Abstract
The two Siz/PIAS SUMO E3 ligases Siz1 and Siz2 are responsible for the vast majority of sumoylation in Saccharomyces cerevisiae. We found that siz1Δ siz2Δ mutants are sensitive to UV light. Epistasis analysis showed that the SIZ genes act in the nucleotide excision repair (NER) pathway, and suggested that they participate both in global genome repair (GGR) and in the Rpb9-dependent subpathway of transcription-coupled repair (TCR), but have minimal role in Rad26-dependent TCR. Quantitative analysis of NER at the single-nucleotide level showed that siz1Δ siz2Δ is deficient in repair of both the transcribed and non-transcribed strands of the DNA. These experiments confirmed that the SIZ genes participate in GGR. Their role in TCR remains unclear. It has been reported previously that mutants deficient for the SUMO conjugating enzyme Ubc9 contain reduced levels of Rad4, the yeast homolog of human XPC. However, our experiments do not support the conclusion that SUMO conjugation affects Rad4 levels. We found that several factors that participate in NER are sumoylated, including Rad4, Rad16, Rad7, Rad1, Rad10, Ssl2, Rad3, and Rpb4. Although Rad16 was heavily sumoylated, elimination of the major SUMO attachment sites in Rad16 had no detectable effect on UV resistance or removal of DNA lesions. SUMO attachment to most of these NER factors was significantly increased by DNA damage. Furthermore, SUMO-modified Rad4 accumulated in NER mutants that block the pathway downstream of Rad4, suggesting that SUMO becomes attached to Rad4 at a specific point during its functional cycle. Collectively, these results suggest that SIZ-dependent sumoylation may modulate the activity of multiple proteins to promote efficient NER.
Keywords: Smt3, ubiquitin, protein modification, protein degradation
1. Introduction
The ubiquitin-related protein SUMO functions by becoming covalently attached to other proteins as a post-translational modification. SUMO conjugation is an essential process in Saccharomyces cerevisiae and in most other eukaryotic cells [1-5]. Sumoylation participates in many cellular processes, including DNA replication and repair [1-5]. In S. cerevisiae, the SUMO conjugation pathway is composed of an E1 activating enzyme Uba2-Aos1, the E2 conjugating enzyme Ubc9 and four E3 ligases: Siz1, Siz2, Mms21, and the meiosis-specific E3 Zip3 [5]. Attachment of SUMO to substrate proteins is a reversible process, and yeast encodes two SUMO-specific deconjugases Ulp1 and Ulp2.
Siz1 and Siz2 belong to a conserved family of SUMO E3 ligases that includes the mammalian PIAS proteins. Siz1 and Siz2 are required for at least 90% of global protein sumoylation in yeast [6]. SIZ1 and SIZ2 each have some distinct substrates, but also show considerable substrate overlap [6-9]. siz1Δ siz2Δ has no obvious phenotypes, other than growth defects related to hyperaccumulation of the native 2μm circle plasmid [10]. In the absence of 2μm, siz1Δ siz2Δ has a near wt growth rate and is not more sensitive than wt to most DNA damaging agents [11]. However, siz1Δ siz2Δ cells show moderate sensitivity to ultra-violet (UV) irradiation [11]. Here we analyzed this phenotype and examined the roles of SIZ1 and SIZ2 in NER.
UV irradiation induces DNA damage predominantly in the form of cyclobutane pyrimidine dimers (CPDs) and pyrimide pyrimidone photoproducts (6-4PPs) [12-14]. Removal of bulky lesions such as these is carried out by the nucleotide excision repair (NER) pathway. NER is catalyzed by at least 30 proteins, and this repair pathway is conserved in all eukaryotes [12-14]. Mutations in genes encoding NER factors are the cause of the human autosomal recessive disorder Xeroderma pigmentosum (XP), which is characterized by a ~2000-fold increase in the rate of skin cancer [12-14].
The lesion recognition step of NER is divided into two subpathways: transcription-coupled repair (TCR) and global genome repair (GGR). TCR repairs DNA damage specifically in actively transcribed regions of the genome and recognizes lesions as the RNA polymerase II (RNAPII) complex becomes stalled at bulky adducts [12-14]. Thus, TCR acts exclusively on the transcribed (template) strand (TS). TCR is partially dependent on the yeast Rad26 protein, homolog of human CSB [15]. Additionally, there is a Rad26-independent TCR subpathway that depends on the RNAPII subunit Rpb9 [16]. GGR can repair damage throughout the genome, including the non-transcribed strand (NTS) of actively transcribed genes. In yeast GGR absolutely requires a multiprotein complex containing Rad7 and Rad16 [17, 18]. The Rad7-Rad16 complex binds specifically to UV-damaged DNA in an ATP-dependent manner [19, 20]. It also has ubiquitin ligase (E3) activity and stimulates conjugation of ubiquitin to the lesion-binding NER protein Rad4 [21]. When TCR is absent or defective, GGR can readily repair transcribed regions of the genome [15, 18]. Consequently, cells with mutations in genes that participate only in TCR tend to be more resistant to UV than mutants in GGR.
Downstream of lesion recognition, the repair process is the same for both TCR and GGR [12-14]. First, repair factors are recruited to the site of DNA damage. Next, DNA is unwound to expose the damaged region by the helicase subunits of TFIIH. Dual-incision of an approximate 25 bp region of DNA containing the damaged bases is carried out by endonucleases Rad1-Rad10 (5′-cleavage) and Rad2 (3′-cleavage). Finally, repair synthesis of the gapped region is conducted by polymerases, and the DNA strands are ligated.
There are two lesion-binding proteins that are required for all NER in yeast. The first of these to bind at the site of repair is Rad4, the yeast homolog of human XPC, which, together with its binding partner Rad23, binds directly to the DNA surrounding the CPD and flips out the damaged bases [22]. Subsequently, Rad14, homolog of human XPA, binds to the lesion together with its binding partners, the endonuclease subunits Rad1 and Rad10. Yeast mutants lacking either RAD4 or RAD14 are completely unable to carry out NER and are extremely sensitive to UV light [13].
No function has yet been described for sumoylation in the NER pathway, although some NER-related factors have been reported to be sumoylated. The yeast RNAPII subunit Rpb1 is sumoylated in response to UV irradiation [23]. Sumoylation of Rpb1 does not affect NER but is instead implicated in regulating UV-induced activation of the checkpoint kinase Rad53 [23]. It has been reported that levels of Rad4 are significantly reduced in yeast mutants that are deficient in the SUMO E2 enzyme Ubc9 [20]. XPC, the human homolog of Rad4, has been shown to be modified by SUMO, and it was proposed that sumoylation of XPC may protect it from degradation after UV irradiation [24, 25].
In the current study we characterized the role of SIZ1 and SIZ2 in NER. Our results indicated that siz1Δ siz2Δ mutants have defects in GGR, and possibly also in TCR. We also showed that several NER proteins exhibit UV-inducible sumoylation, consistent with the possibility that they become sumoylated at a specific stage in the NER process.
2. Materials and Methods
2.1 Media, strains, and genetic techniques
Standard techniques were used [26]. Rich yeast medium containing 2% glucose (YPD) or 2% galactose (YPG) and synthetic yeast medium was prepared as previously described [27]. Strains used in this study are listed in Supplemental Table 1 and are all derived from the JD52 wt strain, except the ubc9-ts strain and the congenic wt. Mutations, deletion alleles, and C-terminal epitope tags were introduced into native loci in chromosomal sequences by transforming strains with appropriate PCR products, as described [28]. The sequence of the His8-HA (hemagglutinin) tag was GHHHHHHHHGYPYDVPDYAAFL. Ssl2 and Tfb2 were tagged with His8 alone, because the His8-HA tag had adverse effects on function. Oligonucleotide sequences are available on request.
2.2 Spot tests and survival curves
For spot tests, cells were grown overnight and diluted to 2 × 107 cells/ml. Ten-fold serial dilutions were made and 3 μl aliquots were spotted onto YPD plates. Plates were either untreated (NT) or UV-irradiated at indicated doses and incubated in the dark at 30°C. Plates were photographed after 2-4 days of incubation. For UV survival curves, overnight cultures were diluted and plated onto YPD at appropriate concentrations. Plates were UV irradiated at indicated doses and grown in the dark for 3 days at 30°C. Colonies were counted and survival was calculated. Values shown represent averages of at least 3 independent experiments; error bars represent standard deviations.
2.3 CPD repair analysis
DNA was prepared as described [29] from yeast that had been resuspended in cold (4°C) PBS at 2 X 107 cells/ml, irradiated at 150 J/m2 using a Stratalinker 1800 (Stratagene, La Jolla, CA) containing 254 nm UV bulbs, resuspended in YPD or YPG, and grown in the dark for the indicated times. Repair of RPB2 and MFA2 gene fragments was analyzed as reported [29-31]. Genomic DNA was digested with HaeIII, followed by cleavage at CPDs using T4 endonuclease V (a gift from Stephen Lloyd, Oregon Health and Science University). DNAs were then annealed to biotinylated primers and bound to Dynabeads M-280 Streptavidin (Invitrogen). After washing, DNA fragments were labeled using [α32P]dATP (3000 Ci/mmol, Perkin Elmer) and Sequenase (Amersham). Labeled fragments were eluted in formamide-containing loading buffer and loaded on 6% denaturing polyacrylamide-sequencing gel and electrophoresed at 40 V/cm for approximately 3 h. The gel was exposed to a phosphorimager screen and imaged. Intensities of bands were quantified with ImageQuant software (GE Heathcare). Sequencing reactions to determine the transcription start site for TS analysis were performed as described [29]. Primers used in CPD analysis: RPB2-TS: 5′ biotin- gatagcTTTTTTCCATCTTAAAATAATTTGTTACCCTGCTTGGC; RPB2-NTS, 5′ biotin-gatagcTTTTTTCCAATGAAAACTTTTCCGCTTTCGCTGTC; MFA-NTS (courtesy of Yumin Teng, Cardiff), 5′ biotingatagcTTTTTTCCCTTGATTATATAGATTGTCTTTCTTTTCAGAGGAT.
Lower case gatagc is a hexamer to prevent steric hindrance between the biotin-bead interaction and Sequenase. Poly T that is the template for labeling with [α32P]dATP is in bold capital letters. The remaining sequence is specific for the strand of interest at the HaeIII site.
2.4 Preparation of cells for Ni-NTA protein purification
~ 1 X 109 yeast cells were suspended in cold PBS at 2 X 107 cells/ml and UV irradiated at 150 J/m2, as described for DNA preparations above. Subsequent steps up to freezing the cells were completed in the dark. Cells were then resuspended in YPD, incubated at 30°C for 30 m, cooled on ice for 10 m, and isolated by centrifugation. Cell pellets were frozen in liquid N2. The cells were lysed in NaOH, followed by TCA precipitation, and HA- and His8-tagged proteins were purified by Ni-NTA affinity chromatography in the presence of 6 M guanidine-HCl as described [10, 32].
2.5 Antibodies and immunoblot analysis
Yeast whole-cells lysates were prepared by lysis in NaOH [33]. Proteins were detected by immunoblotting using either HRP-conjugated secondary Abs and chemiluminescent detection (Supersignal West Pico; Pierce) or fluorescently labeled secondary Abs followed by detection with an Odyssey infrared imaging system (LI-COR Biosciences). The following antibodies were used: affinity-purified rabbit polyclonal antibody (Ab) against Smt3 (SUMO) [28], the 16B12 monoclonal Ab against the HA epitope (Covance Research Products), the Penta His Ab (5 Prime), and a rabbit Ab against Rad4 [21]. An anti-PSTAIR mouse monoclonal antibody (Sigma) was used as a loading control on Rad4 immunoblots. For quantification of immunoblot signals, secondary antibodies coupled to fluorescent dyes IRDye 800 (Rockland Immuno-chemicals) and Alexa Fluor 680 (Molecular Probes) were used.
2.6 Cycloheximide chase
For cycloheximide (CHX) chase, cells were grown to log phase in YPD at 30°C, 0 time point was taken at this time, culture was iced for 10 min and resuspended in cold PBS + 50 μg/ml CHX. Cells were mock- or UV-treated with 150 J/m2 and kept in dark through the remainder of time course. Cells were collected by centrifugation and resuspended in 30°C YPD + 50 μg/ml CHX and samples for whole cell protein lysates were taken at indicated time points.
3. Results
3.1 Siz1 and Siz2 are involved in NER
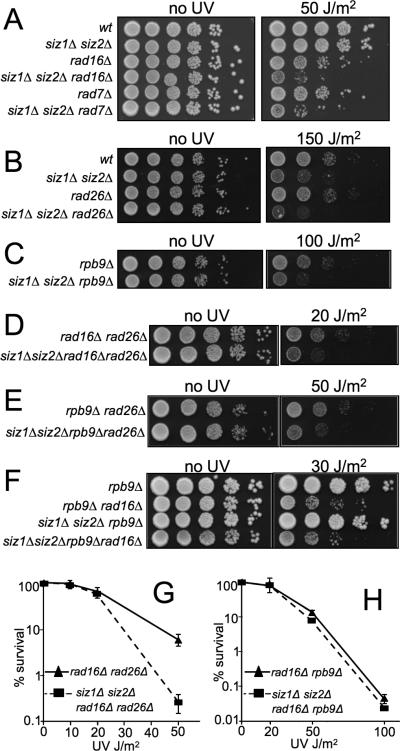
We showed previously that the siz1Δ siz2Δ mutant is modestly sensitive to UV light [11]. Quantitative analysis of this UV sensitivity revealed that SIZ1 contributes more to UV resistance than does SIZ2 (Fig. 1). However, UV sensitivity was increased in a siz1Δ siz2Δ double mutant compared to either single mutant (Fig. 1A). This suggested that both SIZ genes contribute to UV resistance and that neither is individually capable of supporting wt levels of DNA repair.
Fig. 1.
Epistasis between core NER genes and siz1Δ siz2Δ. (A, B) The indicated mutants were plated, UV irradiated at indicated intensities, and allowed to grow for three days in the dark before counting surviving colonies. Values represent averages of three independent experiments and error bars represent standard deviations. (C, D) Overnight cultures of indicated genotypes were serially diluted (10-fold), spotted onto YPD, UV irradiated at indicated doses and incubated for 2-3 days in the dark at 30°C.
Because sensitivity to UV light, but not to other DNA damaging agents, is a hallmark of NER pathway mutants, we hypothesized that SIZ genes may have a role in NER. To determine if SIZ genes function in a common pathway with known NER genes, we combined siz1Δ siz2Δ with deletions of RAD4, RAD14, or RAD1. Deletion of each of these core NER genes completely abolishes all NER activity and confers extreme UV sensitivity [13]. siz1Δ siz2Δ is much less UV sensitive than these mutants, indicating that any effect on NER is only partial. Analyzing UV survival of the triple mutants siz1Δ siz2Δ rad14Δ, siz1Δ siz2Δ rad4Δ, and siz1Δ siz2Δ rad1Δ using a spotting assay showed that none of these triple mutants was more sensitive than the cognate single mutant alone (Fig. 1C). Quantitative survival curves also showed no effect on UV sensitivity of deleting SIZ1 and SIZ2 in a rad14Δ mutant (Fig. 1B). These results demonstrated that NER pathway mutants are epistatic to siz1Δ siz2Δ, suggesting that SIZ1 and SIZ2 participate in NER.
We next asked if the SIZ genes act in a specific subpathway of NER. We first examined the effect of combining siz1Δ siz2Δ with mutants that have partial effects on both GGR and TCR, including rad23Δ, rad33Δ, and rad34Δ [34-37]. Spotting assays showed that all triple mutants lacking SIZ1, SIZ2, and one of these genes showed increased UV sensitivity over the cognate single mutants (Fig. 1D). Next, we tested the effect of combining siz1Δ siz2Δ with deletion mutants in RAD16, which functions exclusively in GGR, or with RAD26 or RPB9, each of which acts in a different TCR subpathway. Deleting SIZ1 and SIZ2 increased the UV sensitivity of all three of these mutants (Fig. 2A-C). If SIZ genes functioned together exclusively with one or another of these genes, then the siz1Δ siz2Δ triple mutant would show no increased sensitivity over the single mutant, as was seen with rad14Δ. Thus, these data suggested that SIZ genes do not act exclusively in a pathway defined by any of these genes. They also suggested that SIZ genes play a role in NER that affects both GGR and TCR. This role could be at a single step that is common to multiple subpathways or at multiple steps in the pathway.
Fig. 2.
Siz1 and Siz2 play a role in Rad16- and Rpb9-mediated NER. (A-F) Spotting of indicated mutants was completed as in Fig. 1. (G, H) Survival analyses of indicated mutants were performed as in Fig. 1.
We next examined whether SIZ1 and SIZ2 are required for the NER that remains when any two of the RAD16-, RAD26-, or RPB9-dependent subpathways are eliminated. To determine if SIZ1 and SIZ2 function in Rad26-independent TCR we introduced siz1Δ siz2Δ into the rad16Δ rad26Δ mutant background and quantified UV survival. The quadruple mutant siz1Δ siz2Δ rad16Δ rad26Δ was more sensitive to UV killing than the rad16Δ rad26Δ mutant alone (Fig. 2D,G). This suggested that Siz1 and Siz2 play a role in the repair that remains when RAD26 and RAD16 are both absent, mostly Rpb9-dependent TCR. Likewise when SIZ1 and SIZ2 were eliminated from rad26Δ rpb9Δ cells, the quadruple mutant displayed increased UV sensitivity, consistent with a role for SIZ genes in Rad16-dependent GGR (Fig. 2E). However, when siz1Δ siz2Δ was introduced into the rad16Δ rpb9Δ mutant background, no change in UV-sensitivity was observed (Fig. 2F,H). Thus, the rad16Δ rpb9Δ double mutant is largely epistatic to siz1Δ siz2Δ, implying that SIZ genes function in the Rad16- and Rpb9-dependent subpathways of NER. This result also implies that SIZ activity plays little or no role in the residual NER that occurs in the rad16Δ rpb9Δ double mutant, which is primarily Rad26-dependent TCR.
3.2 siz1Δ siz2Δ mutants show delayed CPD repair
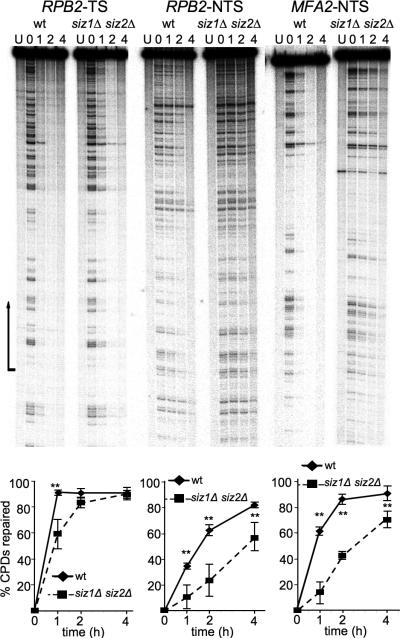
To determine the effect of SIZ1 and SIZ2 on GGR and TCR directly, we next used an assay that quantifies CPD removal at the single nucleotide level [29]. In this assay, cells are UV-irradiated, incubated to allow DNA repair, and used to prepare DNA. DNA is then cleaved with a restriction enzyme and treated with T4 endonuclease V, which cleaves at CPDs. This is followed by isolation of specific DNA fragments, end-labeling, and denaturing electrophoresis. CPDs at specific sites are thereby visualized as bands on a sequencing gel. As CPDs are repaired, these bands disappear. Each strand is assayed individually, so that either the transcribed strand (TS) or non-transcribed strand (NTS) can be assayed. Repair at the RPB2 locus was analyzed because it is actively transcribed throughout the cell cycle and has been used previously to assay NER [16, 31].
In wt cells, repair of the RPB2-TS was approximately 90% completed by one-hour post UV treatment (Fig. 3). The comparable repair profile for siz1Δ siz2Δ was slightly delayed. At the one-hour time point, siz1Δ siz2Δ was roughly 60% repaired and by two hours 83% of TS repair was completed. This showed that the siz1Δ siz2Δ mutant carries out a lower rate of NER in the TS than do wt cells.
Fig. 3.
siz1Δ siz2Δ shows delayed repair of CPDs. (Top) Representative autoradiograms showing repair of the indicated strands of the indicated genes in wt and siz1Δ siz2Δ. Lanes are DNA samples from untreated cells (NT) and UV-irradiated cells following different times of repair incubation (hours) as indicated over each lane. Arrow indicates transcription start site and direction of transcription. For MFA2-NTS, the wt and siz1Δ siz2Δ samples were taken from different gels. (Bottom) Plots of CPD repair for the genes and strands shown above. For TS, the region of quantification included the entire transcribed region; for NTS, the whole gel was quantified. Error bars represent standard deviations of three independent experiments. Two asterisks represents p<0.01.
siz1Δ siz2Δ had a greater effect on repair of the NTS of RPB2 (Fig. 3). wt cells completed 35%, 63% and 83% of NTS repair by the one, two and four hour time points, respectively. In contrast, siz1Δ siz2Δ cells completed only 11%, 24% and 57% in the same time course. To test whether the siz1Δ siz2Δ delay in GGR could also be observed at other loci, we analyzed repair of the NTS of the MFA2 locus (Fig. 3). Again siz1Δ siz2Δ showed a ~3-fold delay in the repair rate. While wt cells repaired more than 60% of CPDs by the 1 hr timepoint, siz1Δ siz2Δ repaired less than 20% in the same period. These results demonstrate a role for SIZ genes in GGR.
The UV-sensitivity data suggesting a role for SIZ1 and SIZ2 in Rpb9-dependent TCR encouraged us to assay directly for SIZ involvement in this pathway. A region of robust Rpb9-dependent repair in the RPB2 locus occurs just downstream of the transcription start site [16, 38]. We asked if Rpb9-dependent repair of this region in rad16Δ rad26Δ mutants was SIZ-dependent. Comparison of repair rates from rad16Δ rad26Δ and siz1Δ siz2Δ rad16Δ rad26Δ cells indicated that this region showed, at most, very slightly delayed repair in the absence of SIZ genes. (not shown). In contrast, rpb9Δ rad16Δ rad26Δ cells exhibited almost no repair, as observed previously [16]. The main conclusion from this experiment is that SIZ activity is not required for the bulk of Rpb9-dependent repair.
3.3 Several NER proteins are sumoylated
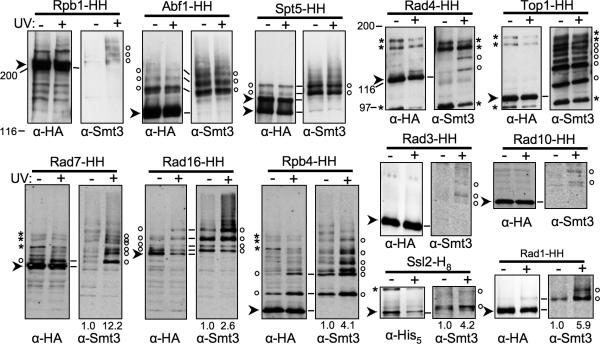
More than 30 proteins function in NER, and the NER defects of siz1Δ siz2Δ suggests that SUMO attachment to one or more of these proteins facilitates NER. Our genetic experiments did not suggest a single specific subpathway that might require sumoylation. Consequently, we decided to test virtually all known NER factors for sumoylation in the presence or absence of UV irradiation. We constructed cells expressing His8- and HA-tagged (HH) versions of NER proteins from their chromosomal loci, purified the tagged proteins using Ni-NTA agarose, and then examined them by immunoblotting using α-HA and α-Smt3 (yeast SUMO) Abs (Fig. 4). We found that the NER-specific factors Rad16, Rad7, and Rad4 were sumoylated, as were a number of factors that participate in NER, but also have other functions, including Rad1, Rad10, Rpb4, Spt5, Ssl2, Rad3, Abf1, and Top1. We also confirmed that Rpb1/Rpo21, the large CTD-containing subunit of RNAPII, is sumoylated. Of these, sumoylation of Rad7, Rad4, Rad3, and Rad10 was almost entirely dependent on UV irradiation of the cells, while SUMO attachment to Rad16, Rpb4, Rad1, Ssl2 and Rpb1 was significantly increased by UV irradiation (Fig. 4). For many of these proteins, several SUMO-modified species were observed, which may represent mono-SUMO attachment at multiple sites, poly-SUMO chains, mixed conjugates that also contain ubiquitin, or a combination of these. Little or no increase in SUMO attachment to Spt5, Abf1, or Top1 was observed upon UV irradiation. We did not detect SUMO attachment to Rad33, Cdc31, Cul3, Rad23, Rad28, Rad14, Rad34, Spt4, Tfb1, Tfb2, Tfb3, Tfb4, Tfb5, Ssl1, Def1, or Rad2 (not shown).
Fig. 4.
NER factors modified by SUMO. His8-, or His8- and HA-tagged versions of indicated proteins from mock- and UV-treated wt strains were purified by Ni-NTA affinity chromatography and analyzed by SDS-PAGE and immunoblotting with Abs against Smt3 (right of each set), and against the HA epitope or His5, as indicated (left of each set). Cells were incubated in YPD for 30 m post-UV treatment. Ab binding in the top five sets of blots was detected using chemiluminescence, while double-label fluorescent detection was used in bottom seven blots. Arrowheads indicate unsumoylated tagged proteins. (There are two for Spt5, which is partially hyperphosphorylated.) Open circles designate sumoylated species. In some samples, sumoylated species are also visible on the HA blot. Asterisks indicate cross-reacting bands. Numbers below the lanes represent relative levels of protein sumoylation, where mock-treated was set at 1. HH, –His8-HA. Lines between HA and Smt3 blots indicate how these blots align. For the top row of blots, the HA and Smt3 blots were performed on different filters (using the same samples), so alignments are approximate. Markers are Bio-Rad broad range markers. Three sets of lanes (Rpb1, Abf1, and Spt5), (Rad4 and Top1), and (Rad7, Rad16, and Rpb4) show the same protein size range from the same exposure(s) of the same blot(s), and so the background and markers for these lanes can be compared to each other.
3.4 SUMO attachment to Rad16
Rad16 sumoylation was remarkable in that greater than 50% of the total pool of Rad16 appeared to become sumoylated upon UV irradiation (Fig. 4). (The exact fraction was difficult to quantify because of background bands in the α-HA blot.) Rad16 was also sumoylated at a fairly high level in unirradiated cells. This contrasts with the Rad16 interaction partner Rad7, which was detectably sumoylated only upon UV irradiation (the bands in the unirradiated sample are mostly background bands). To test whether Rad16 sumoylation might contribute to the role of SIZ genes in NER, we tested whether Rad16 sumoylation depends on SIZ genes (Fig. 5B). The robust SUMO modification of Rad16 was predominantly SIZ1-dependent (~70% decrease in siz1Δ), but not decreased by deletion of SIZ2. However, the siz1Δ siz2Δ double mutant further reduced sumoylation of Rad16 compared to siz1Δ alone, indicating that Siz1 and Siz2 can both contribute to sumoylation of Rad16.
Fig. 5.
Analysis of Rad16 sumoylation. (A) Sumoylation of Rad16-HH and Rad16-HH mutants in wt cells was analyzed as in Fig. 4, using chemiluminescent detection. Designations are as in Fig. 4. The same 4 sumoylated species indicated in Fig. 4 are indicated. Arrows on Smt3 blots indicate position of unmodified Rad16-HH. HH, His8-HA; Rad16ΔN-HH, N-terminal deletion mutant lacking the first 103 residues. Markers are Bio-Rad broad range markers. (B) Sumoylation of Rad16-HH in cells of indicated genotypes, and of SUMO attachment site mutant in wt cells, was analyzed as in Fig. 4, using fluorescent detection. Designations are as in Fig. 4. Arrows on Smt3 blots indicate position of unmodified Rad16-HH. Numbers under the lanes indicate the ratio of Smt3 signal relative to unmodified species, where all ratios are normalized to the wt UV-treated sample. Rad16K3R-HH: Rad16K62,93,103R-HH. Markers are Licor single color markers. (C) Spotting of indicated strains completed as in Fig. 1.
Rad16 has multiple SUMO-modified forms (Figs. 4,5). We initially identified two SUMO consensus motifs at K93 (in IKND) and K103 (in IKEE). Cells expressing Rad16 with both K93R and K103R mutations still contained a single sumoylated form of Rad16 (Fig. 5A). However, cells containing a truncated Rad16 lacking the N-terminal 103 amino acids (rad16-ΔN) did not contain sumoylated Rad16 (Fig. 5A). We then identified a third potential SUMO attachment site at K62 (in VKSP). Mutating this residue along with K93 and K103 (rad16-K3R) abolished the vast majority of Rad16 sumoylation (Fig. 5B, right lanes; one sumoylated form is barely detectable.). Thus the three major SUMO attachment sites in Rad16 are K62, K93, and K103.
We tested the UV sensitivities of rad16-K3R and rad16-ΔN mutants and found that they were not more UV sensitive than wt cells (Fig. 5C). Because other investigators have observed synthetic UV sensitivity phenotypes of certain NER mutants when combined with other NER mutants, we combined rad16-K3R and rad16-ΔN with rad23Δ or rad26Δ. The Rad16 sumoylation site mutants also did not have synthetic UV sensitive phenotypes when combined with these mutants (Fig. 5C, shown for rad16-K3R only; rad16-ΔN, not shown). In quantitative CPD repair analysis in the rad16-K3R strain, no NER defect was observed in the repair of the NTS of MFA2 (not shown). These data indicated that eliminating sumoylation of Rad16 alone has no effect on NER. This may suggest either that Rad16 sumoylation functions in an activity other than NER or that some other NER activity acts redundantly with Rad16 sumoylation. For example, Rad16 forms a multimer including Rad7, so it is possible that sumoylation of Rad16 and Rad7 is functionally redundant.
3.5 No effect of SUMO pathway on Rad4 levels
Ramsey et al. have reported that a ts mutant defective in the SUMO conjugating enzyme Ubc9 contains a striking reduction in Rad4 levels [20]. This could suggest that the SUMO pathway protects Rad4 from degradation. Consequently, we asked if reduced levels of Rad4 explain the NER defects of SUMO pathway mutants. Rad4 levels were measured using quantitative double-labeled fluorescent immunoblots of whole cell lysates, using an Ab against Rad4 and a PSTAIR Ab as a loading control (Fig. 6A). This experiment showed that Rad4 levels were not significantly different between wt and siz1Δ siz2Δ mutants. When we compared Rad4 levels in a ubc9-ts mutant that had been grown at the permissive temperature (22°C) to one that had been shifted to the non-permissive temperature (36°C) for 2 hr, we did see a significant, ~ 2-fold reduction in the level of Rad4 (Fig. 6A). However, the main difference between these samples and wt (grown at 30°C) was that the Rad4 level in ubc9 at the permissive temperature was higher than in wt. However, since the ubc9–ts allele was in a slightly different strain background, this result was hard to interpret (see below). Ramsey et al also reported that rad16Δ mutants contain higher than wt levels of Rad4 [20], but our data also did not confirm this result (Fig. 6A). However, we did reproduce the result by den Dulk et al [39] that the rad33Δ mutant shows a reduction (2-fold in our experiment) in Rad4 levels (Fig. 6A).
Fig. 6.
Rad4 steady state levels are not altered in SUMO pathway mutants. (A) Whole cell lysates from cells of indicated genotype were analyzed by SDS-PAGE and double-label fluorescent immunoblotting with Abs against Rad4, and PSTAIR as a loading control. Ratio of Rad4 to PSTAIR is shown, normalized to wt. Error bars represent standard deviations of 3-4 independent experiments. Two asterisks represent p<0.01. (B) Cultures of indicated genotype were grown to log phase at 22°C and shifted to 36°C for the indicated time (h), except extreme right two lanes, which were grown long-term at 30°C and grown to log phase. Whole cell lysates were analyzed as in (A) using Abs against Rad4 (top panel) and PSTAIR (not shown) or by chemiluminescent immunoblotting with an Ab against Smt3 (bottom panel). Numbers beneath the lanes indicate ratios of Rad4 to PSTAIR, with ratios normalized to the wt 0 time point. Arrowhead indicates Rad4. (C) Cultures of the indicated genotypes were treated with 50 μg/ml CHX immediately before UV or mock treatment. Cells were returned to culture containing CHX, for the indicated time (h). Whole cell lysates were analyzed as in (B). Designations as in (B).
To test the effect of ubc9-ts on Rad4 levels more precisely, we next performed a temperature shift experiment, in which ubc9-ts and a congenic wt strain were both shifted from 22°C to 36°C. In this experiment, levels of Rad4 fell at similar rates in both the wt and ubc9-ts strains (Fig. 6B). We also compared Rad4 levels in wt and ubc9-ts strains that had been grown consistently at the semi-permissive temperature of 30°C, and found no difference (Fig. 6B, right lanes). Control experiments showed that SUMO conjugates were virtually undetectable in the ubc9-ts strain, while the wt strain exhibited robust sumoylation (Fig. 6B, bottom panel). Collectively, these experiments are consistent with the interpretation that Rad4 levels are not affected by sumoylation, but are reduced when yeast are grown at high temperature.
As a final test of the effect of sumoylation on Rad4 stability, we performed cycloheximide chase experiments to measure the rate of Rad4 degradation in mock- and UV-treated cells (Fig. 6C). These experiments showed that wt and siz1Δ siz2Δ initially contained very similar amounts of Rad4, as in Fig. 6A, and that the rate of degradation of Rad4 in either UV-irradiated or untreated cells was also very similar. We cannot rule out slight differences (<20%) in degradation rate. To make sure that our experiments were consistent with previous work, we also performed cycloheximide chase experiments in rad23Δ cells, in which Rad4 is rapidly degraded [36, 40]. Consistent with prior reports, Rad4 levels were low in the rad23Δ cells initially, and Rad4 rapidly disappeared during the chase (Fig. 6C). Thus, Siz1 and Siz2 do not play a major role in maintaining Rad4 levels under normal or UV-challenged conditions.
3.6 Sumoylation of Rad4
In wt cells, sumoylation of Rad4 is strongly induced by UV (Figs. 4, 7A). A previous report on Rad4 has shown that several unidentified modified forms of Rad4 increase in UV-treated rad33Δ cells compared to wt [39]. Based on this report and our finding that Rad4 was sumoylated, we asked if levels of Rad4 sumoylation would be altered in rad33Δ. This experiment showed that the level of UV-induced Rad4 sumoylation was increased by almost 20-fold in rad33Δ cells, compared to wt cells (Fig. 7B). We next asked if this increase in Rad4 sumoylation was unique for rad33Δ or if other NER mutants would have a similar effect. We found that rad1Δ and rad14Δ mutants also accumulated increased levels of SUMO-Rad4 (~10-fold and ~30-fold, respectively, compared to wt), while rad16Δ had no effect (Fig. 7C,D). Levels of SUMO-Rad4 in the mutants, when compared to the wt SUMO/Rad4 ratio, varied somewhat from experiment to experiment, mostly because the low levels of SUMO-Rad4 in wt were difficult to measure. However, rad33Δ, rad1Δ and rad14Δ consistently contained at least 10-fold more SUMO-Rad4 than a paired wt control. Since RAD1 and RAD14 act downstream of RAD4, these results may suggest that Rad4 becomes sumoylated as a part of its functional cycle, and that these downstream mutants accumulate Rad4 at the stage when it is subject to sumoylation. These experiments also showed that the most abundant higher molecular weight modified forms of Rad4 in UV-irradiated NER mutant cells contain SUMO, since the modified forms overlap exactly with the SUMO signal (Fig. 7C, right panels).
Fig. 7.
Analysis of Rad4 sumoylation. Sumoylation of Rad4-HH in strains of the indicated genotypes was analyzed as in Fig. 4, using fluorescent detection. Designations as in Fig. 4. The same 2 sumoylated species indicated in Fig. 4 are indicated. Numbers under the lanes indicate normalized ratio of Smt3 signal to HA signal. In the top panel, ratios were normalized to UV-irradiated wt, while in other panels, ratios were normalized to sample with the highest ratio. rad16Δ and rad1Δ samples were from the same blot, but lanes in between were removed. For the UV-irradiated rad1Δ sample, an overexposed version of the α-HA signal is shown next to the same lane with the α-Smt3 signal overlaid.
In otherwise wt cells, Rad4 sumoylation was mostly dependent on SIZ1: SUMO signal was decreased by 50% in siz1Δ cells, and no decrease was detected in siz2Δ cells (Fig. 7A). Near complete loss of sumoylated Rad4 was observed in siz1Δ siz2Δ cells. In rad14Δ and rad33Δ cells (Fig. 7B,D), SIZ1 and SIZ2 both contributed to SUMO modification of Rad4, while the corresponding siz1Δ siz2Δ mutants nearly completely eliminated SUMO-modified Rad4. Thus, sumoylation of Rad4 depends on Siz proteins and could contribute to the role of SIZ genes in NER.
4. Discussion
Our results demonstrate that Siz1 and Siz2 are required for S. cerevisiae to carry out normal rates of NER. UV sensitivity experiments suggested that SIZ genes participate primarily in GGR and in the RPB9-dependent subpathway of TCR, while having minimal effect on RAD26-dependent TCR. Consistent with these observations, assays of CPD repair showed that the siz1Δ siz2Δ mutant had a ~3-fold reduction in the repair rate of the NTS. This confirms that SIZ genes are required for normal rates of GGR. In contrast, SIZ genes did not have a clear effect on RPB9-dependent TCR in this assay. However, CPD repair experiments did show a statistically significant reduction in the rate of repair of the TS in siz1Δ siz2Δ. It is not clear whether this reflects a modest effect of SIZ genes on TCR or whether this effect is derived from reduced GGR-dependent repair of the TS. Mutants that reduce only GGR, such as rad16Δ and rad7Δ, actually increase the rate of repair of the TS [16], possibly because absence of GGR increases TCR by reducing competition for downstream factors. If this effect also takes place in siz1Δ siz2Δ, the slightly reduced TS repair in this mutant would suggest that SIZ genes affect TCR. However, the partial reduction of GGR in siz1Δ siz2Δ may not cause the same increase in TCR as in rad16Δ. In this case, the siz1Δ siz2Δ effect on TS repair may result primarily from the effect on GGR.
It is likely that the role of Siz proteins is to promote SUMO attachment to one or more NER proteins. However, substrate(s) whose sumoylation enhances NER have not yet been identified. It is not clear whether SUMO attachment to a single substrate could account for the observed effects, or whether these effects result from sumoylation of distinct proteins, or sets of proteins. We found that SUMO attachment to Rad16 could be mostly eliminated without detectable effects on UV survival or the rate of GGR, despite the fact that a large fraction (>50%) of Rad16 can become sumoylated after UV irradiation. A likely explanation for this observation is that SUMO attachment to the Rad16 interaction partner Rad7, or possibly to other proteins, is functionally redundant with sumoylation of Rad16. Rad4 also associates with sites of repair together with the Rad16-Rad7 complex [41], so it is conceivable that SUMO attachment to any of Rad16, Rad7, or Rad4 would have the same effect. Testing this possibility will depend on the identification of the SUMO attachment sites in Rad7 and Rad4 in the future.
We found that UV irradiation of yeast cells induces sumoylation of several proteins associated with NER. Some proteins, including Rad4, Rad7, Rad3 and Rad10, were not detectably sumoylated in unperturbed cells, but became sumoylated upon UV irradiation, while others, including Rad16, Rpb4, Rpb1, Rad1 and the TFIIH helicase Ssl2, were sumoylated constitutively, but were modified at higher levels after UV irradiation. How does UV irradiation induce sumoylation of these proteins? One possibility would be regulation by DNA damage signaling pathways. However, a different, but not mutually exclusive, possibility was suggested by detailed analysis of Rad4 sumoylation, which showed that eliminating downstream factors in the NER pathway, such as Rad14 and Rad1, increased SUMO-Rad4 levels by up to 30-fold. Other investigators have found that the RNAPII subunit Rpb1 is also sumoylated at higher levels in rad14Δ mutants, suggesting that this may be a general effect [23]. These observations suggest that Rad4 and Rpb1 may become sumoylated at a specific point in the NER process. Blocking downstream steps would cause these proteins to accumulate at the step when they become sumoylated, thus increasing sumoylation levels. The increased SUMO-Rad4 in rad33Δ could be explained by this same mechanism if rad33Δ accumulates the same repair intermediates. Such context-dependent sumoylation has been observed before. For example, yeast PCNA is sumoylated primarily when it is bound to DNA, both in vivo and in vitro [42]. Thus it is sumoylated only during DNA replication and repair. Not only Rad4 and Rpb1, but also other proteins that participate in NER may be susceptible to sumoylation primarily at specific steps in the repair process.
Another mechanism for how Rad4 sumoylation might be controlled was suggested by den Dulk et al [39], who showed that an unidentified UV-induced modification was increased in the rad33Δ mutant and also in a rad4 mutant that disrupted the interaction between Rad4 and Rad33. Assuming that this modification is SUMO, one interpretation of this result would be that the interaction with Rad33 inhibits Rad4 sumoylation. Similarly, Rad4 and Rad14 were identified as having a physical interaction in a high-throughput screen for protein complexes [43]. Thus it is also possible that Rad4 interaction with the complex containing Rad14, Rad1 and Rad10 protects Rad4 from sumoylation.
den Dulk et al also found that eliminating RAD26 from rad33Δ cells reduced levels of the unidentified Rad4 modification [39]. Our own results from similar experiments were consistent with this result, but difficult to interpret. We found that deleting RAD26 from rad33Δ or rad14Δ mutants reduced the level of Rad4 sumoylation, similar to the results in den Dulk et al, but that deleting RAD26 had very little effect on Rad4 sumoylation in otherwise wt cells (HRS and EJ, unpublished data). Further studies will be necessary to understand these observations.
den Dulk et al suggested that the UV-inducible modification of Rad4 they observed that was increased in rad33Δ mutants might be ubiquitylation [39]. However, our results suggest that the major, UV-inducible modified forms of Rad4 are sumoylated (Fig. 7C). Of course, ubiquitin could also be present. Because rad33Δ contains decreased Rad4 levels [39] (Fig. 6A), we tested whether elevated SUMO-Rad4 is generally associated with reduced total Rad4, by examining Rad4 levels in rad14Δ. Rad4 levels in rad14Δ were the same as in wt (Fig. 6A). Thus, our results do not support an association between Rad4 sumoylation and reduced Rad4 levels.
Ramsey et al reported that Rad4 levels were reduced in temperature sensitive mutants of UBC9, which encodes the single, essential conjugating enzyme for SUMO [20]. This result would suggest that sumoylation increases Rad4 levels. We were unable to reproduce this result, and did not detect any significant differences in Rad4 levels either in a ubc9 mutant or in siz1Δ siz2Δ. Possible explanations for this discrepancy include a different strain background, a different method for preparing yeast lysates, or the fact that Ramsey et al. were examining a TAP-tagged version of Rad4. To avoid potential problems related to epitope tags, we measured Rad4 levels in a strain where Rad4 was untagged, using an Ab against Rad4.
Wang et al have reported that XPC, the human homolog of Rad4, also shows UV-inducible sumoylation [24, 25]. Thus, sumoylation may play a conserved role in nucleotide excision repair. A full elucidation of the role for SUMO in this process awaits the identification of interaction partners with sumoylated NER proteins and analysis of the SUMO attachment sites in the proteins that become modified during this process, including Rad4 and Rad7 in yeast and XPC in humans.
Supplementary Material
Highlights.
>The Siz SUMO ligases participate in nucleotide excision repair in yeast.
> Several NER factors are sumoylated, including Rad4, Rad16, Rad7, Rad1, and Rad10.
> SUMO attachment to Rad16 is not required for UV resistance or repair of DNA lesions.
> SUMO-modified Rad4 accumulates in NER pathway mutants that block downstream of Rad4.
> Rad4 may become sumoylated at a specific point in its functional cycle.
Acknowledgements
We thank James Webb for technical assistance and help preparing the manuscript. We also thank Stephen Lloyd for his gift of purified T4-endonuclease V. Yumin Teng, Edgar Davidson and Hyejeong Park have been invaluable resources for technical advice on the CPD removal assays, as have members of Hou laboratory, especially Tom Christian, Georges Lahoud and Cuiping Liu. This work was supported by MCB-0820228 from the National Science Foundation to E.J. and NIH GM081601 to YM.H.
Abbreviations
- CPD
cyclobutane pyrimidine dimer
- RNAPII
RNA polymerase II
- TS
transcribed strand
- NTS
non-transcribed strand
- GGR
global genome repair
- TCR
transcription-coupled repair
- CHX
cycloheximide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Gareau JR, Lima CD. The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat Rev Mol Cell Biol. 2010;11:861–871. doi: 10.1038/nrm3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 3.Thomson TM, Guerra-Rebollo M. Ubiquitin and SUMO signalling in DNA repair. Biochem Soc Trans. 2010;38:116–131. doi: 10.1042/BST0380116. [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson KA, Henley JM. Mechanisms, regulation and consequences of protein SUMOylation. Biochem J. 2010;428:133–145. doi: 10.1042/BJ20100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ulrich HD. The SUMO system: an overview. Methods Mol Biol. 2009;497:3–16. doi: 10.1007/978-1-59745-566-4_1. [DOI] [PubMed] [Google Scholar]
- 6.Johnson ES, Gupta AA. An E3-like factor that promotes SUMO conjugation to the yeast septins. Cell. 2001;106:735–744. doi: 10.1016/s0092-8674(01)00491-3. [DOI] [PubMed] [Google Scholar]
- 7.Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 8.Reindle A, Belichenko I, Bylebyl GR, Chen XL, Gandhi N, Johnson ES. Multiple domains in Siz SUMO ligases contribute to substrate selectivity. J Cell Sci. 2006;119:4749–4757. doi: 10.1242/jcs.03243. [DOI] [PubMed] [Google Scholar]
- 9.Sacher M, Pfander B, Hoege C, Jentsch S. Control of Rad52 recombination activity by double-strand break-induced SUMO modification. Nat Cell Biol. 2006 doi: 10.1038/ncb1488. [DOI] [PubMed] [Google Scholar]
- 10.Chen XL, Reindle A, Johnson ES. Misregulation of 2 microm circle copy number in a SUMO pathway mutant. Mol Cell Biol. 2005;25:4311–4320. doi: 10.1128/MCB.25.10.4311-4320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen XL, Silver HR, Xiong L, Belichenko I, Adegite C, Johnson ES. Topoisomerase I-dependent viability loss in Saccharomyces cerevisiae mutants defective in both SUMO conjugation and DNA repair. Genetics. 2007;177:17–30. doi: 10.1534/genetics.107.074708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa RM, Chigancas V, Galhardo Rda S, Carvalho H, Menck CF. The eukaryotic nucleotide excision repair pathway. Biochimie. 2003;85:1083–1099. doi: 10.1016/j.biochi.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Prakash S, Prakash L. Nucleotide excision repair in yeast. Mutat Res. 2000;451:13–24. doi: 10.1016/s0027-5107(00)00037-3. [DOI] [PubMed] [Google Scholar]
- 14.Sugasawa K. Xeroderma pigmentosum genes: functions inside and outside DNA repair. Carcinogenesis. 2008;29:455–465. doi: 10.1093/carcin/bgm282. [DOI] [PubMed] [Google Scholar]
- 15.van Gool AJ, Verhage R, Swagemakers SM, van de Putte P, Brouwer J, Troelstra C, Bootsma D, Hoeijmakers JH. RAD26, the functional S. cerevisiae homolog of the Cockayne syndrome B gene ERCC6. Embo J. 1994;13:5361–5369. doi: 10.1002/j.1460-2075.1994.tb06871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Smerdon MJ. Rpb4 and Rpb9 mediate subpathways of transcription-coupled DNA repair in Saccharomyces cerevisiae. Embo J. 2002;21:5921–5929. doi: 10.1093/emboj/cdf589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzder SN, Sung P, Prakash L, Prakash S. Yeast Rad7-Rad16 complex, specific for the nucleotide excision repair of the nontranscribed DNA strand, is an ATP-dependent DNA damage sensor. J Biol Chem. 1997;272:21665–21668. doi: 10.1074/jbc.272.35.21665. [DOI] [PubMed] [Google Scholar]
- 18.Verhage R, Zeeman AM, de Groot N, Gleig F, Bang DD, van de Putte P, Brouwer J. The RAD7 and RAD16 genes, which are essential for pyrimidine dimer removal from the silent mating type loci, are also required for repair of the nontranscribed strand of an active gene in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:6135–6142. doi: 10.1128/mcb.14.9.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzder SN, Sung P, Prakash L, Prakash S. The DNA-dependent ATPase activity of yeast nucleotide excision repair factor 4 and its role in DNA damage recognition. J Biol Chem. 1998;273:6292–6296. doi: 10.1074/jbc.273.11.6292. [DOI] [PubMed] [Google Scholar]
- 20.Ramsey KL, Smith JJ, Dasgupta A, Maqani N, Grant P, Auble DT. The NEF4 complex regulates Rad4 levels and utilizes Snf2/Swi2-related ATPase activity for nucleotide excision repair. Mol Cell Biol. 2004;24:6362–6378. doi: 10.1128/MCB.24.14.6362-6378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillette TG, Yu S, Zhou Z, Waters R, Johnston SA, Reed SH. Distinct functions of the ubiquitin-proteasome pathway influence nucleotide excision repair. Embo J. 2006;25:2529–2538. doi: 10.1038/sj.emboj.7601120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min JH, Pavletich NP. Recognition of DNA damage by the Rad4 nucleotide excision repair protein. Nature. 2007;449:570–575. doi: 10.1038/nature06155. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Ding B, LeJeune D, Ruggiero C, Li S. Rpb1 sumoylation in response to UV radiation or transcriptional impairment in yeast. PloS one. 2009;4:e5267. doi: 10.1371/journal.pone.0005267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang QE, Praetorius-Ibba M, Zhu Q, El-Mahdy MA, Wani G, Zhao Q, Qin S, Patnaik S, Wani AA. Ubiquitylation-independent degradation of Xeroderma pigmentosum group C protein is required for efficient nucleotide excision repair. Nucleic Acids Res. 2007;35:5338–5350. doi: 10.1093/nar/gkm550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang QE, Zhu Q, Wani G, El-Mahdy MA, Li J, Wani AA. DNA repair factor XPC is modified by SUMO-1 and ubiquitin following UV irradiation. Nucleic Acids Res. 2005;33:4023–4034. doi: 10.1093/nar/gki684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ausubel FM, Brent R, Kingston RE, Moore DD, Smith JA, Seidman JG, Struhl K. Current Protocols in Molecular Biology. Wiley-Interscience; New York: 2000. [Google Scholar]
- 27.Sherman F, Fink GR, Hicks JB. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1986. [Google Scholar]
- 28.Johnson ES, Blobel G. Cell cycle-regulated attachment of the ubiquitin-related protein SUMO to the yeast septins. J Cell Biol. 1999;147:981–994. doi: 10.1083/jcb.147.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teng Y, Yu S, Reed SH, Waters R. Lux ex tenebris: nucleotide resolution DNA repair and nucleosome mapping. Methods. 2009;48:23–34. doi: 10.1016/j.ymeth.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Li S, Waters R, Smerdon MJ. Low- and high-resolution mapping of DNA damage at specific sites. Methods. 2000;22:170–179. doi: 10.1006/meth.2000.1058. [DOI] [PubMed] [Google Scholar]
- 31.Tijsterman M, Tasseron-de Jong JG, van de Putte P, Brouwer J. Transcription-coupled and global genome repair in the Saccharomyces cerevisiae RPB2 gene at nucleotide resolution. Nucleic Acids Res. 1996;24:3499–3506. doi: 10.1093/nar/24.18.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wohlschlegel JA, Johnson ES, Reed SI, Yates JR., 3rd Global analysis of protein sumoylation in Saccharomyces cerevisiae. J Biol Chem. 2004;279:45662–45668. doi: 10.1074/jbc.M409203200. [DOI] [PubMed] [Google Scholar]
- 33.Yaffe MP, Schatz G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc Natl Acad Sci U S A. 1984;81:4819–4823. doi: 10.1073/pnas.81.15.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.den Dulk B, Brandsma JA, Brouwer J. The Rad4 homologue YDR314C is essential for strand-specific repair of RNA polymerase I-transcribed rDNA in Saccharomyces cerevisiae. Mol Microbiol. 2005;56:1518–1526. doi: 10.1111/j.1365-2958.2005.04607.x. [DOI] [PubMed] [Google Scholar]
- 35.den Dulk B, Sun SM, de Ruijter M, Brandsma JA, Brouwer J. Rad33, a new factor involved in nucleotide excision repair in Saccharomyces cerevisiae. DNA Repair (Amst) 2006;5:683–692. doi: 10.1016/j.dnarep.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Ortolan TG, Chen L, Tongaonkar P, Madura K. Rad23 stabilizes Rad4 from degradation by the Ub/proteasome pathway. Nucleic Acids Res. 2004;32:6490–6500. doi: 10.1093/nar/gkh987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schauber C, Chen L, Tongaonkar P, Vega I, Lambertson D, Potts W, Madura K. Rad23 links DNA repair to the ubiquitin/proteasome pathway. Nature. 1998;391:715–718. doi: 10.1038/35661. [DOI] [PubMed] [Google Scholar]
- 38.Ding B, LeJeune D, Li S. The C-terminal repeat domain of Spt5 plays an important role in suppression of Rad26-independent transcription coupled repair. J Biol Chem. 2010;285:5317–5326. doi: 10.1074/jbc.M109.082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.den Dulk B, van Eijk P, de Ruijter M, Brandsma JA, Brouwer J. The NER protein Rad33 shows functional homology to human Centrin2 and is involved in modification of Rad4. DNA Repair (Amst) 2008;7:858–868. doi: 10.1016/j.dnarep.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Mao P, Smerdon MJ. Yeast deubiquitinase Ubp3 interacts with the 26 S proteasome to facilitate Rad4 degradation. J Biol Chem. 2010;285:37542–37550. doi: 10.1074/jbc.M110.170175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guzder SN, Sung P, Prakash L, Prakash S. Synergistic interaction between yeast nucleotide excision repair factors NEF2 and NEF4 in the binding of ultraviolet-damaged DNA. J Biol Chem. 1999;274:24257–24262. doi: 10.1074/jbc.274.34.24257. [DOI] [PubMed] [Google Scholar]
- 42.Ulrich HD. Regulating post-translational modifications of the eukaryotic replication clamp PCNA. DNA Repair (Amst) 2009;8:461–469. doi: 10.1016/j.dnarep.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Ho Y, Gruhler A, Heilbut A, Bader GD, Moore L, Adams SL, Millar A, Taylor P, Bennett K, Boutilier K, Yang L, Wolting C, Donaldson I, Schandorff S, Shewnarane J, Vo M, Taggart J, Goudreault M, Muskat B, Alfarano C, Dewar D, Lin Z, Michalickova K, Willems AR, Sassi H, Nielsen PA, Rasmussen KJ, Andersen JR, Johansen LE, Hansen LH, Jespersen H, Podtelejnikov A, Nielsen E, Crawford J, Poulsen V, Sorensen BD, Matthiesen J, Hendrickson RC, Gleeson F, Pawson T, Moran MF, Durocher D, Mann M, Hogue CW, Figeys D, Tyers M. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature. 2002;415:180–183. doi: 10.1038/415180a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.