Abstract
13C INEPT Diffusion-Ordered NMR Spectroscopy (DOSY) with an internal reference system was developed to study the aggregation state of THF solvated LDA dimeric complex. Six components are clearly identified in the diffusion dimension and their DOSY-generated 13C INEPT spectrum slices agree extremely well with their respective INEPT spectra. The correlation between log D and log FW of the linear least squares fit to reference points of all components is exceptionally high – (r = 0.9985).
Pulsed gradient spin-echo (PGSE) diffusion NMR spectroscopy was designed to measure diffusion coefficients and deduce the hydrodynamic radii of molecules in solution.1 By incorporation of this technique in a two dimensional experiment – now referred to as DOSY2 - one can, in principle, determine formula weights of different components in solution. 3 Reports of 1H-DOSY experiments continue to increase, however very few 13C INEPT DOSY spectra have been reported 4 although they provide better resolution, a wider chemical shift range than proton spectra and absence of homonuclear coupling.
DOSY spectra often include artifacts generated by temperature fluctuation, convection, and viscosity change. 5 Hence we sought to develop an internal reference method 6 to avoid these effects while taking advantage of the benefits of 13C INEPT DOSY spectra. We report that by utilizing known molecules as internal references, we can correlate relative diffusion coefficients with formula weights of aggregates quantitatively. Consequently, we determine aggregation numbers and solvation states accordingly. This methodology extends and simplifies the current NMR techniques such as analysis of quadrapole coupling 7, multiplicity and bimolecular exchange8, HMPA titration9 and Job plots10 that have been employed for determining aggregation states of reactive intermediates in solution.
To establish the effectiveness of the internal reference 13C INEPT DOSY technique, we report initial studies utilizing commercially available THF-solvated lithium diisopropylamide (LDA) dimer because LDA is the most prominent non-nucleophilic base used in organic synthesis and also an ideal candidate for investigating organolithium aggregation states.11 It exists as a single form—THF disolvated dimer—in THF solution as depicted in Figure 1. 12 The solid structure of this aggregate has also been determined by X-ray crystallography so its size and shape are well established. 13 In this study we choose 1-octadecene (ODE), cyclododecene (CDDE) 14 and benzene as the internal references due to their chemical and NMR properties such as: solubility in different solvents, low reactivity or coordination with other species, chemical shift dispersion, and desirable molecular weight distribution.
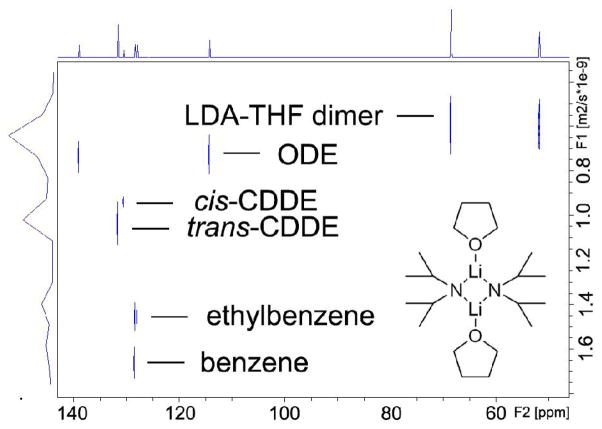
Figure 1.
13C INEPT DOSY of THF solvated LDA dimeric aggregate in toluene-d8 with internal references at 25 °C. The X-axis is the regular 13C INEPT dimension and the Y-axis is the diffusion dimension.
The 13C INEPT DOSY spectrum of THF solvated LDA dimer with the three internal references indicated above in toluene-d8 solution separates into six components in the diffusion dimension. These are clearly identifiable in the DOSY spectrum reproduced in Figure 1. In increasing order of diffusion coefficient (decreasing radii) these are the LDA-THF dimer (C20H44Li2N2O2, mw 351.5), ODE (C18H36, mw 252.3), cis-CDDE (C12H22, mw 166.3), trans-CDDE (C12H22, mw 166.3), ethylbenzene (C8H10, mw 106.2) 15 and benzene (C6H6, mw 78.1). It is noteworthy that diffusion dimension separation (Table S1) of these components was achieved, especially for cis-and trans-CDDE as they have exactly the same formula weight and are observed to exhibit a 3.17% difference in relative diffusion coefficent. The 13C INEPT signals of the oxygen-attached carbons in THF (δ=68.5 ppm) and the LDA methane carbons (δ=51.8 ppm) have identical diffusion coefficients. This evidence corroborates that THF and LDA moieties are in the same aggregate and remain attached under the experimental conditions. The 1H NMR integration also shows that THF and LDA have a 1:1 ratio in the aggregate (Figure S16).
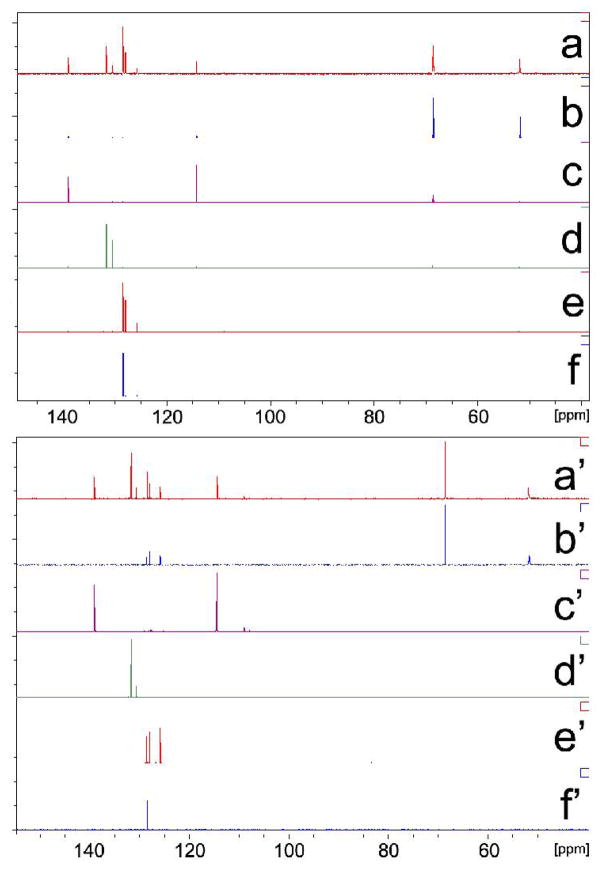
An added bonus of 2D-INEPT 13C-DOSY spectra is to extract a one dimensional 13C-INEPT spectrum slice at the diffusion coefficient of a particular species. Hence, one author has previously referred to the DOSY technique as “chromatography by NMR”. 16 To emphasize this point, we have depicted peaks of LDA (δ = 68.5 ppm) and THF (δ = 51.8 ppm) in the slice of LDA-THF aggregate (Figure 2b) to be compared with the spectrum of the pure, authentic sample (Figure 2b′). Slices taken at other diffusion coefficients of the components agree extremely well with their respective 13C INEPT spectra. Hence, the extracted INEPT spectra determined from the single 2D-INEPT DOSY experiment resolved chemical shift information of every component in the mixture. We note that the 1D- 13C INEPT spectrum of commercially available LDA-THF solution (Figure 2b′) includes signals of ethylbenzene (δ = 125.8, 127.9 and 128.4 ppm), however these signals do not appear in the DOSY slice (Figure 2b). The DOSY slices also illustrate the complete chemical shift resolution of nearly identical signals of benzene (δ = 128.38 ppm) and ethylbenzene (δ = 128.41 ppm) – (Figure S21). We also call attention to the fact that although the difference of relative diffusion coefficient between cis- and trans-CDDE is observed to be only 3.17%, spectra of the pure trans- and nearly pure cis-olefin isomers are resolvable (Figure S22).
Figure 2.
Comparison between slices of 13C INEPT DOSY spectra (a–f) with 13C INEPT NMR spectra of authentic samples (a′–f′). (a) and (a′)-LDA dimer with internal references, (b) and (b′)-LDA dimer without internal references, (c) and (c′)-ODE, (d) and (d′)-cis- and trans-CDDE mixture, (e) and (e′)-ethylbenzene, (f) and (f′)-benzene.
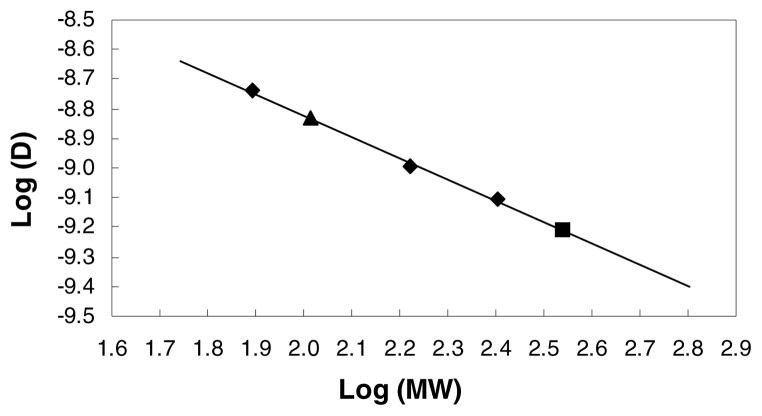
Previously we have proposed a relationship between diffusion coefficient and formula weight utilizing 1H DOSY experiments and we have utilized this correlation to study vinylic lithiation of allylamine derivatives.3a,c Analogously, the 13C INEPT DOSY results strongly suggest that the diffusion coefficients and formula weights (Table S1) of LDA-THF dimer, ethylbenzene and the three internal references, ODE, CDDE and benzene, can also be utilized to define a linear correlation between the relative log D (diffusion coefficient) and log FW (formula weight). The correlation between log FW and log D of the linear least squares fit to reference points of all components in this mixture is extremely high, r = 0.9985 (Figure 3). This remarkable result highlights the ability to use suitable internal references in 13C INEPT DOSY experiments to interpolate relative diffusion coefficients and formula weights, and by inference solvation and aggregation states of the bis-THF solvated LDA dimer.
Figure 3.
13C INEPT DOSY of the five major species in toluene-d8. Diamonds represent reference compounds. Solid square represents THF solvated LDA dimer. Triangle represents ethylbenzene. The solid line is a linear least squares fit to the reference points.
The following issues, noted by a perceptive reviewer, are worthy of consideration – applicability of the Stokes-Einstein equation to nonspherical molecules, secondary solvent shell solvation, ligand exchange and temperature/viscosity dependence. We have also been concerened about these issues and we note that these factors do not interefere with the interpretation of results reported in this manuscript. We are applying this methodology to study the solvation and aggregation of additional organometallic intermediates and we are also probing the scope and limitations of this interal reference DOSY methodology – vis-à-vis the issues noted above.
Supplementary Material
Acknowledgments
This work is supported through the NSF Grant 0718275 and in part by the National Institutes of Health Grant GM-35982.
Footnotes
Supporting Information Avaliable: The 1H NMR, 13C NMR, COSY, HSQC, HMBC and 13C INEPT DOSY spectra are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Cohen Y, Avram L, Frish L. Angew Chem, Internat Ed Engl. 2005;44:520–554. doi: 10.1002/anie.200300637. [DOI] [PubMed] [Google Scholar]; (b) Pregosin PS, Kumar PGA, Fernandez I. Chemical Reviews. 2005;105:2977–2998. doi: 10.1021/cr0406716. [DOI] [PubMed] [Google Scholar]; (c) Stejskal EO, Tanner JE. J Chem Phys. 1965;42:288–292. [Google Scholar]; (d) Tanner JE. Rev Sci Instrum. 1965;36:1086–1087. [Google Scholar]; (e) Stejskal EO. J Chem Phys. 1965;43:3597–3603. [Google Scholar]
- 2.(a) Nilsson M, Morris GA. J Chem Soc, Chem Comm. 2007:933–935. doi: 10.1039/b617761a. [DOI] [PubMed] [Google Scholar]; (b) Johnson CS. Progress in Nuclear Magnetic Resonance Spectroscopy. 1999;34:203–256. [Google Scholar]; (c) Morris KF, Johnson CS. J Am Chem Soc. 1992;114:3139–3141. [Google Scholar]
- 3.(a) Jacobson MA, Keresztes I, Williard PG. J Am Chem Soc. 2005;127:4965–4975. doi: 10.1021/ja0479540. [DOI] [PubMed] [Google Scholar]; (b) Viel S, Capitani D, Mannina L, Serge A. Biomacromoolecules. 2003;4:1843–1847. doi: 10.1021/bm0342638. [DOI] [PubMed] [Google Scholar]; (c) Keresztes I. PhD Dissertation. Brown Univ; 2002. [Google Scholar]; (d) Chen A, Johnson CS. J Am Chem Soc. 1995;117:7965–7970. [Google Scholar]
- 4.(a) Schlorer NE, Cabrita EJ, Berger S. Angewandte Chemie-International Edition. 2002;41:107–109. doi: 10.1002/1521-3773(20020104)41:1<107::aid-anie107>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]; (b) Kapur GS, Findeisen M, Berger S. Fuel. 2000;79:1347–1351. [Google Scholar]; (c) Wu DH, Chen AD, Johnson CS. Journal of Magnetic Resonance Series A. 1996;123:215–218. [Google Scholar]
- 5.Sorland GH, Aksnes D. Magnetic Resonance in Chemistry. 2002;40:S139–S146. [Google Scholar]; (b) Jerschow A, Mueller N. J Mag Res. 1997;125:372. [Google Scholar]
- 6.(a) Groves P, Palczewska M, Molero MD, Batta G, Canada FJ, Jiminez-Barbero J. Anal Biochem. 2004;331:395–7. doi: 10.1016/j.ab.2004.04.038. [DOI] [PubMed] [Google Scholar]; (b) Cabrita EJ, Berger S. Mag Res in Chem. 2001;39:S142–8. [Google Scholar]
- 7.(a) Jackman LM, Cizmeciyan D, Williard PG, Nichols MA. Journal of the American Chemical Society. 1993;115:6262–7. [Google Scholar]; (b) Jackman LM, Chen X. Journal of the American Chemical Society. 1992;114:403–11. [Google Scholar]; (c) Jackman LM, Bortiatynski J. Advances in Carbanion Chemistry. 1992;1:45–87. [Google Scholar]; (d) Jackman LM, Scarmoutzos LM, Smith BD, Williard PG. Journal of the American Chemical Society. 1988;110:6058–63. doi: 10.1021/ja00226a021. [DOI] [PubMed] [Google Scholar]; (e) Jackman LM, Scarmoutzos LM, DeBrosse CW. Journal of the American Chemical Society. 1987;109:5355–61. [Google Scholar]; (f) Jackman LM, Szeverenyi NM. Journal of the American Chemical Society. 1977;99:4954–62. [Google Scholar]
- 8.(a) Fraenkel G, Qiu F. Journal of the American Chemical Society. 2000;122:12806–12812. doi: 10.1021/ja985511o. [DOI] [PubMed] [Google Scholar]; (b) Kim YJ, Streitwieser A, Chow A, Fraenkel G. Organic Letters. 1999;1:2069–2071. doi: 10.1021/ol991088s. [DOI] [PubMed] [Google Scholar]; (c) Fraenkel G, Duncan JH, Martin K, Wang J. Journal of the American Chemical Society. 1999;121:10538–10544. [Google Scholar]; (d) Fraenkel G, Subramanian S, Chow A. Journal of the American Chemical Society. 1995;117:6300–7. [Google Scholar]; (e) Fraenkel G, Martin K. Journal of the American Chemical Society. 1995;117:10336–44. [Google Scholar]; (f) Fraenkel G, Chow A, Winchester WR. Journal of the American Chemical Society. 1990;112:6190–8. [Google Scholar]; (g) Fraenkel G, Henrichs M, Hewitt M, Su BM. Journal of the American Chemical Society. 1984;106:255–6. [Google Scholar]; (h) Fraenkel G, Pramanik P. J Chem Soc, Chem Comm. 1983:1527–9. [Google Scholar]; (i) Fraenkel G, Henrichs M, Hewitt JM, Su BM, Geckle MJ. Journal of the American Chemical Society. 1980;102:3345–50. [Google Scholar]; (j) Fraenkel G, Fraenkel AM, Geckle MJ, Schloss F. Journal of the American Chemical Society. 1979;101:4745–7. [Google Scholar]
- 9.(a) Reich HJ, Goldenberg WS, Sanders AW, Jantzi KL, Tzschucke CC. Journal of the American Chemical Society. 2003;125:3509–3521. doi: 10.1021/ja028301r. [DOI] [PubMed] [Google Scholar]; (b) Reich HJ, Goldenberg WS, Gudmundsson BO, Sanders AW, Kulicke KJ, Simon K, Guzei IA. Journal of the American Chemical Society. 2001;123:8067–8079. doi: 10.1021/ja010489b. [DOI] [PubMed] [Google Scholar]; (c) Reich HJ, Green DP, Medina MA, Goldenberg WS, Gudmundsson BO, Dykstra RR, Phillips NH. Journal of the American Chemical Society. 1998;120:7201–7210. [Google Scholar]; (d) Reich HJ, Gudmundsson BO. Journal of the American Chemical Society. 1996;118:6074–6075. [Google Scholar]; (e) Reich HJ, Borst JP, Dykstra RR, Green PD. Journal of the American Chemical Society. 1993;115:8728–41. [Google Scholar]; (f) Reich HJ, Borst JP. Journal of the American Chemical Society. 1991;113:1835–7. [Google Scholar]; (g) Reich HJ, Green DP. Journal of the American Chemical Society. 1989;111:8729–31. [Google Scholar]
- 10.(a) McNeil AJ, Collum DB. Journal of the American Chemical Society. 2005;127:5655–5661. doi: 10.1021/ja043470s. [DOI] [PubMed] [Google Scholar]; (b) McNeil AJ, Toombes GES, Gruner SM, Lobkovsky E, Collum DB, Chandramouli SV, Vanasse BJ, Ayers TA. Journal of the American Chemical Society. 2004;126:16559–16568. doi: 10.1021/ja045144i. [DOI] [PubMed] [Google Scholar]; (c) McNeil AJ, Toombes GES, Chandramouli SV, Vanasse BJ, Ayers TA, O’Brien MK, Lobkovsky E, Gruner SM, Marohn JA, Collum DB. Journal of the American Chemical Society. 2004;126:5938–5939. doi: 10.1021/ja049245s. [DOI] [PubMed] [Google Scholar]
- 11.(a) Ma Y, Ramirez A, Singh KJ, Keresztes I, Collum DB. Journal of the American Chemical Society. 2006;128:15399–15404. doi: 10.1021/ja060964b. [DOI] [PubMed] [Google Scholar]; (b) Singh KJ, Collum DB. Journal of the American Chemical Society. 2006;128:13753–13760. doi: 10.1021/ja064655x. [DOI] [PubMed] [Google Scholar]; (c) Ramirez A, Sun XF, Collum DB. Journal of the American Chemical Society. 2006;128:10326–10336. doi: 10.1021/ja062147h. [DOI] [PubMed] [Google Scholar]
- 12.Collum DB, McNeil AJ, Ramirez A. Angewandte Chemie-International Edition. 2007;46:3002–3017. doi: 10.1002/anie.200603038. [DOI] [PubMed] [Google Scholar]
- 13.Williard PG, Salvino JM. Journal of Organic Chemistry. 1993;58:1–3. [Google Scholar]
- 14.Cyclododecene (CDDE) was purchased from Aldrich Chemical Company as a trans:cis (60:40) mixture. Olefin stereochemistry was confirmed by HSQC and 1H-NMR homo-decoupling experiments utilizing 1H-CW off resonance spectra. See: Radeglia R. J Prakt Chem/Chem-Ztg. 1993;335:291–3.Radeglia R, Poleschner H, Haufe G. Magn Reson Chem. 1993;31:1054–6.Radeglia R, Poleschner H, Haufe G. Magn Reson Chem. 1993;31:639–41.Radeglia R, Poleschner H, Theil F. J Prakt Chem/Chem-Ztg. 1993;335:673–9.(e) supporting info Figs. S1 & S10.
- 15.Ethylbenzene is present in the LDA•THF solution obtained from Aldrich Chemical Company. Supporting information Figure S2 and S3.
- 16.Gounarides JS, Chen A, Shapiro MJ. Journal of Chromatography, B: Biomedical Sciences and Applications. 1999;725(1):79–90. doi: 10.1016/s0378-4347(98)00512-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.