Abstract
Heart failure (HF) and atrial fibrillation (AF) are two of the most common cardiovascular diseases encountered in clinical practice, and the prevalence of these diseases continues to grow world-wide with the aging of the global population.
While recognizing that AF is a heterogeneous disorder, we submit that the parallels between AF and HF may arise because many cases of AF and HF result from the cumulative exposure of the atria and ventricles to a common set of systemic cardiovascular risk factors. Over time, exposure to risk factors promotes development of atrial and ventricular structural and functional abnormalities via activation of several biological pathways in concert: up-regulation of neurohormonal signaling cascades, release of inflammatory mediators, programmed cell death and fibrosis. Cardiac structural remodeling occurs in concert with electrophysiologic remodeling, both of which contribute to atrial and ventricular rhythm disturbances, including AF.
AF and HF, instead of representing distinct disease processes, often represent different endpoints along a disease continuum. By reviewing some of the mechanistic parallels between AF and HF, we hope to emphasize the connection between established cardiovascular risk factors, cardiac remodeling and AF, with a view to promoting strategies for AF prevention.
Keywords: atrial fibrillation, heart failure, atrial fibrillation prevention, epidemiology
Introduction
In his Shattuck lecture, Dr. Eugene Braunwald identified two epidemics of cardiovascular disease that continue to emerge despite advances in cardiovascular medicine: heart failure (HF) and atrial fibrillation (AF).[1] AF is now the most common arrhythmia encountered in clinical practice.[2] The prevalence of AF increases dramatically with age and it is present in 9% of individuals by the age of 80 years.[3] AF is associated with a lower quality of life, increased hospitalization rates, and a greater risk of stroke and mortality.[4] HF is also highly prevalent and is a major cause of morbidity and mortality worldwide.[5] Like AF, HF incidence increases with age and the condition is associated with substantial morbidity, mortality, and an impaired quality of life.[2]
HF and AF frequently coexist and each condition predisposes to the other.[2] AF is exceedingly common in HF, affecting 30% of all individuals with HF.[6] Men and women with AF have a 3- and 11-fold increased risk, respectively, of the combined endpoint of HF and all-cause mortality when compared to those in sinus rhythm.[2,5] Conversely, the development of HF is associated with a 4.5–5.9 fold increased risk of future AF.[5,6] As with HF, the increase in new cases of AF is attributable in part to the aging of the population and a concomitant increase in the prevalence of comorbidities, including the burden of cardiovascular risk factors.
The striking epidemiological similarities between AF and HF are mirrored by experimental and clinical studies that suggest significant mechanistic connections between AF and HF.[2,5,7,8] These similarities may be explained by the fact that many cases of AF and HF arise from cumulative exposure of the atria and ventricles, respectively, to a common set of cardiovascular risk factors (Figures 1 and 2).[9] These risk factors lead to atrial and ventricular sub-clinical structural and electrophysiological remodeling via activation of several biological pathways in concert: up-regulation of neurohormonal signaling cascades, release of inflammatory mediators, metabolic stress, programmed cell death and myocardial fibrosis.[7,8] The direct mechanisms linking sub-clinical cardiac remodeling to clinical AF are poorly defined, but structural atrial remodeling clearly facilitates AF in many patients.
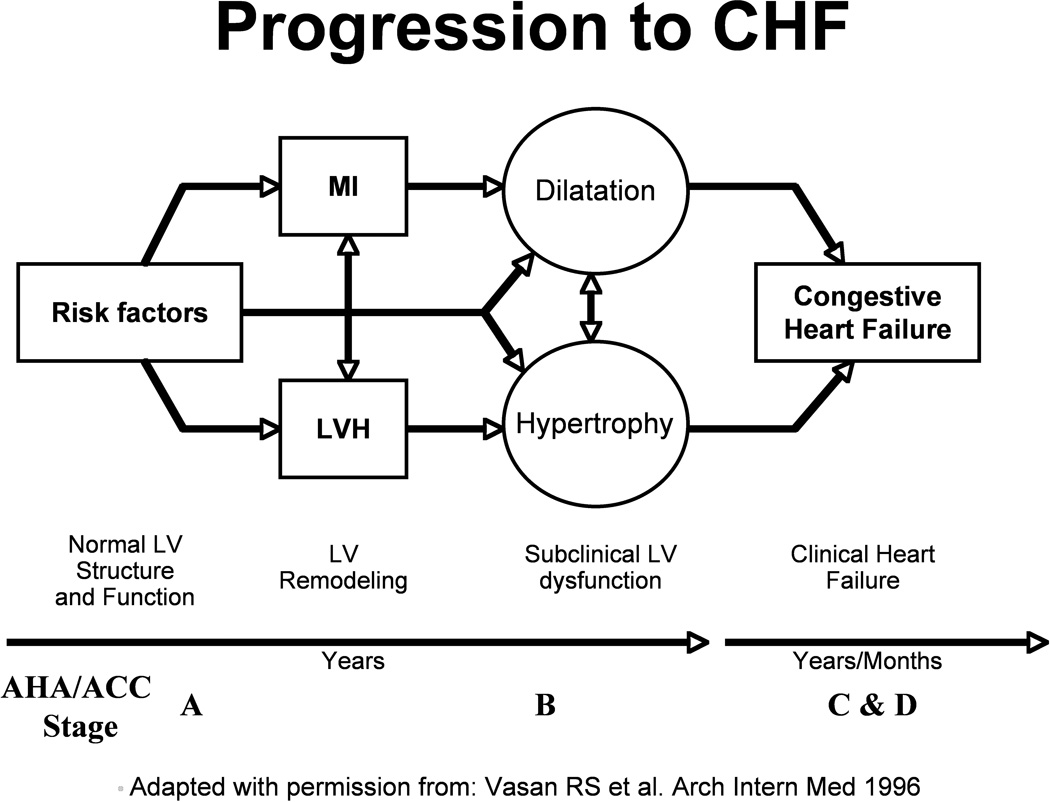
Figure 1.
Model of Progression to HF
LV = left ventricle, MI = myocardial infarction
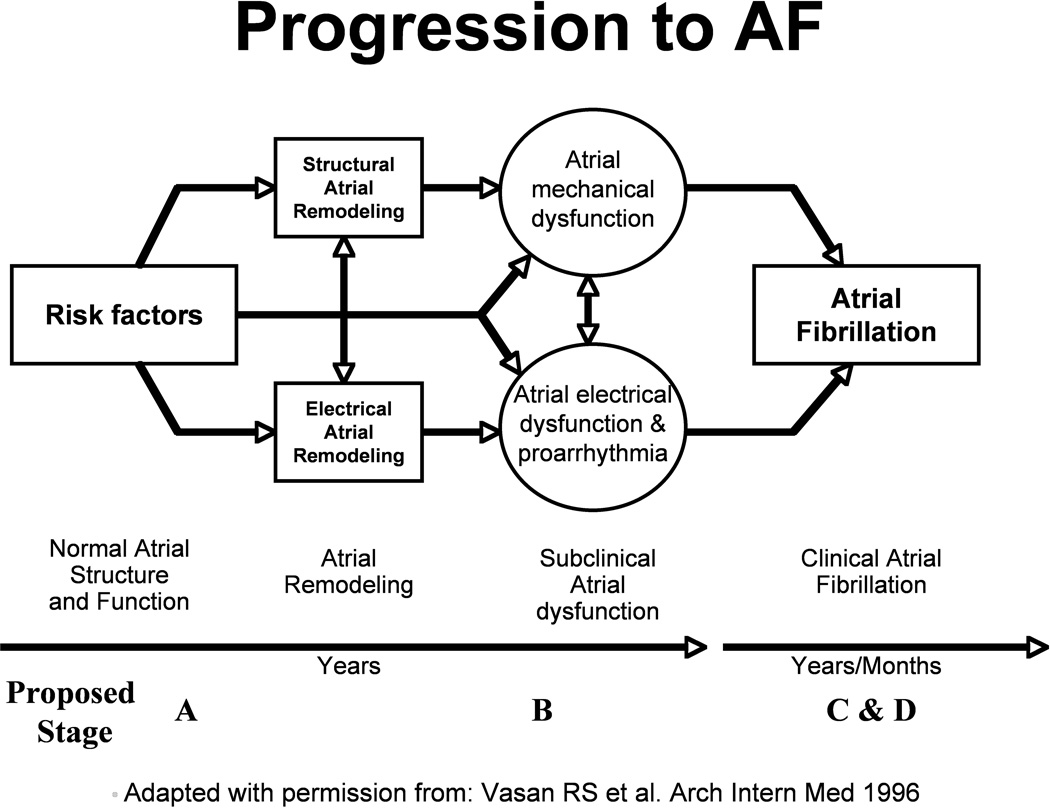
Figure 2.
Model of Progression to AF
AF = Atrial Fibrillation
While the pathophysiology of AF is still debated, significant discoveries have increased our understanding of this arrhythmia by highlighting the importance of focal triggers in initiating AF.[9] These observations have led to the development of new therapies for individuals with AF, including percutaneous and surgical AF ablation.[10] It is notable, however, that anti-arrhythmic and ablative therapies have greater efficacy in younger patients with paroxysmal AF than in older patients with multiple cardiovascular risk factors and persistent AF,[11] possibly because AF is a heterogeneous disorder with complex initiating and perpetuating mechanisms.[12,13] Pathological atrial structural remodeling often precedes development of AF and the presence of atrial enlargement is strongly associated with increased AF risk.[14] While enhanced automaticity plays a key role in triggering AF, pathological atrial remodeling also contributes substantially to AF initiation and maintenance in many individuals.
The conventional paradigm of regarding AF and HF as distinct disorders is challenged by the important epidemiological and mechanistic similarities between these disorders. In patients with sub-clinical ventricular remodeling, HF is often the clinical manifestation of underlying ventricular myopathy. Likewise, AF is often the clinical manifestation of atrial myopathy, especially in older individuals and those with cardiovascular disease risk factors. The interplay between genetic factors and the nature, chronicity and severity of risk factor exposure may help to explain why all individuals with AF do not develop HF, and vice versa. The purpose of this work is to review the genetic, mechanistic and epidemiological parallels between HF and AF, and to emphasize the strong connections between these diseases and cardiovascular risk factors. In contrast to prior reviews examining the dual epidemics of AF and HF,[7,8] we have drawn from the available literature in order to promote strategies for AF prevention in the community (Table 1).
Table 1.
Mechanisms and Features Common to both Atrial Fibrillation and Heart Failure
| Common Cardiovascular Risk Factors | Age, hypertension, diabetes, valvular disease, MI |
| Common Genetic Associations | Parental history, variation in angiotensin converting enzyme, angiotensinogen, angiotensin II type I receptor, IL-6, endothelial nitric oxide synthase genes |
| Common Morphologic and Hemodynamic Features | Increased intra-atrial and intra-ventricular volume and pressure, reduced CO |
| Common Intermediate Phenotypes | LV hypertrophy, LV enlargement, LA enlargement, reduced LV and LA EF |
| Common Mechanisms of Electrophysiologic Remodeling | |
| Stretch-related Mechanisms | Abnormal calcium handling, shortened action potential duration, decreased refractoriness, dispersion of the refractory period |
| Fibrosis-related Mechanisms | Disrupted cell-cell junctions, areas of delayed conduction, facilitation of re-entry |
| Independent Mechanisms | altered ion channel expression and density |
| Common Mechanisms of Structural Remodeling | |
| Neurohormonal signaling mechanisms | Renin-angiotensin-aldosterone system, TGF-β1, sympathetic nervous system |
| Tissue-level inflammatory mediators | CRP, TNF-α, IL-6 |
| Factors associated with extracellular remodeling | Increased collagen deposition, increased MMP activity |
| Cellular oxidative stress response | Myocyte apoptosis, myolysis |
MI = myocardial infarction, CO = cardiac output, LV = left ventricular, EF = ejection fraction, LA = left atrial, TGF = tissue growth factor, CRP =C-reactive protein, TNF = tumor necrosis factor, IL = interleukin, AII = angiotensin II, MMP = matrix metalloproteinase
AF and HF: Shared risk factors
AF shares strong epidemiologic associations with other cardiovascular diseases (CVD) and risk factors such as coronary artery disease, valvular heart disease, diabetes mellitus hypertension, obesity and obstructive sleep apnea (OSA).[15–18] These factors have been termed “upstream” risk factors, but the relationship between concomitant CVD and AF is incompletely understood and complex. In the Framingham Heart Study, history of myocardial infarction (MI) increased the risk for AF in men by 40%.[19] AF occurs often in post-MI patients and is independently associated with increased short- and long-term mortality in this setting.[20] History of valvular heart disease confers a 1.8- and 3.4-fold increased risk of AF in men and women, respectively.[19] Similarly, a 20-mmHg increase in pulse pressure confers a 26% increased risk of AF.[21] Diabetes mellitus and hypertension may have a synergistic effect on likelihood of developing AF, as diabetes has been associated with a 49% increased risk of AF in individuals with hypertension.[22] Obesity is another significant risk factor for AF, with each unit increase in body mass index conferring a 4% increased risk of AF.[17] Importantly, the association between obesity and AF appears to be mediated by atrial enlargement. It is also significant that, even after adjustment for body mass, OSA remains associated with a 2.2-fold increased risk of incident AF.[18]
Many of the conditions associated with AF also predispose to HF.[23] Hypertension confers a 2- to 3-fold increased risk of HF.[23] History of MI is associated with a 2-fold increased risk of HF.[23] Diabetes mellitus is associated with up to a 5-fold increased risk of HF.[24] As with AF, valvular heart disease is a risk factor for HF, accounting for about 1 in 10 cases.[23] Evidence suggests that obesity is also associated with increased risk of HF, with each unit increase in body mass index conferring a 5% and 7% risk of HF for men and women, respectively.[25] After adjustment for body mass, sleep apnea remains associated with a 2.4-fold increased risk of prevalent HF.[26]
AF and HF: Shared intermediate phenotypes
Cardiovascular risk factors impose hemodynamic, inflammatory, catecholaminergic, neurohumoral and metabolic stress on the atria and the ventricles. Sub-clinical phenotypes such as ventricular hypertrophy or atrial enlargement develop in response to these stressors and serve as useful markers of increased HF and AF risk, respectively. These intermediate phenotypes may appear to be initially adaptive, enabling the heart to compensate for increases in afterload and/or preload, or decreased contractility. With the passage of time, however, these initially compensatory mechanisms become maladaptive, leading to a decline in atrial and/or ventricular function. As the atria and ventricles are inexorably linked, any functional abnormality of one chamber may affect the other.
Echocardiographic and electrocardiographic measures of ventricular and atrial remodeling provide support for the hypothesis that AF and HF share common intermediate phenotypes.[27,28] Left ventricular wall thickness and electrocardiographic left ventricular hypertrophy, two well-established markers of hypertensive ventricular remodeling and HF, are also strongly associated with atrial enlargement and AF.[27] Left ventricular dilatation and systolic dysfunction post-MI predict future HF and also predict AF.[27,28] Pressure and volume-overload in left-sided valvular heart disease are associated with both ventricular and atrial enlargement.[29] Dilatation of either the atria or the ventricles in valvular as well as coronary heart disease predicts AF, HF and mortality.[30,31] In sum, ventricular remodeling, regardless of etiology, is associated with increased AF risk just as atrial enlargement is associated with increased HF risk.[32,33]
AF and HF: Shared neurohormonal signaling pathways
Structural remodeling seen in atria of individuals with AF is similar to that seen in myopathic ventricles from individuals with HF.[34–37] Chronic intra-atrial or intra-ventricular pressure overload activates common stress signaling pathways, most notably the renin angiotensin-aldosterone system (RAAS), which results in impaired myocardial vascular growth, myocyte apoptosis and interstitial fibrosis.[38]
Chronic RAAS activation has been shown to contribute to fibrosis and chamber remodeling in both AF and HF experimental studies.[35–37,39] Angiotensin-converting enzyme, Angiotensin II (AII) and transforming growth factor-beta 1 (TGF-β1) are up-regulated in a systemic and tissue-specific manner in response to atrial and ventricular stretch.[40] Mechanical stretch also induces fibroblasts to synthesize AII and TGF-β1.[41] AII and TGF-β1 induce downstream factors that promote local extracellular ventricular and atrial fibrosis.[42] AII and TGF-β1 also promote atrial and ventricular myocyte apoptosis and chamber dilatation. Experimental data suggest that the RAAS may also affect potassium channel function, action potential duration, and facilitate intra-atrial re-entry. In this fashion, the RAAS may directly promote AF independent of its effect on atrial structure or function.[43]
AF and HF: Shared mechanisms of inflammation and oxidative stress
Inflammation and oxidative stress play an important role in the pathogenesis of AF.[42] Histologic changes consistent with inflammation are seen in two-thirds of patients with AF and these changes are often seen prior to the onset of AF.[43] Over-expression of tumor necrosis factor-α (TNF-α), a well established inflammatory mediator, has been associated with atrial fibrosis, abnormal calcium handling, altered ion channel function, prolonged action potential duration and increased susceptibility to AF.[43] Circulating levels of C-reactive protein and interleukin-6, two other systemic inflammatory cytokines, are also predictive of future AF.[43]
It is well established that pro-inflammatory cytokines also contribute to the pathogenesis of ventricular dysfunction and HF.[44] Circulating levels of these cytokines independently predict worsened functional class and mortality in individuals with HF.[45] As is true in individuals with AF, TNF-α and interleukin-6 are important regulators of ventricular myocyte cell death in individuals with left ventricular remodeling and HF.[45]
AF and HF: Shared mechanisms of extracellular fibrosis
Atrial fibrosis is the predominant pathologic abnormality seen in AF-related structural remodeling and the degree of fibrosis has clinical significance.[14] Fibrosis results from increased deposition of dense, disorganized collagen in the extracellular space and occurs in the setting of myocyte atrophy and apoptosis.[14] Atrial fibrosis is mediated by altered expression of matrix metalloproteinases (MMPs) in response to neurohormonal signaling, inflammation and oxidative stress.[35–37] Increased MMP activity correlates with both degree of fibrosis and duration of AF.[46] Atrial interstitial fibrosis increases AF vulnerability in animal and transgenic models for selective fibrosis.[46] Atrial fibrosis often precedes development of AF and the degree of fibrosis correlates with AF persistence.[46] The severity of fibrosis also correlates with the duration of AF as well as with the likelihood of restoration of sinus rhythm with cardioversion.[47]
As in AF, accumulation of extracellular collagen and fibrosis play important roles in both ventricular dilatation and hypertrophic ventricular remodeling in HF.[48] Fibrosis typically occurs in a localized fashion in ischemic cardiomyopathy and diffusely in pressure-overload and hypertrophic cardiomyopathies. MMPs have also been shown to mediate ventricular remodeling in HF.[48] Increased MMP expression predicts transition from hypertrophy to HF and is inversely correlated with ventricular function.[48]
AF and HF: Electrophysiological Parallels
Pacing-induced HF models have yielded valuable information about how atrial structural remodeling affects the electrophysiological properties of the atrium.[49] These models have convincingly demonstrated that atrial ion channel expression, distribution and function are profoundly linked to ventricular structure and function.[50] Atrial fibrosis develops in these models and disrupts atrial cell-to-cell junctions and myocyte coupling, causing regional electrical silence, abnormal calcium handling, decreased atrial refractoriness, and dispersion of the atrial refractory period.[50,51] Atrial fibrosis causes localized conduction slowing and unidirectional block, thereby increasing conduction heterogeneity and providing a substrate for both macro-reentrant and focal tachyarrhythmias.[14] Mechanical stretch may directly modulate atrial myocyte electrical activity via a pro-arrhythmic mechanism known as mechano-electric feedback and indirectly by up-regulation AII expression.[52] Another pro-arrhythmic mechanism seen in HF models is the dysregulated expression of ion channels and modulator proteins in the atrium. This results in shortening of the atrial effective refractory period, promotion of multiple wave reentry and facilitation of AF.[53,54] Atrial structural remodeling promotes atrial electrical anisotropy, thereby facilitating AF and providing a plausible electrophysiologic mechanism linking risk factors to AF as well as justification for AF prevention through risk factor modification.
AF and HF: Common Genetic Associations
Parental history of HF is associated with an almost 70% increase in HF risk and parental history of AF is associated with an almost two-fold increase in AF risk among offspring.[55,56] Traditional mapping and cloning studies have shown that mutations in ion-channel genes, including several encoding sodium and potassium channels, explain a small proportion of AF cases.[57] Case-control and genome-wide association studies suggest that a much larger proportion of AF cases may be explained by common variation in a small number of genes.[57] Interestingly, a number of these genetic polymorphisms occur in genes controlling the production of angiotensin converting enzyme, the AII receptor, interleukin-6, and endothelial nitric oxide synthase.[57] As has been discussed previously, these gene products are involved in myocardial fibrosis, inflammation and cardiac remodeling, respectively. Importantly, many of these genes and gene products have also been associated with ventricular hypertrophy, ventricular enlargement and HF.[58] Ongoing genome-wide association research promises to yield further insight into the genetic predictors of AF and HF. Genetic polymorphisms may help to explain the considerable variance in clinical presentation seen among patients exposed to cardiovascular risk factors.
AF: Lessons for AF Prevention from HF epidemiology
Several schemes have been proposed for the classification of AF, but none fully account for all aspects of AF.[59] Characterization of AF based on presence or absence of symptoms, duration or response to cardioversion, while clinically relevant, do not adequately describe the underlying mechanisms of this arrhythmia. The limitations of these descriptors make individualized treatment and clinical trial design as well as interpretation difficult. For example, a patient with paroxysmal and symptomatic AF may have as severe an atrial myopathy as another patient with persistent and asymptomatic AF.
When thinking about AF prevention and treatment, it may be helpful to consider the HF disease model proposed by the American College of Cardiology/American Heart Association in 2001 (Figure 1).[60] The advantage of this pathophysiological model is that it recognizes the importance of cardiovascular risk factors for the development of HF and emphasizes that risk factors evolve over the adult life course. Like HF, AF is often a phenotypic manifestation of exposure of the heart to cardiovascular risk factors. Though AF develops suddenly, underlying atrial electrical and structural remodeling often precedes clinical AF and is strongly linked to cardiovascular risk factors (Figure 2). As was recently emphasized by an expert panel, AF prevention has received relatively little attention despite an increasing prevalence.[61] Parallels between AF and HF would suggest that an effort should be made to develop AF prevention strategies that focus on patients with cardiovascular risk factors and/or intermediate phenotypes associated with AF. Adaptation of current conceptual models of AF to accommodate the existence and importance of AF risk factors and intermediate phenotypes may enhance disease prevention efforts through more aggressive risk factor modification in at-risk individuals. Although existing data have not yet convincingly demonstrated that treatment of hypertension, diabetes or coronary artery disease modifies the natural history of AF, the risk signals are sufficiently strong to justify enhanced attention to guideline-directed therapies for these conditions as a plausible way of reducing the incidence of AF.
Limitations
It is clear that while AF results from structural and electrophysiologic remodeling in many patients, the complexity of this arrhythmia dictates recognition that this is not the case for all patients with AF. The disease model proposed by the American College of Cardiology/American Heart Association for HF has limited value when applied to those patients with AF due exclusively to enhanced pulmonary vein automaticity. Though the electrocardiographic manifestation of AF may be similar in the patient with AF but no structural heart disease and the patient with AF and structural heart disease, patients with truly isolated AF likely have a distinct electrophysiologic disorder strongly linked to familial predisposition. While lone AF has been associated in some patients with development of ventricular myopathy and HF, the majority of these patients do not have significant atrial myopathy.
Conclusions
AF is likely to affect 3% of the population by 2050. One in four individuals 40 years of age and older will develop AF during their lifetime.[2] The weight of current evidence suggests that most cases of AF and HF result from exposure of the heart to a common set of systemic cardiovascular risk factors and that HF and AF share common genetic predictors as well as mechanisms of structural and electrophysiologic remodeling.[2,7,8] Early identification and treatment of these risk factors may help to slow or prevent the onset of AF. Reframing AF as a disease process that develops over the life course of an individual may add a useful dimension to our thinking by helping to emphasize that established risk factors and cardiac remodeling are essential for the development and maintenance of AF in many patients. Further investigation is needed to evaluate the role of CV risk modification, particularly treatment of hypertension, on the development of future AF.
Acknowledgements
Our work was supported by the National Heart, Lung and Blood Institute's Framingham Heart Study (Contract No. N01-HC-25195), 6R01-NS 17950, 2 K24 HL04334, RO1HL080124. The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology.[69]
References
- 1.Braunwald E. Shattuck lecture--cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 3.Page RL. Clinical practice. Newly diagnosed atrial fibrillation. N Engl J Med. 2004;351:2408–2416. doi: 10.1056/NEJMcp041956. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson WG, Stevenson LW, Middlekauff HR, et al. Improving survival for patients with atrial fibrillation and advanced heart failure. J Am Coll Cardiol. 1996;28:1458–1463. doi: 10.1016/s0735-1097(96)00358-0. [DOI] [PubMed] [Google Scholar]
- 7.Anter E, Jessup M, Callans D. Atrial Fibrillation and Heart Failure Treatment Considerations for a Dual Epidemic. Circulation. 2009;119:2516–2525. doi: 10.1161/CIRCULATIONAHA.108.821306. [DOI] [PubMed] [Google Scholar]
- 8.Morrison B, Bunch J, Gersh BJ. Pathophysiology of concomitant atrial fibrillation and heart failure: implications for management. Nat Clin Pract Cardiovasc Med. 2009;6:45–56. doi: 10.1038/ncpcardio1414. [DOI] [PubMed] [Google Scholar]
- 9.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 10.Pappone C, Rosanio S, Oreto G, et al. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. 2000;102:2619–2628. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- 11.Hsu LF, Jais P, Sanders P, et al. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351:2373–2383. doi: 10.1056/NEJMoa041018. [DOI] [PubMed] [Google Scholar]
- 12.Ehrlich JR, Nattel S, Hohnloser SH. Atrial fibrillation and congestive heart failure: specific considerations at the intersection of two common and important cardiac disease sets. J Cardiovasc Electrophysiol. 2002;13:399–405. doi: 10.1046/j.1540-8167.2002.00399.x. [DOI] [PubMed] [Google Scholar]
- 13.Allessie MA, Boyden PA, Camm AJ, et al. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001;103:769–777. doi: 10.1161/01.cir.103.5.769. [DOI] [PubMed] [Google Scholar]
- 14.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 15.Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 16.Psaty BM, Furberg CD, Kuller LH, et al. Isolated systolic hypertension and subclinical cardiovascular disease in the elderly. Initial findings from the Cardiovascular Health Study. JAMA. 1992;268:1287–1291. [PubMed] [Google Scholar]
- 17.Wang TJ, Parise H, Levy D, et al. Obesity and the Risk of New-Onset Atrial Fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 18.Kanagala R, Murali NS, Friedman PA, et al. Obstructive Sleep Apnea and the Recurrence of Atrial Fibrillation. Circulation. 2003:2589–2594. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 19.Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008;92:17–40. ix. doi: 10.1016/j.mcna.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes RD, Pieper KS, Horton JR, et al. Short- and Long-Term Outcomes Following Atrial Fibrillation in Patients With Acute Coronary Syndromes With or Without ST-Segment Elevation. Heart. 2008;94:867–873. doi: 10.1136/hrt.2007.134486. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell GF, Vasan RS, Keyes MJ, et al. Pulse pressure and risk of new-onset atrial fibrillation. JAMA. 2007;297:709–715. doi: 10.1001/jama.297.7.709. [DOI] [PubMed] [Google Scholar]
- 22.Aksnes TA, Schmieder RE, Kjeldsen SE, Ghani S, Hua TA, Julius S. Impact of New-Onset Diabetes Mellitus on Development of Atrial Fibrillation and Heart Failure in High-Risk Hypertension (from the VALUE Trial) Am J Cardiol. 2008;101:634–638. doi: 10.1016/j.amjcard.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 24.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 25.Kenchaiah S, Evans JC, Levy D, et al. Obesity and Risk of Heart Failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 26.Shahar E, Whitney C, Redline S, et al. Sleep-disordered breathing and Cardiovascular Disease: Cross-Sectional Results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 27.Tsang TS, Barnes ME, Bailey KR, et al. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76:467–475. doi: 10.4065/76.5.467. [DOI] [PubMed] [Google Scholar]
- 28.Braunwald E, Pfeffer MA. Ventricular enlargement and remodeling following acute myocardial infarction: mechanisms and management. Am J Cardiol. 1991;68:1D–6D. doi: 10.1016/0002-9149(91)90255-j. [DOI] [PubMed] [Google Scholar]
- 29.Boudoulas H, Boudoulas D, Sparks EA, Pearson AC, Nagaraja HN, Wooley CF. Left atrial performances indices in chronic mitral valve disease. J Heart Valve Dis. 1995;4 Suppl 2:S242–S247. discussion S248. [PubMed] [Google Scholar]
- 30.Goldberg RJ, Seeley D, Becker RC, et al. Impact of atrial fibrillation on the in-hospital and long-term survival of patients with acute myocardial infarction: a community-wide perspective. Am Heart J. 1990;119:996–1001. doi: 10.1016/s0002-8703(05)80227-3. [DOI] [PubMed] [Google Scholar]
- 31.Osranek M, Fatema K, Qaddoura F, et al. Left atrial volume predicts the risk of atrial fibrillation after cardiac surgery: a prospective study. J Am Coll Cardiol. 2006;48:779–786. doi: 10.1016/j.jacc.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 32.Ristow B, Ali S, Whooley MA, Schiller NB. Usefulness of left atrial volume index to predict heart failure hospitalization and mortality in ambulatory patients with coronary heart disease and comparison to left ventricular ejection fraction (from the Heart and Soul Study) Am J Cardiol. 2008;102(1):70–76. doi: 10.1016/j.amjcard.2008.02.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciaroni S, Cuenoud L, Bloch A. Clinical study to investigate the predictive parameters for the onset of atrial fibrillation in patients with essential hypertension. Am Heart J. 2000;139(5):814–819. doi: 10.1016/s0002-8703(00)90012-7. [DOI] [PubMed] [Google Scholar]
- 34.Bauer A, McDonald AD, Donahue JK. Pathophysiological findings in a model of persistent atrial fibrillation and severe congestive heart failure. Cardiovasc Res. 2004;61:764–770. doi: 10.1016/j.cardiores.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Fedak PW, Verma S, Weisel RD, Li RK. Cardiac remodeling and failure From molecules to man (Part II) Cardiovasc Pathol. 2005;14:49–60. doi: 10.1016/j.carpath.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Fedak PW, Verma S, Weisel RD, Li RK. Cardiac remodeling and failure: from molecules to man (Part I) Cardiovasc Pathol. 2005;14:1–11. doi: 10.1016/j.carpath.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Fedak PW, Verma S, Weisel RD, Skrtic M, Li RK. Cardiac remodeling and failure: from molecules to man (Part III) Cardiovasc Pathol. 2005;14:109–119. doi: 10.1016/j.carpath.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Brundel BJ, Henning RH, Kampinga HH, Van Gelder IC, Crijns HJ. Molecular mechanisms of remodeling in human atrial fibrillation. Cardiovasc Res. 2002;54:315–324. doi: 10.1016/s0008-6363(02)00222-5. [DOI] [PubMed] [Google Scholar]
- 39.Ehrlich JR, Hohnloser SH, Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. Eur Heart J. 2006;27:512–518. doi: 10.1093/eurheartj/ehi668. [DOI] [PubMed] [Google Scholar]
- 40.Goette A, Lendeckel U, Klein HU. Signal transduction systems and atrial fibrillation. Cardiovasc Res. 2002;54:247–258. doi: 10.1016/s0008-6363(01)00521-1. [DOI] [PubMed] [Google Scholar]
- 41.Carver W, Nagpal ML, Nachtigal M, Borg TK, Terracio L. Collagen expression in mechanically stimulated cardiac fibroblasts. Circ Res. 1991;69:116–122. doi: 10.1161/01.res.69.1.116. [DOI] [PubMed] [Google Scholar]
- 42.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 43.Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol. 2007;50:2021–2028. doi: 10.1016/j.jacc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 44.Testa M, Yeh M, Lee P, et al. Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J Am Coll Cardiol. 1996;28:964–971. doi: 10.1016/s0735-1097(96)00268-9. [DOI] [PubMed] [Google Scholar]
- 45.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 46.Xu J, Cui G, Esmailian F, et al. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation. 2004;109:363–368. doi: 10.1161/01.CIR.0000109495.02213.52. [DOI] [PubMed] [Google Scholar]
- 47.Bailey GW, Braniff BA, Hancock EW, Cohn KE. Relation of left atrial pathology to atrial fibrillation in mitral valvular disease. Ann Intern Med. 1968;69:13–20. doi: 10.7326/0003-4819-69-1-13. [DOI] [PubMed] [Google Scholar]
- 48.Weber KT, Brilla CG, Janicki JS. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res. 1993;27:341–348. doi: 10.1093/cvr/27.3.341. [DOI] [PubMed] [Google Scholar]
- 49.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100(1):87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 50.Savelieva I, Camm J. Update on atrial fibrillation: part I. Clin Cardiol. 2008;31:55–62. doi: 10.1002/clc.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Everett TH, Olgin JE. Atrial fibrosis and mechanisms of atrial fibrillation. Heart Rhythm. 2007;4(3 Suppl):S24–S27. doi: 10.1016/j.hrthm.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamkin A, Kiseleva I, Lozinsky I, Scholz H. Electrical interaction of mechanosensitive fibroblasts and myocytes in the heart. Basic Res Cardiol. 2005;100:337–345. doi: 10.1007/s00395-005-0529-4. [DOI] [PubMed] [Google Scholar]
- 53.Everett TH, Li H, Mangrum JM, et al. Electrical, morphological, and ultrastructural remodeling and reverse remodeling in a canine model of chronic atrial fibrillation. Circulation. 2000;102:1454–1460. doi: 10.1161/01.cir.102.12.1454. [DOI] [PubMed] [Google Scholar]
- 54.Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res. 1997;81:512–525. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]
- 55.Lee DS, Pencina MJ, Benjamin EJ, et al. Association of Parental Heart Failure with Risk of Heart Failure in Offspring. New Engl J Med. 2006;355:138–147. doi: 10.1056/NEJMoa052948. [DOI] [PubMed] [Google Scholar]
- 56.Fox CS, Parise H, D’Agostino RB, et al. Parental Atrial Fibrillation as a Risk Factor for Atrial Fibrillation in Offspring. JAMA. 2004;291(23):2851–2855. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- 57.Tsai CT, Lai LP, Hwang JJ, Lin JL, Chiang FT. Molecular genetics of atrial fibrillation. J Am Coll Cardiol. 2008;52(4):241–250. doi: 10.1016/j.jacc.2008.02.072. [DOI] [PubMed] [Google Scholar]
- 58.Bleumink GS, Schut AF, Sturkenboom MC, Deckers JW, van Duijn CM, Stricker BH. Genetic polymorphisms and heart failure. Genet Med. 2004;6(6):465–474. doi: 10.1097/01.gim.0000144061.70494.95. [DOI] [PubMed] [Google Scholar]
- 59.Fuster V, Ryden LE, Cannom DS, et al. American College of Cardiology/American Heart Association/European Society of Cardiology 2006 Guidelines for the Management of Patients with Atrial Fibrillation. J Am Coll Cardiol. 2006;48:e149–e246. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 60.Hunt SA, Baker DW, Chin MH, et al. ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation. 2001;104:2996–3007. doi: 10.1161/hc4901.102568. [DOI] [PubMed] [Google Scholar]
- 61.Benjamin EJ, Chen PS, Bild DE, et al. Prevention of Atrial Fibrillation: Report From a National Heart, Lung, and Blood Institute Workshop. Circulation. 2009;119:606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pedersen OD, Bagger H, Kober L, et al. Trandolapril reduces the incidence of atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation. 1999 Jul 27;100(4):376–380. doi: 10.1161/01.cir.100.4.376. [DOI] [PubMed] [Google Scholar]
- 63.Madrid AH, Bueno MG, Rebollo JM, et al. Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: a prospective and randomized study. Circulation. 2002 Jul 16;106(3):331–336. doi: 10.1161/01.cir.0000022665.18619.83. [DOI] [PubMed] [Google Scholar]
- 64.ACTIVE Investigators. Rationale and design of ACTIVE: the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events. Am Heart J. 2006 Jun;151(6):1187–1193. doi: 10.1016/j.ahj.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 65.Cleland JG, Coletta AP, Yassin A, et al. Clinical trials update from the European Society of Cardiology Meeting 2009: AAA, RELY, PROTECT, ACTIVE-I, European CRT survey, German pre-SCD II registry, and MADIT-CRT. Eur J Heart Fail. 2009 Dec 11;12:1214–1219. doi: 10.1093/eurjhf/hfp162. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Zhang P, Mu Y, et al. The role of renin-angiotensin system blockade therapy in the prevention of atrial fibrillation: a meta-analysis of randomized controlled trials. Clin Pharmacol Ther. 2010 Oct;88(4):521–531. doi: 10.1038/clpt.2010.123. [DOI] [PubMed] [Google Scholar]
- 67.Moro C, Hernández-Madrid A, Matía R. Non-antiarrhythmic drugs to prevent atrial fibrillation. Am J Cardiovasc Drugs. 2010;10(3):165–173. doi: 10.2165/11537270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 68.Young-Xu Y, Jabbour S, Goldberg R, et al. Usefulness of statin drugs in protecting against atrial fibrillation in patients with coronary artery disease. Am J Cardiol. 2003 Dec 15;92(12):1379–1383. doi: 10.1016/j.amjcard.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 69.Coats AJ. Ethical authorship and publishing. Int J Cardiol. 2009;131:149–150. doi: 10.1016/j.ijcard.2008.11.048. [DOI] [PubMed] [Google Scholar]