Abstract
The role of Wnt signaling in osteoblastogenesis in the embryo remains to be fully established. Although β-catenin, a multifunctional protein also mediating canonical Wnt signaling, is indispensable for embryonic osteoblast differentiation, the roles of the key Wnt co-receptors Lrp5 and Lrp6 are unclear. Indeed, global deletion of either Lrp5 or Lrp6 did not overtly affect osteoblast differentiation in the mouse embryo. Here, we generated mice lacking both receptors specifically in the embryonic mesenchyme and observed an absence of osteoblasts in the embryo. In addition, the double-deficient embryos developed supernumerary cartilage elements in the zeugopod, revealing an important role for mesenchymal Lrp5/6 signaling in limb patterning. Importantly, the phenotypes of the Lrp5/6 mutant closely resembled those of the β-catenin-deficient embryos. These phenotypes are likely independent of any effect on the adherens junction, as deletion of α-catenin, another component of the complex, did not cause similar defects. Thus, Lrp5 and 6 redundantly control embryonic skeletal development, likely through β-catenin signaling.
Introduction
The Wnt family of glycoproteins plays essential roles conserved from flies to human in many developmental processes including cell differentiation. In one mode of action, Wnt proteins engage both the low-density lipoprotein receptor-related protein Lrp5 or 6, and a member of the Frizzled receptor family, to activate an intracellular signaling cascade that culminates in β-catenin-dependent transcriptional regulation; this mechanism is commonly known as the canonical pathway (Huelsken and Birchmeier, 2001; Mao et al., 2001; Pinson et al., 2000; Tamai et al., 2000; Wehrli et al., 2000; Wodarz and Nusse, 1998; Wu et al., 2008).
Genetic studies of Lrp5 have indicated a critical role for Wnt signaling in postnatal bone formation. LRP-5 was found to be inactivated in patients with the osteoporosis-pseudoglioma syndrome (Gong et al., 2001). Conversely, an activating mutation in LRP-5 has been linked in two separate cases to patients with a high bone density syndrome (Boyden et al., 2002; Little et al., 2002). Similarly, the Lrp5−/− mice developed a low bone mass phenotype postnatally due to reduced osteoblast proliferation and function (Kato et al., 2002), and Lrp5−/−;Lrp6+/− compound mutants exhibited a further reduction in bone mass (Holmen et al., 2004). Although Yadav et al reported that Lrp5 controlled bone formation indirectly through gut-derived serotonin (Yadav et al., 2008), Cui et al recently provided evidence that Lrp5 functioned directly in the osteoblast lineage (Cui et al., 2011). Other proteins that participate in Wnt signaling have also been implicated in postnatal bone formation, including the extracellular Wnt antagonist sclerostin (Balemans et al., 2001; Balemans et al., 2002; Brunkow et al., 2001; Li et al., 2008; Semenov et al., 2005), and Wnt10b (Bennett et al., 2005).
Wnt signaling has also been implicated in osteoblast development during embryogenesis. Loss of Wnt7b led to a delay in osteoblast differentiation in the embryo (Tu et al., 2007). Deletion of β-catenin in the embryonic mesenchyme resulted in a lack of mature osteoblasts in the mouse embryo, due to the failure of progressing through the early stages of differentiation, although deletion in the more mature osteoblast-lineage cells did not have a similar effect (Day et al., 2005; Glass et al., 2005; Hill et al., 2005; Holmen et al., 2005; Hu et al., 2005; Rodda and McMahon, 2006). However, because of the multiple functions of β-catenin beyond Wnt signaling, it remains uncertain whether the phenotype caused by β-catenin deletion faithfully reflects the role of Wnt signaling during osteoblastogenesis.
Here we genetically deleted Lrp5 and 6, either singly or in combination, in the embryonic mesenchyme, and analyzed the skeletal phenotype. We further compared these mutants with embryos deficient in either α-catenin- or β-catenin. The results provide evidence that Lrp5 and Lrp6 redundantly mediate Wnt signaling to control both osteoblastogenesis and cartilage development in the embryo.
Results
Deletion of either Lrp5 or 6 has little effect on embryonic skeleton
As a first step to investigate the roles of Lrp5 and 6 in skeletogenesis, we generated Lrp5 and Lrp6 floxed alleles (Lrp5f and Lrp6f, respectively) through homologous recombination (Fig. 1A, B). Mice homozygous for either or both floxed alleles developed and reproduced normally, indicating that both alleles had a normal function. We subsequently generated null alleles (Lrp5n or Lrp6n) from mice harboring both the respective floxed allele and a germ-cell active CMV-Cre transgene. Mice homozygous for Lrp5n appeared grossly normal, but displayed low bone mass postnatally, as previously reported (Kato et al., 2002) (data not shown). Embryos homozygous for Lrp6 deletion in all cells (CMV-Cre; Lrp6f/f) died at birth with multiple developmental defects, recapitulating the phenotype previously reported for Lrp6-deficient embryos (Pinson et al., 2000) (Fig. 1C). These results therefore validated our targeting strategy.
Figure 1.
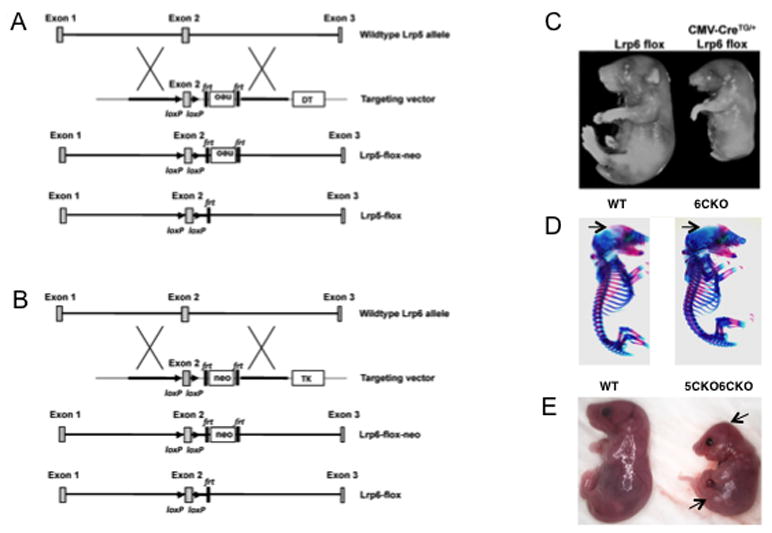
Generation of mice deficient in Lrp5 and/or Lrp6. (A) Gene targeting strategy for Lrp5. Neo: neomycin resistance gene expression cassette for positive selection; Frt: Flippase recognition target; LoxP: Cre recognition site; DT: diphtheria toxin expression cassette for negative selection. (B) Gene targeting strategy for Lrp6. TK: thymidine kinase expression cassette for negative selection. (C) Images of E18.5 littermate embryos with or without global deletion of Lrp6. (D) Whole-mouse skeletal staining at E17.5. Arrows denote frontal bone area undermineralized in Dermo1Cre;Lrp6f/f (6CKO). (E) Photographs of wild type versus Dermo1Cre;Lrp5f/f;Lrp6f/f (5CKO6CKO) littermates at E18.5. Arrows denote abnormal skull and hindlimb morphology.
We next deleted Lrp6 specifically in the early mesenchyme, which contain precursors for the skeletal tissues. Specifically, we used the Dermo1-Cre strain to generate progenies with the genotype of either Dermo1-Cre; Lrp6n/f or Dermo1-Cre; Lrp6f/f (6CKO) (Yu et al., 2003). Mice of either genotype were born at the Mendelian ratio with no obvious phenotype. Indeed, whole-mount skeletal staining of the 6CKO embryo revealed a relatively normal skeleton, exhibiting only a slight delay in ossification of the skull at E17.5 (Fig. 1D). The relatively normal skeleton of the 6CKO embryonic skeleton was similar to that of the Lrp5n/n (5KO) or the Dermo1Cre; Lrp5f/f (5CKO) embryo (Kato et al., 2002) (data not shown). Thus, skeletal development proceeds normally in embryos that lack either Lrp5 or Lrp6 in the skeletogenic mesenchyme.
Loss of both Lrp5 and 6 causes profound skeletal defects in the embryo
We next deleted both Lrp5 and 6 in the embryonic mesenchyme. In one approach, we generated embryos with the genotype of Dermo1Cre;Lrp5f/f;Lrp6f/f (5CKO6CKO), wherein deletion of Lrp5 and Lrp6 were both mediated by Dermo1-Cre. Anticipating that Cre-mediated recombination might be insufficient, we also created mice of the genotype Dermo1Cre;Lrp5n/n;Lrp6f/f (5KO6CKO) which lacked Lrp5 globally but Lrp6 tissue-specifically. Embryos of either genotype developed to term but died shortly after birth, exhibiting similar gross abnormalities including reduced body size, misshaped skull and limbs (Fig. 1E). Whole-mount skeletal staining confirmed the shortening of all skeletal elements, and revealed a profound defect in the ossification of the craniofacial, the axial and the appendicular skeleton (Fig. 2). In addition, the sternum failed to fuse in the mutant embryos of either genotype, likely contributing to their neonatal lethality. Interestingly, both mutant types displayed extra cartilage elements (typically 4) in the zeugopod, and in some cases also in the autopod. However, whereas the zeugopod elements in the 5KO6CKO embryo remained completely cartilaginous, those in 5CKO6CKO showed signs of ossification. Residual ossification was also observed in other skeletal elements of the 5CKO6CKO embryo, although the scapula and the ileum were partially ossified in both mutants. Finally, the hindlimbs of the 5KO6CKO embryo possessed an additional element bridging the zeugopod and the stylopod. The discrepancies between the two genotypes likely reflected a slight delay in sufficient Lrp5/6 deletion, or a residual Lrp5/6 activity in the 5CKO6CKO embryo, and prompted us to focus subsequent analyses on the 5KO6CKO mutant. Overall, these results indicate that Lrp5 and Lrp6 together control multiple aspects of skeletal development in a dose-dependent manner.
Figure 2.
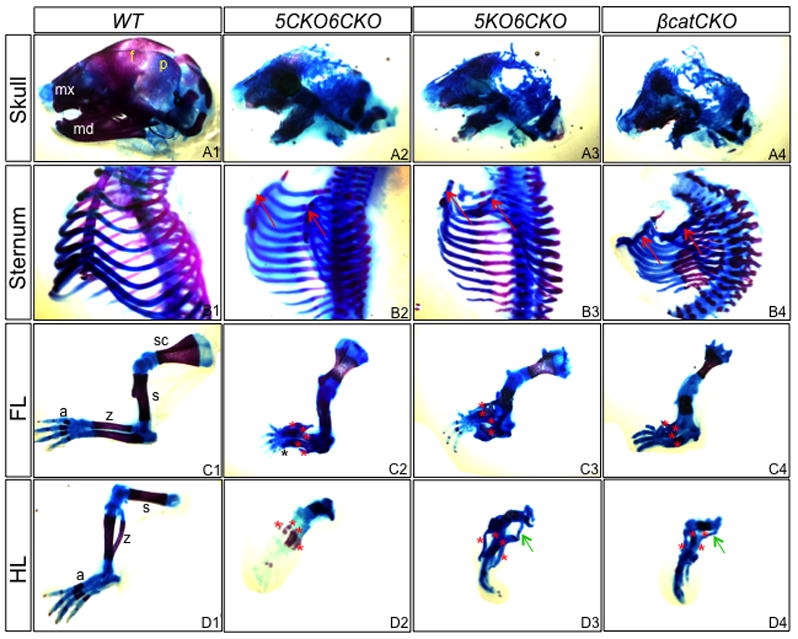
Whole-mount skeletal staining of E18.5 embryos. Blue stains unmineralized cartilage, whereas red stains bone and mineralized cartilage. (A1–A4) Skull. mx: maxilla; md: mandible; f: frontal bone; p: parietal bone. (B1–B2) Sternum. Arrows denote unfused sternum primordia. (C1–C4) Forelimb. (D1–D4) Hindlimb. a: autopod; z: zeugopod; s: stylopod; sc: scapula. Red asterisks denote cartilage elements in zeugopod; black asterisk: additional digit; green arrow: ectopic cartilage. Note ossification in scapula of all genotypes, and in zeugopod of 5CKO6CKO.
To assess the relationship between Lrp5/6 and β-catenin in skeletal development, we compared 5KO6CKO mutants with embryos of the Dermo1Cre;β-cateninf/f genotype (βcatCKO). As previously reported, the βcatCKO embryos exhibited shortening of all skeletal elements, and a general lack of ossification (except for the scapula and the ileum), both features shared with the 5KO6CKO mutant (Fig. 2A4–D4). Other common defects between the two mutants included unfused sterna and supernumerary cartilage elements in the limbs. Finally, histological analyses revealed that both the 5KO6CKO and the βcatCKO embryos, but not 5CKO6CKO lacked joints at multiple locations including the knee (Fig. 3A–D). Overall, the 5KO6CKO and the βcatCKO embryos exhibited very similar morphological defects in their skeleton.
Figure 3.
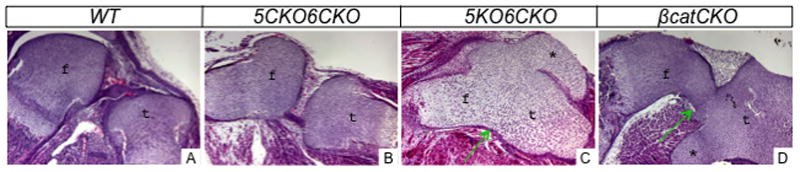
Histology of the knee joint area in E18.5 embryos. f: femur; t: tibia; *:ectopic cartilage. Arrows denote lack of joint.
Lrp5/6 signaling is indispensable for osteoblast differentiation
We next focused on the roles of Lrp5/6 in osteoblast differentiation. H&E or picrosirius red/alcian blue staining of the humerus sections detected no obvious defect in either cartilage or bone in 5KO or 6CKO embryos (Fig. 4A1–A4, B1–B4, C1–C4). On the other hand, the 5CKO6CKO embryo exhibited a partial bone collar but no trabecular bone (Fig. 4D1–D4), whereas the 5KO6CKO mutant possessed no bone collar or trabecular bone (Fig. 4E1–E4). Moreover, in both 5CKO6CKO and 5KO6CKO embryos, the epiphyseal cartilage exhibited abnormal morphology, and ectopic cartilage was present at the diaphysis (Fig. 4D1, E1). Particularly, in the 5KO6CKO embryo, ectopic cartilage surrounded the entire marrow cavity (Fig. 4E3). Both the lack of bone and the presence of ectopic cartilage were also observed in the βcatCKO embryo, although here ectopic cartilage was often seen to extend from the diaphyseal perichondrium into the marrow cavity (Fig. 4F1–F4) (Hilton et al., 2005; Hu et al., 2005).
Figure 4.
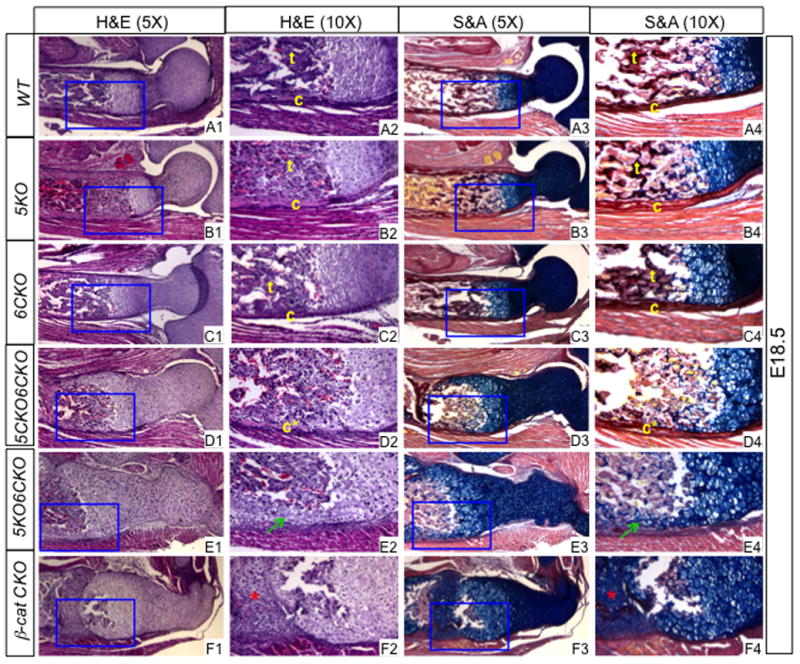
Histology of the humerus at E18.5. (A1–F1) H&E images at a lower magnification. (A2–F2) Boxed regions in (A1–F1) at a higher magnification. (A3–F3) Picrosirius red and alcian blue staining at a lower magnification. (A4–F4) Boxed regions in (A3–F3) at a higher magnification. c: bone collar; c*: partial bone collar; t: trabecular bone. Picrosirius red stains bone matrix dark red whereas alcian blue stains cartilage matrix blue. Green arrows: ectopic cartilage surrounding marrow cavity; red asterisk: ectopic cartilage within presumptive marrow cavity.
The absence of bone prompted us to assess the osteoblast lineage at the molecular level by in situ hybridization. In E18.5 5KO6CKO embryos, the presumptive osteogenic cells in the perichondrium expressed early osteoblast markers including Runx2 and alkaline phosphatase (AP), indicating that the osteogenic program was initiated (Fig. 5B1–B2). However, osterix (Osx), a critical transcription factor downstream of Runx2, was virtually absent, except in occasional small areas (Fig. 5B3). Importantly, the mature osteoblast marker osteocalcin (OC) could not be detected in the 5KO6CKO embryos (Fig. 5B4). This molecular profile of the osteoblast lineage was identical to that in the βcatCKO embryo (Fig. 5C1–C4) (Hu et al., 2005). Thus, in both 5KO6CKO and βcatCKO embryos, mature osteoblasts failed to form likely due to the loss of Osx expression.
Figure 5.
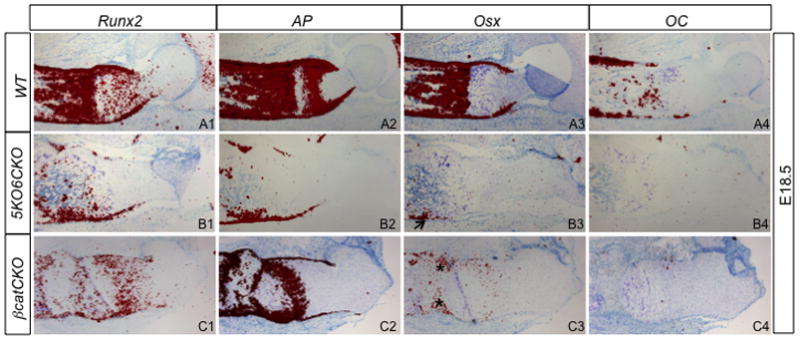
In situ hybridization of osteoblast markers at E18.5. Signal is in red. Arrow denotes occasional Osx signal; asterisks: Osx signal in ectopic cartilage.
To gain further insight about Osx expression in the mutant embryos, we examined E14.5 and E15.5 embryos. Consistent with our previous finding, Osx was either undetectable (E14.5) or barely (E15.5) detectable in the perichondrium of βcatCKO embryos (Fig. 6C3, E2) (Hu et al., 2005). However, in the 5KO6CKO mutant the perichondral expression of Osx was relatively normal at E14.5 (Fig. 6B3). Thus, Osx expression appeared to initiate normally but failed to maintain in the perichondrium in 5KO6CKO mutants. To explore whether the initial expression of Osx could be attributed to residual Wnt signaling in the 5KO6CKO embryo, we examined the expression of Dkk1, a known target of canonical Wnt signaling in the perichondrium. Indeed, Dkk1 was detected in the perichondrium of the 5KO6CKO sample (Fig. 6B4), but was completely absent in βcatCKO at both E14.5 and E15.5 (Fig. 6C4, E3) (Hu et al., 2005). Thus, canonical Wnt signaling appears to be required for both initiation and maintenance of Osx expression.
Figure 6.
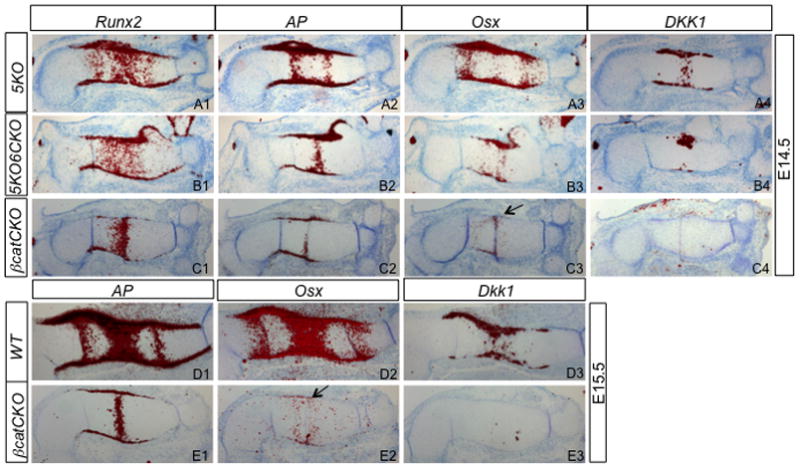
In situ hybridization. 5KO embryos used as normal controls. Arrow denotes no or little Osx expression in the perichondrium of βcatCKO sample.
Lrp5/6 signaling promotes chondrocyte hypertrophy
The severe shortening of the endochondral skeletal elements in the Lrp5/6-deficient embryos indicated that growth of the cartilage anlagen was likely defective. We therefore examined the status of chondrocyte hypertrophy, a major driving force for skeletal linear growth. In the humerus, a distinct zone of hypertrophy normally emerges around E14.5, and this event was undisturbed in the 5KO embryo, as confirmed by both histology and in situ hybridization of Col10a1, a specific marker for hypertropohic chondrocytes (Fig. 7A1–A3). However, in the 5KO6CKO littermate embryo, few hypertrophic chondrocytes were evident morphologically, and the expression domain of Col10a1 was greatly reduced (Fig. 7B1–B3), indicating a marked delay in chondrocyte hypertrophy. The delay was reminiscent of, but slightly less severe than that in the βcatCKO mutant (Hu et al., 2005). Indeed, in the βcatCKO embryo, there was little sign of cellular hypertrophy even at E15 when a hypertrophic zone was normally fully developed in the humerus, consistent with the very limited expression of Col10a1 in the mutant (Fig. 7C1–C3, D1–D3). Thus, loss of Lrp5/6 signaling resulted in a marked delay in chondrocyte hypertrophy that was similar to that caused by β-catenin deficiency.
Figure 7.
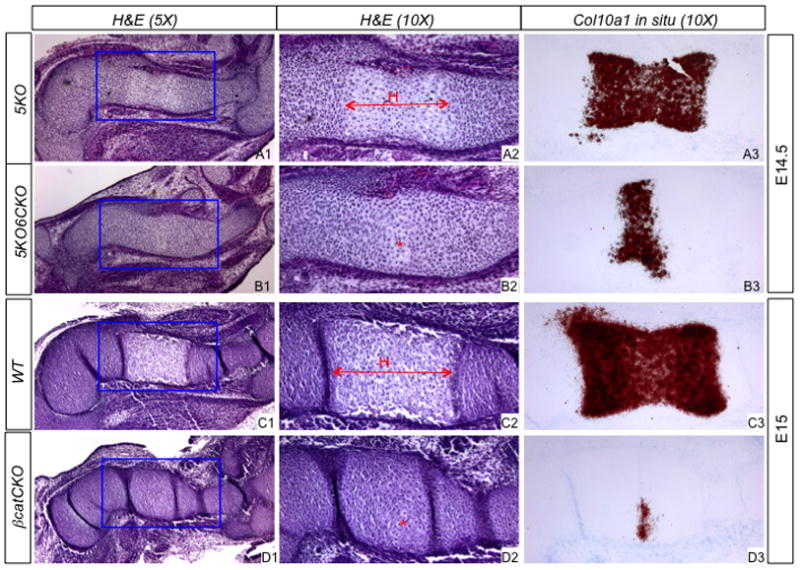
Analyses of chondrocyte hypertrophy. (A1–D1) H&E at a lower magnification. (A2–D2) Boxed regions in (A1–D1) at a higher magnification. (A3–D3) In situ hybridization of Col10a1. H: hypertrophic zone; *: emerging hypertrophic cells.
Deletion of α-catenin has little effect on embryonic skeleton
To discern the potential role of the adherens junction on skeletal development, we deleted α-catenin with Dermo1-Cre. Mice with the genotype of Dermo1-Cre;α-cateninf/f (αCatCKO) were grossly normal. A careful examination of the E18.5 skeleton revealed only a minor delay in ossification in the skull, and a slight shortening of the bone collar in the long bones (Fig. 8A). Histological analyses showed that formation of the marrow cavity appeared to be slightly delayed in the tibia (Fig. 8B). Overall, loss of α-catenin in the limb mesenchyme, in contrast to that of β-catenin, had minimal effects on skeletal development.
Figure 8.
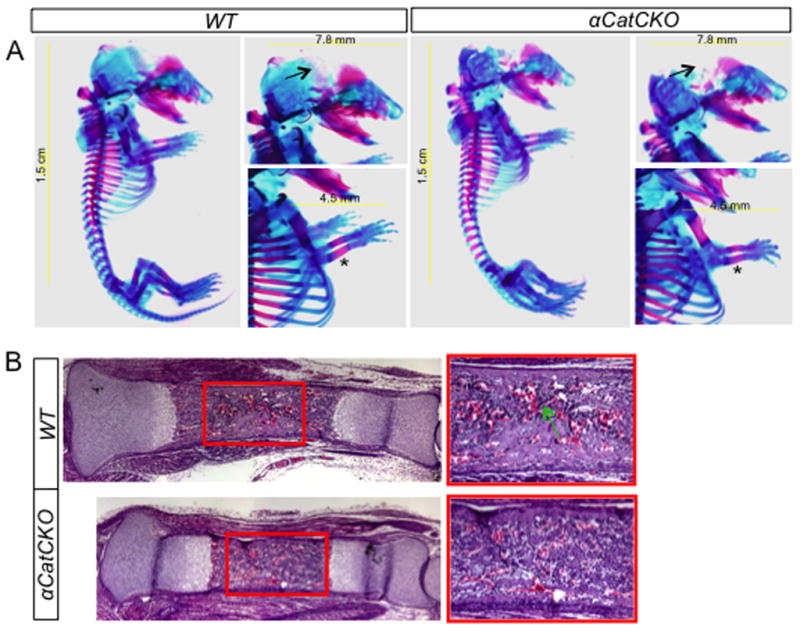
Analyses of the α-catenin CKO embryos at E18.5. (A) Whole-mount skeletal preparations. Arrows denote delay in mineralization of skull in α-catenin CKO mutant; asterisks indicate slight shortening of bone collar in the mutant. (B) Histology of the tibia. Boxed regions shown at a higher magnification to the right. Arrow denotes more abundant myeloid cells present in the wild type bone marrow.
Discussion
By employing genetic deletion of Lrp5 and 6 either singly or in combination, we have established that the two receptors together are indispensable for normal skeletal development in the embryo. The double mutant embryos exhibited a multitude of skeletal defects affecting bone, cartilage and the joints. These findings are not only consistent with the expression of multiple Wnt ligands in the developing skeleton, but more importantly, they recapitulate many phenotypes of the β-catenin-deficient embryos. Therefore, the current results support the concept that canonical Wnt signaling critically controls skeletal development during embryogenesis.
We have focused on the role of Lrp5/6 and β-catenin in osteoblast differentiation. Mice lacking either component failed to produce mature osteoblasts, likely due to the loss of Osx expression. However, a temporal analysis revealed a clear difference between the two mutants. Whereas the β-catenin mutant never initiated a strong Osx expression in the perichondrium, the Lrp5/6 embryos appeared to express Osx normally at E14.5 but failed to maintain it at later stages. This discrepancy could be explained by incomplete deletion of Lrp5/6 at E14.5, as Dkk1, a known target of canonical Wnt signaling (Chamorro et al., 2005; Niida et al., 2004), was still detectable in the perichondrium of these but not the β-catenin embryos. Thus, canonical Wnt signaling through Lrp5/6 and β-catenin could be necessary for both initiation and maintenance of Osx expression, although the data do not exclude that β-catenin may control Osx initiation independently of Lrp5/6. Moreover, a role for β-catenin in Osx maintenance remains to be formally examined, even though β-catenin is necessary for further differentiation of Osx-positive cells (Rodda and McMahon, 2006). Whatever the exact relationship between Lrp5/6 and β-catenin in regulating Osx expression, the current study clearly establishes that Lrp5/6 signaling is indispensable for the formation of mature osteoblasts.
Our study also demonstrates a clear role for Lrp5 in osteoblast differentiation. Such a role has been a matter of debate, because Lrp5n/n mice exhibit normal embryonic bone development, and a deficit in bone mass becomes apparent only later in life (Kato et al., 2002). Moreover, a recent study proposed that Lrp5 affected bone mass indirectly through action in the gut, challenging the role of direct Wnt/Lrp5 signaling in bone (Yadav et al., 2008). We now show that whereas deletion of either Lrp5 or Lrp6 in the skeletogenic mesenchyme caused no obvious defect, a result consistent with what was previously reported for Lrp5 (Yadav et al., 2010), deletion of both abolished the formation of mature osteoblasts. Although a more severe phenotype was observed in embryos with Lrp5 global and Lrp6 tissue-specific deletion, a marked defect was also evident in embryos containing tissue-specific deletions of both genes. The discrepancy in severity between the two types of mutants was likely due to potential differences in the efficiency or timing of Cre-mediated recombination (two versus four floxed alleles). Overall, the results provide strong evidence that Lrp5 is required for osteoblast differentiation when Lrp6 is absent.
The study also revealed an important role for Lrp5/6 signaling in skeletal patterning of the limb. In both 5CKO6CKO and 5KO6CKO embryos, supernumerary cartilage elements were observed in the limbs, a phenotype also seen in the βcatCKO mutants. Interestingly, we previously observed that Lrp5+/−;Lrp6+/− or Lrp5−/−;Lrp6+/− mice also exhibited limb patterning defects, with mice often missing digits (Holmen et al., 2004). Similarly, others have reported that the Lrp6−/− embryos had fewer skeletal elements in both zeugopod and autopod (Collette et al., 2010). The discrepancy in phenotype between the previous and the present mutants may reflect the difference between global (both ectoderm and mesenchyme of the limb) versus mesenchyme-specific deficiency of Lrp5/6 signaling. Overall, Lrp5/6 and β-catenin signaling in the limb mesenchyme critically regulates both early skeletal patterning and subsequent bone formation.
Materials and Methods
Mouse strains
Mouse strains of Dermo1-Cre or β-cateninf/f are as previously described (Brault et al., 2001; Yu et al., 2003). Embryos of Dermo1-Cre;β-cateninf/f were generated as previously described (Hu et al., 2005). The floxed alleles for Lrp5 and Lrp6 were generated using homologous recombination in CJ7 embryonic stem (ES) cell (Swiatek and Gridley, 1993). The Lrp5 targeting vector consisted of a 3.8kb 5′ region of homology (ROH) containing genomic DNA from intron 1 of Lrp5 and a 1.9kb 3′ ROH within intron 2. LoxP sites were placed around exon 2 of Lrp5. For clone selections, an frt-flanked neomycin selection cassette in reverse orientation was included before the 3′ ROH and a Diphtheria toxin selection cassette was placed after the 3′ ROH. The Lrp6 targeting vector consisted of a 2.9 kb 5′ ROH containing genomic DNA from intron 1 of Lrp6 and a 3.0 kb 3′ ROH within intron 2 of Lrp6. LoxP sites were placed around exon 2 of Lrp6. An frt-flanked neomycin selection cassette was included just before the 3′ ROH for positive selection, and a TK cassette was placed after the 3′ROH for negative selection.
Mouse embryo analyses
Whole-mount skeletal staining of embryos was performed according to McLeod with modifications (McLeod, 1980). For sections of E14.5 and E15.5 embryos, tissues were collected in PBS, fixed in 10% Formalin overnight at room temperature, then processed and embedded in paraffin prior to sectioning at 6 μm. For sections of E17.5 and E18.5 embryos, limbs were decalcified in 14% EDTA in PBS, pH 7.4 for 48 hours after fixation and prior to processing. In situ hybridization was performed as described previously by using 35S-labeled riboprobes (Long et al., 2001). All in situ probes were as previously described (Hu et al., 2005; Joeng and Long, 2009; Long et al., 2004; Long et al., 2001).
Highlights.
Single deletion of either Lrp5 or Lrp6 had little effect on embryonic skeleton
Double deletion of Lrp5 and Lrp6 in limb mesenchyme phenocopied β-catenin deletion
Deletion of α-catenin in limb mesenchyme had little effect on skeletal development
Acknowledgments
The work is supported by NIH grants AR060456 (FL), DK065789 (FL), AR053293 (BOW), and by the Van Andel Research Institute (BOW). We thank David Nadziejka for editorial assistance in the preparation of this manuscript, Bryn Eagleson and the VARI vivarium staff for outstanding animal husbandry, and other members of the VARI Center for Skeletal Disease Research, the Williams Laboratory, and the Long Laboratory for discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39:91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68:577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro MN, Schwartz DR, Vonica A, Brivanlou AH, Cho KR, Varmus HE. FGF-20 and DKK1 are transcriptional targets of beta-catenin and FGF-20 is implicated in cancer and development. Embo J. 2005;24:73–84. doi: 10.1038/sj.emboj.7600460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette NM, Genetos DC, Murugesh D, Harland RM, Loots GG. Genetic evidence that SOST inhibits WNT signaling in the limb. Developmental Biology. 2010;342:169–179. doi: 10.1016/j.ydbio.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Niziolek PJ, Macdonald BT, Zylstra CR, Alenina N, Robinson DR, Zhong Z, Matthes S, Jacobsen CM, Conlon RA, Brommage R, Liu Q, Mseeh F, Powell DR, Yang QM, Zambrowicz B, Gerrits H, Gossen JA, He X, Bader M, Williams BO, Warman ML, Robling AG. Lrp5 functions in bone to regulate bone mass. Nature medicine. 2011;17:684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Hilton MJ, Tu X, Cook J, Hu H, Long F. Ihh controls cartilage development by antagonizing Gli3, but requires additional effectors to regulate osteoblast and vascular development. Development. 2005;132:4339–4351. doi: 10.1242/dev.02025. [DOI] [PubMed] [Google Scholar]
- Holmen SL, Giambernardi TA, Zylstra CR, Buckner-Berghuis BD, Resau JH, Hess JF, Glatt V, Bouxsein ML, Ai M, Warman ML, Williams BO. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2004;19:2033–2040. doi: 10.1359/JBMR.040907. [DOI] [PubMed] [Google Scholar]
- Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML, Deng L, Clemens TL, Williams BO. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005;280:21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Birchmeier W. New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev. 2001;11:547–553. doi: 10.1016/s0959-437x(00)00231-8. [DOI] [PubMed] [Google Scholar]
- Joeng KS, Long F. The Gli2 transcriptional activator is a crucial effector for Ihh signaling in osteoblast development and cartilage vascularization. Development. 2009;136:4177–4185. doi: 10.1242/dev.041624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23:860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long F, Chung UI, Ohba S, McMahon J, Kronenberg HM, McMahon AP. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development. 2004;131:1309–1318. doi: 10.1242/dev.01006. [DOI] [PubMed] [Google Scholar]
- Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- McLeod MJ. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology. 1980;22:299–301. doi: 10.1002/tera.1420220306. [DOI] [PubMed] [Google Scholar]
- Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. The Journal of biological chemistry. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- Swiatek PJ, Gridley T. Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes & development. 1993;7:2071–2084. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- Tu X, Joeng KS, Nakayama KI, Nakayama K, Rajagopal J, Carroll TJ, McMahon AP, Long F. Noncanonical Wnt Signaling through G Protein-Linked PKCdelta Activation Promotes Bone Formation. Dev Cell. 2007;12:113–127. doi: 10.1016/j.devcel.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Arantes HP, Barros ER, Lazaretti-Castro M, Ducy P. Genetic analysis of Lrp5 function in osteoblast progenitors. Calcified tissue international. 2010;86:382–388. doi: 10.1007/s00223-010-9350-7. [DOI] [PubMed] [Google Scholar]
- Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]


