Abstract
Corticosteroids inhibit organic cation transporters (OCTs) that play an important role in drug absorption, tissue distribution and elimination. Corticosteroid sensitivity of bronchodilator trafficking in the airway tissue, however, is poorly understood. To assess the effects of inhaled corticosteroids on airway absorption and disposal mechanisms of long-acting β2-agonists, human airway epithelial and smooth muscle cell uptake of tritiated formoterol and salmeterol was measured in vitro. Corticosteroids caused a rapid, concentration-dependent inhibition of uptake of the cationic formoterol by airway smooth muscle cells, but not airway epithelial cells. Uptake of the noncharged lipophilic salmeterol was corticosteroid-insensitive in both cell types. In smooth muscle cells, inhaled corticosteroids inhibited formoterol uptake with a novel potency rank order: des-ciclesonide > budesonide > beclomethasone 17-monopropionate > beclomethasone dipropionate > ciclesonide > fluticasone. The inhibitory action was rapidly reversible, and was not enhanced by prolonged corticosteroid exposure or sensitive to a transcription inhibitor. Suppression of OCT3 expression using lentivirus-mediated production of shRNA reduced corticosteroid sensitivity of formoterol uptake by smooth muscle cells. Our data support a corticosteroid insensitive absorption and a corticosteroid sensitive disposition mechanism for cationic long-acting β2-agonist bronchodilators in the airway. Potency rank order and other ‘classical’ features of anti-inflammatory effects do not apply to inhaled corticosteroids’ rapid drug transport actions.
Keywords: bronchial smooth muscle, organic cation transporter, inhaled corticosteroids, nongenomic, formoterol, asthma
1. INTRODUCTION
Corticosteroid/long-acting β2-agonist combination inhalers are increasingly used in asthma management [1, 2]. Combined drug administration provides more effective control in mild-to-moderate asthma: patients have better lung function, better symptom control, and lower incidence of exacerbation compared with those given either drug alone [3]. Combination inhalers can simultaneously deliver drugs that have complementary actions on asthma pathophysiology: corticosteroids effectively suppress airway inflammation, whereas long-acting β2-agonists, acting as bronchodilators, provide long-lasting relaxation of airway smooth muscle [4]. Importantly, drug-drug interactions are now recognized to have a major role in the superior effects of combined drug administration; however, the molecular mechanisms of the interactions have not been fully elucidated [5]. In addition, it is still uncertain whether the choice of inhaled corticosteroid and long-acting β2-agonist in combination preparations makes a difference to these beneficial interactions.
There is a growing body of evidence that inhaled corticosteroids, in addition to anti-inflammatory actions, could prevent the loss of function and improve the effects of β2-agonist bronchodilators. Among other ‘classical’ delayed (transcriptional) actions, corticosteroids could increase β2-receptor numbers and restore β2-receptor coupling to G proteins that mediate adenylyl cyclase stimulation [6, 7]. In contrast, corticosteroids may also improve β2-adrenergic bronchodilation through rapid (non-transcriptional) mechanisms [8, 9]. We showed that inhaled corticosteroids may limit the vascular clearance of inhaled bronchodilators by causing rapid vasoconstriction in the airway [10]. Furthermore, we recently demonstrated that corticosteroids rapidly interfere with the disposal of cationic β2-agonist bronchodilators by smooth muscle cells in the airway [11]. By rapidly potentiating β2-adrenergic bronchodilation, these mechanisms could be relevant to the use of combination inhalers both as maintenance therapy and rescue medications for symptom relief [12].
In our prior study, we showed expression of a corticosteroid sensitive cationic drug transport machinery in human airways, and the inhibitory corticosteroid effect on cationic drug uptake by airway vascular and bronchial smooth muscle [11]. To further understand corticosteroid sensitivity of inhaled drug trafficking (i.e. absorption and disposal mechanisms) in the airway, we examined here how inhaled corticosteroids influence uptake of two long-acting β2-agonists, the cationic formoterol and the lipophilic salmeterol, by human airway epithelial and smooth muscle cells. We also assessed whether drug transport actions of inhaled corticosteroids are mediated via the classical transcriptional pathway of steroid action.
2. MATERIAL AND METHODS
2.1. Human tissues and airway epithelial cell isolation
Tracheas and large bronchi (≥ third generation) were obtained from organ donors whose lungs were rejected for transplantation because they failed to meet standard selection criteria [13]. Consent for research was obtained by the Life Alliance Organ Recovery Agency of the University of Miami. All consents were IRB-approved and conformed to the Declaration of Helsinki. Primary airway epithelial cells were isolated from airway tissues as previously described [14]. Briefly, the mucosa was dissected from the underlying cartilage under sterile conditions and incubated in 0.05% protease (type XIV; Sigma, St. Louis, MO) in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA) overnight at 4°C. After protease treatment, epithelial cells were released by vigorous shaking and cells were harvested by centrifugation. Human tracheobronchial epithelial cells were counted, and their viability was determined by Trypan Blue exclusion (viability was always > 80%).
2.2. Cell cultures
Freshly isolated airway epithelial cells were deposited onto human placental (type IV) collagen-coated culture dishes at a density of 0.5 × 106 cells/cm2, and maintained ≤ 48 hours in DMEM supplemented with 5% fetal bovine serum (Hyclone, Logan, UT), 100 U/ml penicillin, and 100 μg/ml streptomycin within a humidified atmosphere containing 5% CO2 at 37 °C [15].
Primary human bronchial smooth muscle cells were purchased from Lonza (Walkersville, MD), and grown in their recommended optimal medium (Smooth Muscle Cell Growth Medium; Lonza) supplemented with 5% fetal bovine serum (Hyclone, Logan, UT), 100 U/ml penicillin, and 100 μg/ml streptomycin within a humidified atmosphere containing 5% CO2 at 37 °C. Smooth muscle cells were serum-deprived for 24 hours before transport measurements.
2.3 Transport assays
To examine drug transport in vitro, uptake of the cationic [3H]-formoterol (7.03 Ci/mmol; AstraZeneca, Loughborough, United Kingdom), the non-charged lipophilic [3H]-salmeterol (19.1 Ci/mmol; AstraZeneca), and the organic cation transporter-carried [3H]-N-methyl-4-phenylpyridinium (MPP+; 81 Ci/mmol; PerkinElmer, Boston, MA) was measured in human airway cells. Experiments were performed at 37°C by applying 200 nmol/l [3H]-formoterol or [3H]-salmeterol, or 5 nmol/l [3H]-MPP+ to monolayer cell cultures. Beclomethasone dipropionate and beclomethasone 17-monopropionate were generously provided by Chiesi Farmaceutici (Parma, Italy), and ciclesonide and des-ciclesonide by Nycomed (Konstanz, Germany). Other corticosteroids were purchased from Sigma. Corticosteroids were dissolved in ethanol (final concentration ≤ 0.1%), a solvent without significant effect on drug transport levels as confirmed by controls using vehicle only. Incubation was stopped after 15 min by rinsing the cells with ice-cold PBS. Subsequently, the cells were solubilized and radioactivity was measured by liquid scintillation counting. Protein was measured by the BCA protein assay (Pierce, Rockford, IL).
2.4 Organic cation transporter 3 (OCT3) silencing with shRNA lentiviruses
We used a third-generation, propagation-deficient HIV-pseudotyped lentivirus expressing OCT3 targeted shRNA to knock down OCT3 gene expression. Proviral plasmids (pLKO.1) with anti-OCT3 shRNA sequences (shRNA set ID RHS4533-NM_021977) were purchased from Open Biosystems (Huntsville, AL). In preliminary experiments, one sequence, GCACCAAACTTCCCTGTGTTT (Clone ID TRCN0000038609), was found to be highly effective and thus chosen for the construction of lentivirus for transduction and shRNA expression. Same vector without OCT3 targeted shRNA sequence was used as control. The pLKO.1 lentiviral vector contained a selectable marker for resistance to puromycin. Replication-deficient lentiviruses were prepared by co-transfecting vector and the packaging plasmids pMDLg/pRRE#54 pRSV-Rev and pMDLgVSVG, into HEK 293T cells by calcium phosphate coprecipitation, as previously described [16]. Viruses were collected daily for 3 days beginning 24 hours after removing the precipitates. Viruses were concentrated by precipitation with the addition of polyethylene glycol to 11% and centrifugation. Virus titers were estimated by measuring p24 by ELISA (Perkin-Elmer, Shelton, CT).
Human bronchial smooth muscle cells were infected with OCT3 lentiviruses, selected with 1 μg/ml puromycin until uninfected cells were dead, typically 3–5 days. Date and lung donor matched cultures that were infected with non-targeted shRNA lentiviruses (selected with puromycin) were used as controls.
2.5. OCT3 mRNA measurement
Total RNA was extracted from primary bronchial smooth muscle cells using the RNeasy Protect Mini Kit (Qiagen, Valencia, CA). RNA samples were treated with RNase-free DNase (Qiagen) and quantified spectrophotometrically at 260 nm. RNA integrity was confirmed using RNA 6,000 LabChip Kit (Agilent Technologies, Palo Alto, CA) and a bioanalyzer (model 2100, Agilent Technologies) provided by the University of Miami DNA Microarray Facility.
First-strand cDNA was synthesized using the Quantitect Reverse Transcription Kit (Qiagen). OCT3 mRNA expression levels were measured with quantitative, real-time PCR and our custom-designed gene-specific primers as described previously [15]: 5’-TGC CTA CTT CAT CCC CAA CTG G (forward) and 5’-TTC CGA GTA ATC AGC CAA CGG G (reverse). Real-time PCR amplification reactions were performed with Quantitect SYBR Green PCR Kit (Qiagen). The cycling conditions comprised of 15 min polymerase activation at 95°C, and 45 cycles at 95°C for 15 s, 57°C for 30 s, and at 72°C for 30 s. Reactions were run on an iCycler IQ5 Real-Time PCR Detection System (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. Control reactions were performed in the absence of RT. Specificity of the amplificons were confirmed by purification on QiAquick PCR Purification Kit silica spin columns (Qiagen) and sequencing (DNA Core Laboratory, University of Miami). Gene expression levels were calculated by normalizing the data to the endogenous control gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
2.6. Statistical analysis
Results were expressed as mean ± SD. Each experiment was repeated with cells from at least three different human donors. Groups were compared by one-way ANOVA and, if a significant difference was found, by the Tuckey-Kramer honestly significant difference test using JMP software (SAS, Cary, NC). p < 0.05 was accepted as significant.
3. RESULTS
3.1. Corticosteroid sensitivity of long-acting β2-agonist uptake by bronchial smooth muscle and airway epithelial cells
To assess cellular mechanisms of bronchodilator absorption and disposal in the airway, we measured drug uptake by airway epithelial and bronchial smooth muscle cells, respectively. To look for functional evidence of a corticosteroid-sensitive transport machinery for cationic drugs, we measured uptake of the organic cation transporter-carried MPP+ in these cells. The cellular uptake of MPP+ uptake was 0.21 ± 0.02 and 0.18 ± 0.03 pmol/mg protein/15 min in smooth muscle and epithelial cells, respectively. In our prior study on airway smooth muscle cells, a nearly identical uptake level was measured [11]. After 15-min incubation, the classical OCT inhibitor corticosterone (5 μmol/l) significantly reduced MPP+ uptake by bronchial smooth muscle, but not airway epithelial cells (see Figure 1). By measuring the cellular transport of long-acting β2-agonists, uptake of the largely cationic formoterol was higher in smooth muscle than airway epithelial cells (28.1 ± 1.6 vs. 8.9 ± 0.5 pmol/mg protein/15 min, respectively; p < 0.05). Similar to MPP+, the rapid inhibitory effect of corticosterone was detectable only in bronchial smooth muscle cells, but not in epithelial cells. In contrast to the cationic formoterol, uptake of lipophilic salmeterol was significantly higher in both bronchial smooth muscle and epithelial cells (306.9 ± 13.3 and 46.6 ± 1.9 pmol/mg protein/15 min, respectively), and insensitive to corticosterone.
Figure 1.
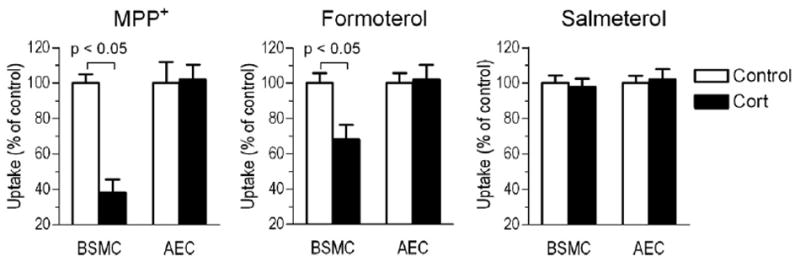
Drug uptake and rapid (15 min) inhibitory effect of corticosterone (Cort; 5 μM) in human bronchial smooth muscle cells (BSMC) and airway epithelial cells (AEC). Bars represent mean ± SD (n = 6 experiments).
3.2. Drug transport inhibitory potency: a novel rank order for inhaled corticosteroids?
To establish their rank order for inhibitory potency on airway drug transport, we measured the effects of various steroids on long-acting β2-agonists uptake. Corticosteroid pro-drugs (i.e. inactive forms that are converted to active metabolites with high glucocorticoid receptor affinity by airway cells) [17] and the corresponding active compounds were also included in the measurements to examine the relationship between cytoplasmic glucocorticoid receptor binding affinity and drug transport action. After a 15-min incubation, corticosteroids caused a concentration dependent inhibition of formoterol by bronchial smooth muscle cells with mean IC50 values in the range of 0.43 to 6.28 μmol/l (see Table 1).
Table 1.
Potency rank order for corticosteroids in rapidly inhibiting formoterol uptake by human bronchial smooth muscle cells. Values are means and 95% confidence intervals (C.I.) (n = 6 experiments).
| IC50 (μM) | 95% C.I. | |
|---|---|---|
| Corticosterone | 0.43 | 0.37, 0.51 |
| des-ciclesonide** | 0.65 | 0.47, 0.91 |
| Hydrocortisone | 1.37 | 0.93, 2,04 |
| Budesonide | 1.92 | 1.49, 2.46 |
| Beclomethasone 17-monopropionate## | 4.04 | 3.38, 4.83 |
| Beclomethasone dipropionate# | 4.39 | 3.75, 5.14 |
| Ciclesonide* | 5.29 | 4.52, 6.59 |
| Fluticasone propionate | 6.29 | 5.37, 7.41 |
corticosteroid pro-drugs
corresponding active compounds
In contrast, corticosteroids had no significant effect on formoterol accumulation in airway epithelial cells. Salmeterol uptake was insensitive to corticosteroids in both cell types (data not shown).
3.3. Drug transport inhibition via a nongenomic corticosteroid action
To investigate whether the rapid corticosteroid action is mediated by the classical steroid pathways (i.e., cytoplasmic receptor binding and nuclear translocation followed by actions on gene transcription), we assessed its time-dependence, immediate reversibility, and sensitivity to transcription inhibition. First, by measuring the uptake of formoterol into human bronchial smooth muscle cells, the rapid inhibitory action of corticosterone (5 μmol/l) on drug uptake was not enhanced by corticosterone pretreatment for 45 or 90 min (see Figure 2A). In further experiments, by assessing its gene transcription dependence, the corticosterone action on formoterol uptake was insensitive to 100 μM actinomycin D (transcription inhibitor) that was added to the incubation medium 60 min prior to transport measurements (see Figure 2B). Finally, to assess the steroid action’s reversibility, cells were exposed to a corticosterone-containing medium for 15 min and then rapidly transferred to corticosterone-free medium just before the uptake measurements. Under this condition, no inhibition of formoterol uptake was seen (see Figure 2C).
Figure 2.
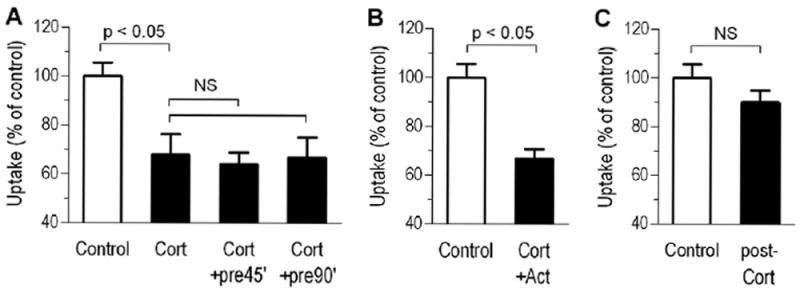
Nongenomic, inhibitory effect of corticosterone (Cort; 5 μM) on formoterol uptake by human bronchial smooth muscle cells. A, The rapid (15 min) inhibitory corticosteroid effect was not enhanced by prolonged corticosteroid pretreatment for 45 and 90 min (+pre45’ and +pre90’, respectively). B, Corticosterone effect in the presence of the transcription inhibitor actinomycin D (Act; 100 μM). C, Formoterol uptake in cells exposed to corticosterone for 15 min followed by corticosterone washout prior to transport measurements (post-Cort). Bars represent mean ± SD (n = 6 experiments).
3.4. OCT3 knockdown with shRNA reduces drug transport inhibition by corticosteroids
To assess the relation between OCT3 knockdown and corticosteroid sensitivity of long-acting β2-agonist uptake, shRNA-expressing lentiviruses were used to specifically reduce OCT3 mRNA expression in bronchial smooth muscle cells. Human immunodeficiency virus-pseudotyped lentiviruses expressing OCT3 targeted shRNAs with a selectable puromycin resistance gene were evaluated for mRNA knockdown. Cells were infected with OCT3 targeted or non-targeted (negative control) shRNA-expressing lentiviruses, maintained in the presence of puromycin, and tested for OCT3 mRNA with quantitative RT-PCR. The OCT3 targeted lentivirus construct reduced mRNA expression consistently to less than 15% of the non-targeted control shRNA (see Figure 3A).
Figure 3.
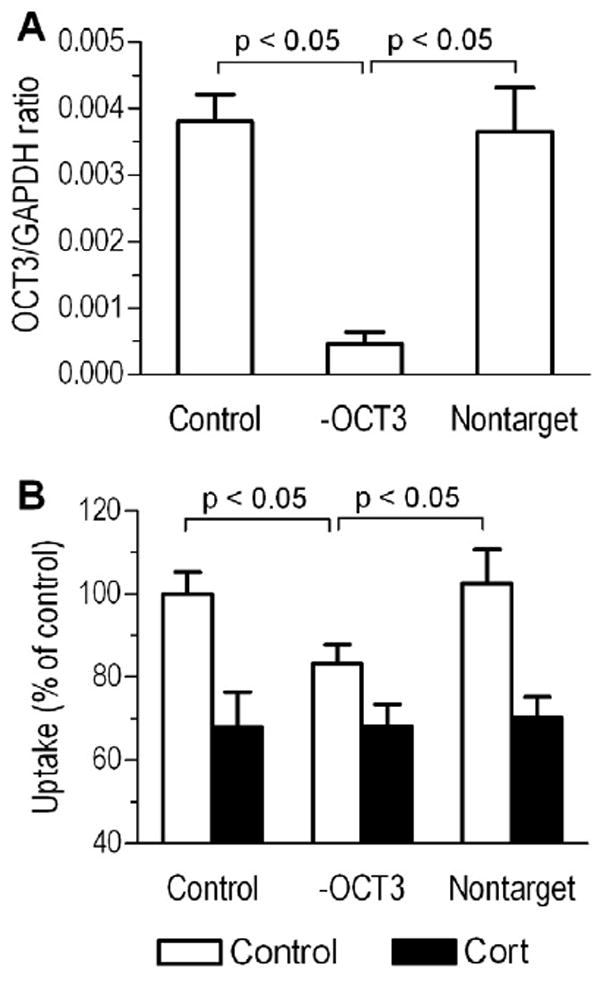
The role of OCT3 in rapidly inhibiting formoterol uptake into human bronchial smooth muscle cells. A, OCT3 mRNA relative expression in cells that were infected with OCT3 targeted (-OCT3) and non-targeted shRNA-containing lentivirus. B, OCT3 mRNA suppression reduces formoterol uptake and the inhibitory effect of corticosterone (Cort). Results represent mean ± SD (n = 4 experiments).
By measuring cellular drug transport, formoterol uptake was significantly reduced in bronchial smooth muscle cells that were infected with OCT3 targeted shRNA comparing to infection to non-targeted shRNA (23.4 ± 1.3 vs. 28.7 ± 1.6 pmol/mg protein/15 min; p < 0.05). OCT3 knockdown also reduced the corticosteroid effect on formoterol uptake (see Figure 3B). In contrast, infection with OCT3 targeted and non-targeted shRNAs had no significant effects on cellular uptake of salmeterol (302.5 ± 14.3 for and 298.3 ± 15.6 pmol/mg protein/15 min; p > 0.05 vs. control for both). Together, these data support the hypothesis that cellular uptake of the cationic formoterol occurs at least in part via the corticosteroid sensitive OCT3.
4. DISCUSSION
Our present in vitro study supports the use of corticosteroid/long-acting β2-agonist combination inhalers in asthma therapy by demonstrating a novel ‘hierarchy’ for inhaled corticosteroids to limit the cellular mechanisms of cationic long-acting β2-agonist disposal, but not absorption into the airway through the airway epithelium. We provide pharmacological evidence that the corticosteroid action on airway drug transport does not require involvement of the ‘classical’ intracellular pathway of steroid action (i.e. cytoplasmic receptor binding, nuclear translocation, and transcription regulation). By demonstrating a role for the corticosteroid-sensitive OCT3 in uptake of formoterol and likely other cationic bronchodilators into bronchial smooth muscle cells, we also identified a molecular target for inhaled corticosteroids. We suggest considering these rapid interactions of inhaled corticosteroids and long-acting β2-agonists to identify superior drug combinations for asthma therapy.
In contrast to lipophilic compounds that can easily pass through cellular barriers, carriers such as the polyspecific organic cation transporters (OCT1-3) and organic cation/carnitine transporters (OCTN1-2) are required for many cation drug molecules to reach their cellular targets [18, 19]. OCTs and OCTNs are expressed and functional in airway epithelia [15, 20] and smooth muscle [11]. However, their roles in airway drug absorption and elimination have not been fully elucidated [21]. In this study, we focused on OCT regulation by corticosteroids, a potential mechanism by which inhaled corticosteroids may influence the pharmacological actions of other inhaled drugs including cationic bronchodilators (i.e. most β2-agonists and anticholinergics) [22]. In primary cultures of human airway smooth muscle cells dominantly expressing corticosteroid sensitive OCTs [11, 15], our transport assays revealed inhibitory actions of inhaled corticosteroids on formoterol uptake with IC50 values between 0.6 to 6.3 μM. Considering the time point immediately after deposition in the airway, inhaled corticosteroids may peak in this concentration range in the airway tissue, sufficient to inhibit OCTs [23, 24]. Despite the similar substrate profiles and the lack of selective inhibitors of OCTs [18], we were able to provide a proof for the role of OCT3 in long-acting β2-agonist uptake by human airway smooth muscle cells. In these cells, by selectively suppressing transporter gene expression, we confirmed that the cationic formoterol is carried by corticosteroid-sensitive OCT3. Importantly, our data also supported that inhaled corticosteroids do not interfere with the transport of cationic drugs in airway epithelial cells, an interference that would limit absorption and thus access of inhaled β2-agonists to their targets on smooth muscle cells. This is consistent with the findings of our prior study demonstrating a pH-dependent rather than corticosteroid-sensitive uptake mechanism (i.e. consistent with OCTN1/2) of a cationic fluorescent dye in airway epithelial cells [15].
Although rapid actions of steroids were revealed many years before their transcriptional effects [25], rapid airway actions of inhaled corticosteroids were demonstrated only in recent years [10, 26, 27]. These actions and their diverse mechanistic pathways (i.e. other than up- or downregulation of target genes) have received particular attention recently [28], but we just started understanding the true nature of these actions. In the present study, we examined airway transport of inhaled bronchodilators as a possible target for inhaled corticosteroids. By using various pharmacological approaches to demonstrate its nongenomic characteristics, we showed that corticosteroid action on cationic long-acting β2-agonist bronchodilator transport by human bronchial smooth muscle is largely reversible in minutes, doesn’t require prolonged corticosteroid exposure, and is independent of gene transcription mechanisms. The nongenomic pathway was further supported by identifying a novel potency rank order for corticosteroids that is not related to their glucocorticoid receptor binding affinity, glucocorticoid-receptor half life, or relative lipophilicity [29]. Our data give a new perspective for the pharmacology of inhaled corticosteroids, however, the clinical relevance of these rapid actions still needs to be explored in vivo.
Asthma guidelines recommend that a long-acting β2-agonist should be added to the treatment regimen of patients not adequately controlled on a low-to-moderate dose of inhaled corticosteroids [30]. Combination therapy leads to effective asthma control primarily because these drugs have complementary modes of actions (i.e. suppressing airway inflammation and inducing bronchodilation). Although these drugs act through separate and distinct pathways, there is scientific evidence that genomic interactions of corticosteroids and long-acting β2-agonists can also contribute to the good results with the combination therapy. Interestingly, recent studies also suggested rapid interactions that may enhance β2-adrenergic bronchodilation; however, the mechanisms of these interactions are still largely unknown [12, 26]. One such mechanism could be that corticosteroids boost the effects of β2-agonists by causing airway vasoconstriction to delay the vascular clearance of the inhaled bronchodilators [31]. Another possible mechanism is that corticosteroids interfere with the carrier-mediated cellular disposition of inhaled cationic drugs in the airway [11]. The present study further explored drug transporter actions by demonstrating beneficial interactions between the currently available inhaled corticosteroids and a cationic (i.e. formoterol), but not a lipophilic long-acting β2-agonist bronchodilator (i.e. salmeterol). A recent in vivo study supports the existence of these interactions by showing that the single inhalation of a corticosteroid enhances airway vascular effects of a simultaneously inhaled short-acting β2-agonist [10]. Interestingly, our present data also suggest that simultaneous administration may not be ideal for every drug combination. Since conversion of the inhaled pro-drug ciclesonide into the potent transporter inhibitor des-ciclesonide takes minutes to hours in airway cells [32], we can speculate that a sequential inhalation scheme (i.e. ciclesonide followed by the β2-agonist) may allow sufficient time for des-ciclesonide production leading to more prominent inhibitory effect on β2-agonist disposal, and further improve bronchodilation.
The combination of long-acting β2-agonists and inhaled corticosteroids provides numerous clinically important advantages in the treatment of obstructive lung disease. However, we still need to explore the pharmacological rationale of drug selection for these combinations. Our experiments demonstrated nongenomic properties (i.e. OCT inhibition) of inhaled corticosteroids and the chemical structure (i.e. substrate affinity to OCTs) of long-acting β2-agonists as important determinants of their superior combination effects. However, the significance of these heretofore poorly recognized interactions will have to await further testing in vivo.
We examine corticosteroid actions on long-acting β2-agonist uptake by airway cells.
We find an acute inbibition of smooth muscle uptake of the cationic formoterol.
The rapid effect does not require the ‘classical’ pathway of steroid action.
We also show a novel hierarchy for corticosteroids to limit formoterol uptake.
These rapid interactions may have a role in the superior effect of drug combinations.
Acknowledgments
This work was supported in part by an academic research grant from AstraZeneca and National Institutes of Health Grants HL-60644 (to M. S.) and HL-66125 (to G. E. C.). G.H. is a recipient of the Bolyai Fellowship of the Hungarian Academy of Sciences.
LIST OF NONSTANDARD ABBREVIATIONS
- OCT
organic cation transporter
- OCTN
organic cation/carnitine transporter
- MPP+
N-methyl-4-phenylpyridinium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sears MR. The addition of long-acting beta-agonists to inhaled corticosteroids in asthma. Curr Opin Pulm Med. 2011;17(1):23–8. doi: 10.1097/MCP.0b013e328341004c. [DOI] [PubMed] [Google Scholar]
- 2.Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010;(4):CD005533. doi: 10.1002/14651858.CD005533.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller-Larsson A, Selroos O. Advances in asthma and COPD treatment: combination therapy with inhaled corticosteroids and long-acting beta2-agonists. Curr Pharm Des. 2006;12(25):3261–79. doi: 10.2174/138161206778194187. [DOI] [PubMed] [Google Scholar]
- 4.Suissa S, Dell’Aniello S, Ernst P. Effectiveness of combination therapies in asthma: an observational study. Pulm Pharmacol Ther. 2009;22(3):194–8. doi: 10.1016/j.pupt.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Chung KF, Caramori G, Adcock IM. Inhaled corticosteroids as combination therapy with beta-adrenergic agonists in airways disease: present and future. Eur J Clin Pharmacol. 2009;65(9):853–71. doi: 10.1007/s00228-009-0682-z. [DOI] [PubMed] [Google Scholar]
- 6.Humbert M, Andersson TL, Buhl R. Budesonide/formoterol for maintenance and reliever therapy in the management of moderate to severe asthma. Allergy. 2008;63(12):1567–80. doi: 10.1111/j.1398-9995.2008.01863.x. [DOI] [PubMed] [Google Scholar]
- 7.Cooper PR, Panettieri RA., Jr Steroids completely reverse albuterol-induced beta(2)-adrenergic receptor tolerance in human small airways. J Allergy Clin Immunol. 2008;122(4):734–40. doi: 10.1016/j.jaci.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 8.Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4(10):525–33. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- 9.Wendler A, Wehling M. Translational research on rapid steroid actions. Steroids. 2009 doi: 10.1016/j.steroids.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Mendes ES, Horvath G, Campos M, Wanner A. Rapid corticosteroid effect on beta(2)-adrenergic airway and airway vascular reactivity in patients with mild asthma. J Allergy Clin Immunol. 2008;121(3):700–4. doi: 10.1016/j.jaci.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 11.Horvath G, Mendes ES, Schmid N, Schmid A, Conner GE, Salathe M, et al. The effect of corticosteroids on the disposal of long-acting beta2-agonists by airway smooth muscle cells. J Allergy Clin Immunol. 2007;120(5):1103–9. doi: 10.1016/j.jaci.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 12.Papi A, Caramori G, Adcock IM, Barnes PJ. Rescue treatment in asthma. More than as-needed bronchodilation. Chest. 2009;135(6):1628–33. doi: 10.1378/chest.08-2536. [DOI] [PubMed] [Google Scholar]
- 13.Frost AE. Donor criteria and evaluation. Clinics In Chest Medicine. 1997;18(2):231–237. doi: 10.1016/s0272-5231(05)70374-9. [DOI] [PubMed] [Google Scholar]
- 14.Nlend MC, Bookman RJ, Conner GE, Salathe M. Regulator of G-protein signaling protein 2 modulates purinergic calcium and ciliary beat frequency responses in airway epithelia. American Journal of Respiratory Cell and Molecular Biology. 2002;27(4):436–45. doi: 10.1165/rcmb.2002-0012OC. [DOI] [PubMed] [Google Scholar]
- 15.Horvath G, Schmid N, Fragoso MA, Schmid A, Conner GE, Salathe M, et al. Epithelial organic cation transporters ensure pH dependent drug absorption in the airway. Am J Respir Cell Mol Biol. 2007;36(1):53–60. doi: 10.1165/rcmb.2006-0230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmid A, Bai G, Schmid N, Zaccolo M, Ostrowski LE, Conner GE, et al. Real-time analysis of cAMP-mediated regulation of ciliary motility in single primary human airway epithelial cells. J Cell Sci. 2006;119(Pt 20):4176–86. doi: 10.1242/jcs.03181. [DOI] [PubMed] [Google Scholar]
- 17.Kelly HW. Comparison of inhaled corticosteroids: an update. Ann Pharmacother. 2009;43(3):519–27. doi: 10.1345/aph.1L546. [DOI] [PubMed] [Google Scholar]
- 18.Koepsell H. Polyspecific organic cation transporters: their functions and interactions with drugs. Trends in Pharmacological Sciences. 2004;25(7):375–381. doi: 10.1016/j.tips.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Nies AT, Koepsell H, Damme K, Schwab M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol. 2011;201:105–67. doi: 10.1007/978-3-642-14541-4_3. [DOI] [PubMed] [Google Scholar]
- 20.Ehrhardt C, Kneuer C, Bies C, Lehr CM, Kim KJ, Bakowsky U. Salbutamol is actively absorbed across human bronchial epithelial cell layers. Pulm Pharmacol Ther. 2005;18(3):165–70. doi: 10.1016/j.pupt.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Dobson PD, Kell DB. Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule? Nat Rev Drug Discov. 2008;7(3):205–20. doi: 10.1038/nrd2438. [DOI] [PubMed] [Google Scholar]
- 22.Patton JS, Brain JD, Davies LA, Fiegel J, Gumbleton M, Kim KJ, et al. The particle has landed--characterizing the fate of inhaled pharmaceuticals. J Aerosol Med Pulm Drug Deliv. 2010;23(Suppl 2):S71–87. doi: 10.1089/jamp.2010.0836. [DOI] [PubMed] [Google Scholar]
- 23.Miller-Larsson A, Mattsson H, Hjertberg E, Dahlback M, Tunek A, Brattsand R. Reversible fatty acid conjugation of budesonide. Novel mechanism for prolonged retention of topically applied steroid in airway tissue. Drug Metab Dispos. 1998;26(7):623–30. [PubMed] [Google Scholar]
- 24.Ewing P, Ryrfeldt A, Sjoberg CO, Andersson P, Edsbacker S, Gerde P. Vasoconstriction after inhalation of budesonide: a study in the isolated and perfused rat lung. Pulm Pharmacol Ther. 2010;23(1):9–14. doi: 10.1016/j.pupt.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Selye H. The anesthetic effect of steroid hormones. Proc Soc Exp Biol Med. 1941;46:116–21. [Google Scholar]
- 26.Rodrigo GJ. Rapid effects of inhaled corticosteroids in acute asthma: an evidence-based evaluation. Chest. 2006;130(5):1301–11. doi: 10.1378/chest.130.5.1301. [DOI] [PubMed] [Google Scholar]
- 27.Volovitz B. Inhaled budesonide in the management of acute worsenings and exacerbations of asthma: a review of the evidence. Respir Med. 2007;101(4):685–95. doi: 10.1016/j.rmed.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Revollo JR, Cidlowski JA. Mechanisms generating diversity in glucocorticoid receptor signaling. Ann N Y Acad Sci. 2009;1179:167–78. doi: 10.1111/j.1749-6632.2009.04986.x. [DOI] [PubMed] [Google Scholar]
- 29.Johnson M. Pharmacodynamics and pharmacokinetics of inhaled glucocorticoids. Journal of Allergy and Clinical Immunology. 1996;97(1 Pt 2):169–76. doi: 10.1016/s0091-6749(96)80217-x. [DOI] [PubMed] [Google Scholar]
- 30.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31(1):143–78. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 31.Horvath G, Wanner A. Inhaled corticosteroids: effects on the airway vasculature in bronchial asthma. Eur Respir J. 2006;27(1):172–87. doi: 10.1183/09031936.06.00048605. [DOI] [PubMed] [Google Scholar]
- 32.Nonaka T, Nave R, McCracken N, Kawashimo A, Katsuura Y. Ciclesonide uptake and metabolism in human alveolar type II epithelial cells (A549) BMC Pharmacol. 2007;7:12. doi: 10.1186/1471-2210-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


