–59. doi: 10.1016/j.jbiotec.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 48.Graff A, Fraysse-Ailhas C, Palivan CG, Grzelakowski M, Friedrich T, Vebert C, Gescheidt G, Meier W. Amphiphilic Copolymer Membranes Promote Nadh:Ubiquinone Oxidoreductase Activity: Towards an Electron-Transfer Nanodevice. Macromol Chem Physic. 2010;211:229–238. [Google Scholar]
- 49.Nardin C, Widmer J, Winterhalter M, Meier W. Amphiphilic Block Copolymer Nanocontainers as Bioreactors. Eur Phys J E. 2001;4:403–410. [Google Scholar]
- 50.Kim AJ, Kaucher MS, Davis KP, Peterca M, Imam MR, Christian NA, Levine DH, Bates FS, Percec V, Hammer DA. Proton Transport from Dendritic Helical-Pore-Incorporated Polymersomes. Advanced Functional Materials. 2009;19:2930–2936. [Google Scholar]
- 51.Cheng ZL, Tsourkas A. Paramagnetic Porous Polymersomes. Langmuir. 2008;24:8169–8173. doi: 10.1021/la801027q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng ZL, Thorek DLJ, Tsourkas A. Porous Polymersomes with Encapsulated Gd-Labeled Dendrimers as Highly Efficient Mri Contrast Agents. Advanced Functional Materials. 2009;19:3753–3759. doi: 10.1002/adfm.200901253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Checot F, Lecommandoux S, Klok HA, Gnanou Y. From Supramolecular Polymersomes to Stimuli-Responsive Nano-Capsules Based on Poly(Diene-B-Peptide) Diblock Copolymers. Eur Phys J E. 2003;10:25–35. doi: 10.1140/epje/e2003-00006-1. [DOI] [PubMed] [Google Scholar]
- 54.Gohy JF, Willet N, Varshney S, Zhang JX, Jerome R. Core-Shell-Corona Micelles with a Responsive Shell. Angewandte Chemie-International Edition. 2001;40:3314–3316. doi: 10.1002/1521-3773(20010903)40:17<3214::AID-ANIE3214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 55.Meng FH, Zhong ZY, Feijen J. Stimuli-Responsive Polymersomes for Programmed Drug Delivery. Biomacromolecules. 200;10:197–209. doi: 10.1021/bm801127d. [DOI] [PubMed] [Google Scholar]
- 56.Katz JS, Burdick JA. Light-Responsive Biomaterials: Development and Applications. Macromolecular Bioscience. 2010;10:339–348. doi: 10.1002/mabi.200900297. [DOI] [PubMed] [Google Scholar]
- 57.Li MH, Keller P. Stimuli-Responsive Polymer Vesicles. Soft Matter. 2009;5:927–937. [Google Scholar]
- 58.Onaca O, Enea R, Hughes DW, Meier W. Stimuli-Responsive Polymersomes as Nanocarriers for Drug and Gene Delivery. Macromolecular Bioscience. 2009;9:129–139. doi: 10.1002/mabi.200800248. [DOI] [PubMed] [Google Scholar]
- 59.Berndl K, JK, Lipowsky R, Sackmann E, Seifert U. Shape Transformations of Giant Vesicles: Extreme Sensitivity to Bilayer Asymetry. Europhysics Letters. 1990;13:659–664. [Google Scholar]
- 60.Sens P, Johannes L, Bassereau P. Biophysical Approaches to Protein-Induced Membrane Deformations in Trafficking. Curr Opin Cell Biol. 2008;20:476–82. doi: 10.1016/j.ceb.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 61.Farrens DL, Altenbach C, Yang K, Hubbell WL, Khorana HG. Requirement of Rigid-Body Motion of Transmembrane Helices for Light Activation of Rhodopsin. Science (New York, NY. 1996;274:768–770. doi: 10.1126/science.274.5288.768. [DOI] [PubMed] [Google Scholar]
- 62.Goodman R, Blank M. Insights into Electromagnetic Interaction Mechanisms. J Cell Physiol. 2002;192:16–22. doi: 10.1002/jcp.10098. [DOI] [PubMed] [Google Scholar]
- 63.Kamat NP, Robbins GP, Rawson J, Therien MJ, Dmochowski IJ, Hammer DA. A Generalized System for Photoresponsive Membrane Rupture in Polymersomes. Adv Funct Mater. 2010;20:2588–2596. doi: 10.1002/adfm.201000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mabrouk E, Cuvelier D, Brochard-Wyart F, Nassoy P, Li MH. Bursting of Sensitive Polymersomes Induced by Curling. Proc Natl Acad Sci U S A. 2009;106:7294–8. doi: 10.1073/pnas.0813157106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DeClue MS, Monnard PA, Bailey JA, Maurer SE, Collis GE, Ziock HJ, Rasmussen S, Boncella JM. Nucleobase Mediated, Photocatalytic Vesicle Formation from an Ester Precursor. J Am Chem Soc. 2009;131:931–933. doi: 10.1021/ja808200n. [DOI] [PubMed] [Google Scholar]
- 66.Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DIC, Whitesides GM, Ingber DE. Engineering Cell-Shape and Function. Science (New York, NY. 1994;264:696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- 67.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Micropatterned Surfaces for Control of Cell Shape, Position, and Function. Biotechnol Prog. 1998;14:356–363. doi: 10.1021/bp980031m. [DOI] [PubMed] [Google Scholar]
- 68.Noguchi H, Gompper G. Shape Transitions of Fluid Vesicles and Red Blood Cells in Capillary Flows. Proc Natl Acad Sci U S A. 2005;102:14159–14164. doi: 10.1073/pnas.0504243102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu J, Hayward RC. Hierarchically Structured Microparticles Formed by Interfacial Instabilities of Emulsion Droplets Containing Amphiphilic Block Copolymers. Angewandte Chemie-International Edition. 2008;47:2113–2116. doi: 10.1002/anie.200704863. [DOI] [PubMed] [Google Scholar]
- 70.Kim KT, Zhu JH, Meeuwissen SA, Cornelissen J, Pochan DJ, Nolte RJM, van Hest JCM. Polymersome Stomatocytes: Controlled Shape Transformation in Polymer Vesicles. J Am Chem Soc. 2010;132:12522–12524. doi: 10.1021/ja104154t. [DOI] [PubMed] [Google Scholar]
- 71.Azzam T, Eisenberg A. Fully Collapsed (Kippah) Vesicles: Preparation and Characterization. Langmuir. 2010;26:10513–10523. doi: 10.1021/la1004837. [DOI] [PubMed] [Google Scholar]
- 72.Battaglia G, Ryan AJ. Neuron-Like Tubular Membranes Made of Diblock Copolymer Amphiphiles. Angewandte Chemie-International Edition. 2006;45:2052–2056. doi: 10.1002/anie.200503334. [DOI] [PubMed] [Google Scholar]
- 73.Antonietti M, Forster S. Vesicles and Liposomes: A Self-Assembly Principle Beyond Lipids. Adv Mater. 2003;15:1323–1333. [Google Scholar]
- 74.Farsad K, De Camilli P. Mechanisms of Membrane Deformation. Curr Opin Cell Biol. 2003;15:372–81. doi: 10.1016/s0955-0674(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 75.McMahon HT, Gallop JL. Membrane Curvature and Mechanisms of Dynamic Cell Membrane Remodelling. Nature. 2005;438:590–6. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 76.Simon J, Kuhner M, Ringsdorf H, Sackmann E. Polymer-Induced Shape Changes and Capping in Giant Liposomes. Chem Phys Lipids. 1995;76:241–258. [Google Scholar]
- 77.Dietrich C, Angelova M, Pouligny B. Adhesion of Latex Spheres to Giant Phospholipid Vesicles: Statics and Dynamics. J Phys II. 1997;7:1651–1682. [Google Scholar]
- 78.Yu SY, Azzam T, Rouiller I, Eisenberg A. “Breathing” Vesicles. J Am Chem Soc. 2009;131:10557–10566. doi: 10.1021/ja902869q. [DOI] [PubMed] [Google Scholar]
- 79.Moughton AO, Patterson JP, O’Reilly RK. Reversible Morphological Switching of Nanostructures in Solution. Chem Commun. 2011;47:355–357. doi: 10.1039/c0cc02160a. [DOI] [PubMed] [Google Scholar]
- 80.Jiang JQ, Shu QZ, Chen X, Yang YQ, Yi CL, Song XQ, Liu XY, Chen MQ. Photoinduced Morphology Switching of Polymer Nanoaggregates in Aqueous Solution. Langmuir. 2010;26:14247–14254. doi: 10.1021/la102771h. [DOI] [PubMed] [Google Scholar]
- 81.Jin QA, Liu GY, Liu XS, Ji JA. Photo-Responsive Supramolecular Self-Assembly and Disassembly of an Azobenzene-Containing Block Copolymer. Soft Matter. 2010;6:5589–5595. [Google Scholar]
- 82.Roodbeen R, van Hest JCM. Synthetic Cells and Organelles: Compartmentalization Strategies. Bioessays. 2009;31:1299–1308. doi: 10.1002/bies.200900106. [DOI] [PubMed] [Google Scholar]
- 83.Chiu HC, Lin YW, Huang YF, Chuang CK, Chern CS. Polymer Vesicles Containing Small Vesicles within Interior Aqueous Compartments and Ph-Responsive Transmembrane Channels. Angewandte Chemie-International Edition. 2008;47:1875–1878. doi: 10.1002/anie.200704078. [DOI] [PubMed] [Google Scholar]
- 84.Fu ZK, Ochsner MA, de Hoog HPM, Tomczak N, Nallani M. Multicompartmentalized Polymersomes for Selective Encapsulation of Biomacromolecules. Chem Commun. 2011;47:2862–2864. doi: 10.1039/c0cc03971c. [DOI] [PubMed] [Google Scholar]
- 85.Chu LY, Utada AS, Shah RK, Kim JW, Weitz DA. Controllable Monodisperse Multiple Emulsions. Angewandte Chemie-International Edition. 2007;46:8970–8974. doi: 10.1002/anie.200701358. [DOI] [PubMed] [Google Scholar]
- 86.Sun BJ, Shum HC, Holtze C, Weitz DA. Microfluidic Melt Emulsification for Encapsulation and Release of Actives. ACS Appl Mater Interfaces. 2010;2:3411–3416. doi: 10.1021/am100860b. [DOI] [PubMed] [Google Scholar]
- 87.Shum HC, Zhao YJ, Kim SH, Weitz DA. Multicompartment Polymersomes from Double Emulsions. Angewandte Chemie-International Edition. 2011;50:1648–1651. doi: 10.1002/anie.201006023. [DOI] [PubMed] [Google Scholar]
- 88.Renggli K, Baumann P, Langowska K, Onaca O, Bruns N, Meier W. Selective and Responsive Nanoreactors. 2011;21:1241–1259. [Google Scholar]
- 89.Kim KT, Meeuwissen SA, Nolte RJM, van Hest JCM. Smart Nanocontainers and Nanoreactors. Nanoscale. 2010;2:844–858. doi: 10.1039/b9nr00409b. [DOI] [PubMed] [Google Scholar]
- 90.van Dongen SFM, Nallani M, Cornelissen J, Nolte RJM, van Hest JCM. A Three-Enzyme Cascade Reaction through Positional Assembly of Enzymes in a Polymersome Nanoreactor. Chem Eur J. 200;15:1107–1114. doi: 10.1002/chem.200802114. [DOI] [PubMed] [Google Scholar]
- 91.van Dongen SFM, Verdurmen WPR, Peters R, Nolte RJM, Brock R, van Hest JCM. Cellular Integration of an Enzyme-Loaded Polymersome Nanoreactor. Angewandte Chemie-International Edition. 201(49):7213–7216. doi: 10.1002/anie.201002655. [DOI] [PubMed] [Google Scholar]
- 92.Kuiper SM, Nallani M, Vriezema DM, Cornelissen J, van Hest JCM, Nolte RJM, Rowan AE. Enzymes Containing Porous Polymersomes as Nano Reaction Vessels for Cascade Reactions. Organic & Biomolecular Chemistry. 2008;6:4315–4318. doi: 10.1039/b811196k. [DOI] [PubMed] [Google Scholar]
- 93.Choi HJ, Montemagno CD. Artificial Organelle: Atp Synthesis from Cellular Mimetic Polymersomes. Nano Lett. 2005;5:2538–2542. doi: 10.1021/nl051896e. [DOI] [PubMed] [Google Scholar]
- 94.Martin JG, Gupta M, Xu YM, Akella S, Liu J, Dordick JS, Linhardt RJ. Toward an Artificial Golgi: Redesigning the Biological Activities of Heparan Sulfate on a Digital Microfluidic Chip. J Am Chem Soc. 2009;131:11041–11048. doi: 10.1021/ja903038d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baumler H, Georgieva R. Coupled Enzyme Reactions in Multicompartment Microparticles. Biomacromolecules. 2010;11:1480–1487. doi: 10.1021/bm1001125. [DOI] [PubMed] [Google Scholar]
- 96.Grzelakowski M, Onaca O, Rigler P, Kumar M, Meier W. Immobilized Protein-Polymer Nanoreactors. Small. 2009;5:2545–2548. doi: 10.1002/smll.200900603. [DOI] [PubMed] [Google Scholar]
- 97.Stano P, Luisi PL. Achievements and Open Questions in the Self-Reproduction of Vesicles and Synthetic Minimal Cells. Chem Commun. 2010;46:3639–3653. doi: 10.1039/b913997d. [DOI] [PubMed] [Google Scholar]
- 98.Channon K, Bromley EHC, Woolfson DN. Synthetic Biology through Biomolecular Design and Engineering. Curr Opin Struct Biol. 2008;18:491–498. doi: 10.1016/j.sbi.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 99.Noireaux V, Bar-Ziv R, Godefroy J, Salman H, Libchaber A. Toward an Artificial Cell Based on Gene Expression in Vesicles. Phys Biol. 2005;2:P1–P8. doi: 10.1088/1478-3975/2/3/P01. [DOI] [PubMed] [Google Scholar]
- 100.Ota S, Yoshizawa S, Takeuchi S. Microfluidic Formation of Monodisperse, Cell-Sized, and Unilamellar Vesicles. Angewandte Chemie-International Edition. 2009;48:6533–6537. doi: 10.1002/anie.200902182. [DOI] [PubMed] [Google Scholar]
- 101.Ichihashi N, Matsuura T, Kita H, Hosoda K, Sunami T, Tsukada K, Yomo T. Importance of Translation-Replication Balance for Efficient Replication by the Self-Encoded Replicase. ChemBioChem. 2008;9:3023–3028. doi: 10.1002/cbic.200800518. [DOI] [PubMed] [Google Scholar]
- 102.Pollard TD, Borisy GG. Cellular Motility Driven by Assembly and Disassembly of Actin Filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 103.van Oudenaarden A, Theriot JA. Cooperative Symmetry-Breaking by Actin Polymerization in a Model for Cell Motility. Nat Cell Biol. 1999;1:493–499. doi: 10.1038/70281. [DOI] [PubMed] [Google Scholar]
- 104.Stachowiak JC, Richmond DL, Li TH, Brochard-Wyart F, Fletcher DA. Inkjet Formation of Unilamellar Lipid Vesicles for Cell-Like Encapsulation. Lab Chip. 2009;9:2003–2009. doi: 10.1039/b904984c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu J, Sigworth FJ, LaVan DA. Synthetic Protocells to Mimic and Test Cell Function. Adv Mater. 2010;22:120–127. doi: 10.1002/adma.200901945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ebbens SJ, Howse JR. In Pursuit of Propulsion at the Nanoscale. Soft Matter. 201(6):726–738. [Google Scholar]
- 107.Solon J, Streicher P, Richter R, Brochard-Wyart F, Bassereau P. Vesicles Surfing on a Lipid Bilayer: Self-Induced Haptotactic Motion. Proc Natl Acad Sci U S A. 2006;103:12382–12387. doi: 10.1073/pnas.0601400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cordova-Figueroa UM, Brady JF. Osmotic Propulsion: The Osmotic Motor. Phys Rev Lett. 2008;100 doi: 10.1103/PhysRevLett.100.158303. [DOI] [PubMed] [Google Scholar]
- 109.Usta OB, Alexeev A, Zhu G, Balazs AC. Modeling Microcapsules That Communicate through Nanoparticles to Undergo Self-Propelled Motion. Acs Nano. 2008;2:471–476. doi: 10.1021/nn700379v. [DOI] [PubMed] [Google Scholar]