Abstract
Ovarian cancer remains the leading cause of death due to gynecologic malignancies. Most patients present with advanced disease at the time of diagnosis. Although many have a good initial response to surgical debulking and platinum-based chemotherapy, relapse is common, with the eventual development of chemotherapy resistance. Innovative treatments are needed in the remission setting to prolong the disease-free interval or prevent recurrence. Abagovomab is a murine monoclonal anti-idiotypic antibody (molecular wieght: 165–175 kDa) that functionally imitates the tumor-associated antigen, CA-125. It has been shown to be well tolerated and to induce a sustained immune response in initial Phase I and II clinical trials. An ongoing, double-blind, placebo-controlled, multicenter, Phase III trial (MIMOSA) completed its double-blind period in December 2010 and will compare abagovomab maintenance therapy to placebo, which will definitively determine the efficacy of this immunotherapeutic approach in patients with ovarian cancer.
Keywords: abagovomab, anti-idiotype, CA-125, ovarian cancer, vaccine
Ovarian cancer is the fifth most common cancer in women and the leading cause of death related to gynecological cancer in both the USA and in Europe [1]. At the time of diagnosis, approximately 67% of patients will have advanced (stage III or IV) disease. The standard treatment for these patients consists of aggressive surgical debulking followed by platinum-based chemotherapy, which can result in a clinical complete remission in 70% of patients. However, the majority will relapse and, while retreatment can produce tumor regression, chemotherapy resistance eventually develops, resulting in a 5-year survival rate for advanced disease of 20–30% [2–4].
The optimal management of patients with advanced ovarian cancer who obtain a clinical remission, or have minimal residual disease after first-line treatment with surgery and platinum-based chemotherapy remains to be determined. A randomized study compared intravenous paclitaxel for 12 versus three cycles (every 28 days) as consolidation therapy in women with a clinical complete remission following initial treatment with surgical debulking and platinum-based chemotherapy; the longer administration period showed a favorable progression-free survival (21 vs 28 months after three and 12 cycles, respectively; p = 0.0035) [5]. This trial was stopped early due to the noted progression-free survival difference by the Data Safety Monitoring Board, but on additional follow-up, no impact in overall survival has been observed (53 vs 48 months; p = 0.34) [6]. The Gynecology Oncology Group (GOG) is currently conducting GOG Group Trial 212 (NCT 00108745), which randomizes patients to receive paclitaxel, paclitaxel poliglumex or observation in first remission, to definitively ascertain the impact of paclitaxel consolidation on overall survival in a prospective fashion. Numerous other negative randomized consolidation approaches include both subcutaneous and intraperitoneal IFN-α [7,8], high-dose chemotherapy [9], continued intravenous carboplatin versus whole abdominal radiotherapy (WART) [10], chemotherapy versus observation versus WART [11], intraperitoneal radioactive phosphorus (32P) [12], ‘noncross resistant’ chemotherapy in the form of cisplatin and 5-fluorouracil for three cycles [13] or topo-tecan for four cycles [14], the monoclonal antibody oregovomab, which targets CA-125 [15] and the SMART study [16]. To date, the application of extended standard cytotoxic agents as a consolidative chemotherapy regimen for patients in a complete remission following primary treatment has been disappointing. No strategy has prolonged overall survival [17].
With the development of novel agents with targets beyond that of standard cytotoxic chemotherapy, particularly in the area of immunotherapeutics, the minimal disease population following standard first-line therapy remains an ideal group in which to intervene, with the hope of prolonging the disease-free interval or, ultimately, preventing relapse.
Overview of the market
According to the National Cancer Institute (NCI), there were an estimated 21,880 new cases of ovarian cancer diagnosed in 2010 and 13,850 women died from this disease [1]. A total of 70% of patients with advanced disease will be in a complete clinical remission following initial debulking surgery and platinum-based chemotherapy [3,18]. However, second-look surgical assessments have historically shown that more than 60% of these patients will actually have persistent disease [19]. Both in vitro and in vivo data suggest that immuno-therapy is most rationally applied to patients with a competent immune system and minimal residual disease state, where the goal is to eradicate remaining malignant cells with minimal associated toxicity [20,21]. An effective immunotherapeutic strategy in the remission patient group (either with a clinically meaningful progression-free or overall survival benefit) would be adopted for general use. Previous standard cytotoxic strategies evaluated in the remission setting have not shown an acceptable risk:benefit ratio or impact on overall survival. The only exception is paclitaxel given as consolidation for 12 months, which was not widely adopted due to its toxicity profile (alopecia and neuropathy) and the lack of proven survival benefit [6]. Current first-line trials are also evaluating the addition of bevacizumab to primary therapy, which includes a consolidation portion following the completion of chemotherapy with paclitaxel and carboplatin, including the GOG (NCT 00262847) and the Medical Research Council ICON 7 (NCT 00483782) studies. The GOG study showed a hazard ratio for first progression of 0.717 (95% CI: 0.625–0.824) in favor of consolidation bevacizumab for a total of 22 cycles. No survival difference has been observed [22]. The clinical importance of this finding is under discussion. If these trials confirm a clinically relevant role for continuing consolidation bevacizumab into the remission setting and abagovomab is also shown to be effective in the ongoing Phase III study (NCT 00418574), it will be logical to evaluate the coadministration of both agents for patients having completed primary therapy and who have achieved a complete response. Alternatively, if both agents have similar efficacy, the adverse-event profile will be important to consider. To date, monoclonal antibody-derived approaches have been distinguished by minimal toxicity, which remains a potential advantage [23]. Interest in the immunomodulatory properties of chemotherapy, when given in combination with immune-directed therapy, is also increasing. Combination studies with abagovomab and other biologic or chemotherapeutic agents will be reasonable if there is evidence of efficacy in the current trial.
Introduction to the compound
Tumor development results from the accumulation of mutational changes that alter normal cell growth and survival pathways. Through the process of immunosurveillance, the innate cellular and humoral immune system of the host is charged with recognizing and destroying mutated cells, providing protection from the development of primary cancers [24]. At the early stages of carcinogenesis, stimulation of an active antitumor immune response is associated with suppression of tumor development. Cancer cells overcome immunosurveillance through the outgrowth of poorly immunogenic tumor cell variants (immunoediting) and through subversion of the immune system (immunosubversion). It is the goal of immuno-therapy to induce new, or re-establish waning, effective antitumor immune responses [25].
Immunotherapy has evolved over the years from nonspecific stimulants such as Bacillus Calmette-Guerin, to autologous cell lysates, to recent advances specifically targeting tumor-associated antigens (TAAs).
Optimal TAAs are preferentially located on tumor cells, such as prostate-specific antigen in prostate cancer, gp100 in melanoma, the bcl/abl rearrangement protein in chronic myeloid leukemia, and CA-125 in ovarian cancer [26]. Typically, they are protein products of genes with unique rearrangements or mutations, differentiation antigens, or other self proteins. CA-125 is a cell-surface high molecular weight (MW) mucin (MUC16) that is expressed by over 80% of nonmucinous epithelial ovarian cancers, and changes in its value are closely associated with disease recurrence and progression [27–29]. MUC16 expression has been directly correlated with platinum resistance and tumor invasiveness [30,31]. Two major obstacles have hampered the development of CA-125-directed immunotherapy. First, these peptides are self-antigens that are tolerated by the host, and attempts at vaccination with irradiated autologous or allogeneic tumor cells or tumor lysates have not produced meaningful immune responses [26,32]. Second, the successful cloning of CA-125, categorizing it as a complex mucin (MUC16), occurred only recently, recognizing it as a massive transmembrane glycoprotein with more than 60 repeat domains and an amino terminus [33]. While it has made the development of directly targeted, synthetic, immunogenic constructs possible, work is necessary to understand which parts of the larger structure need to be included in a vaccine approach, since size prohibits immunization with the entire construct [34–36].
Over the last decade, while awaiting the development of more directly targeted immune strategies towards MUC16, antibodies against CA-125 have been raised (avoiding the need to understand the exact structure of the antigen) and used as immunogens in a variety of studies. Antibodies used as cancer immuno-therapy can initiate tumor antigen-specific immune responses and have been shown to induce antibody-dependent cellular cytotoxicity, trigger the idiotypic network and promote antibody-targeted cross-presentation of tumor antigens [23].
Oregovomab (MAb B43.13), which is an IgG1k subclass murine monoclonal antibody that binds with high affinity (1.16 × 1010/M) to circulating CA-125, was recently evaluated. In addition to the expected human anti-mouse-antibody (HAMA) response, both a cellular and humoral immune response has been observed with the production of anti-oregovomab antibodies (Ab2), T-helper cells, and cytotoxic T cells [37–39]. Retrospective and prospective Phase II trials have consistently associated a longer overall survival with immune response [40,41]. A randomized, placebo-controlled trial of 145 patients with stage III or IV epithelial ovarian cancer in first clinical remission, receiving intravenous oregovomab showed a median time to progression from randomization post-front-line therapy of 13.3 months for patients receiving oregovomab versus 10.3 months for those receiving placebo (p = 0.71). In a favorable subgroup of patients (≤ 2 cm residual at debulking, CA-125 ≤65 U/ml before third cycle, and CA-125 ≤35 U/ml at entry), time to progression for patients receiving oregovomab versus placebo was 24 months versus 10.8 months (HR: 0.543; 95% CI: 0.287–1.025) [15]. Subsequently, using the eligibility criteria of the subgroup as hypothesis generating, the investigators prospectively enrolled 354 patients in first clinical remission in a recently reported Phase III trial. Median time to progression was 10.3 months (95% CI: 9.7–13.0 months) for the oregovomab group, and 12.9 months (95% CI: 10.1–17.4 months) for the placebo group (p = 0.29), demonstrating no benefit to oregovomab immunotherapy [42].
Another antibody strategy is represented by the use of anti-idiotype (anti-id) vaccines, which attempt to increase the antigenicity of the immunogen (in this case the antibody) by presenting the desired epitope to the now tolerant host in a different molecular environment. The ‘immune network hypothesis,’ which provides support for this mechanism was first proposed in the 1970s and describes an interconnected group of idiotypes expressed by antibodies [43].
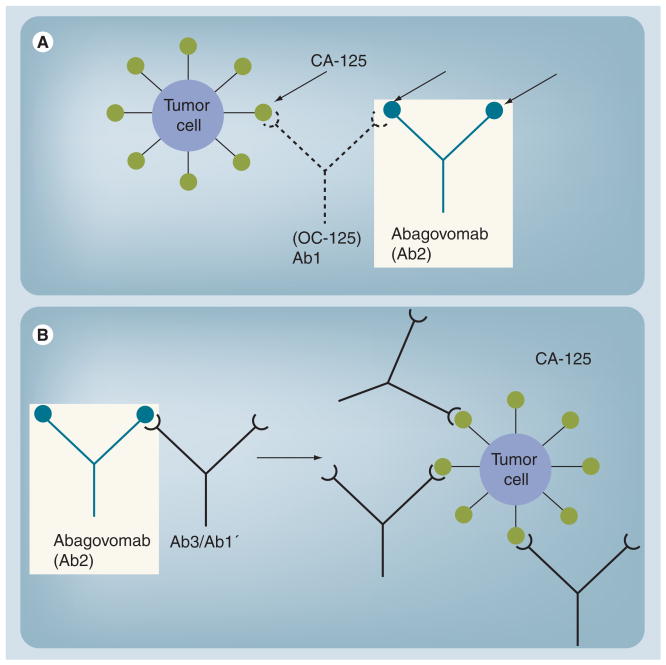
This approach assumes that immunization with a given antigen will generate the production of antibodies against this antigen (termed antibody 1 [Ab1]). Ab1 can generate anti-idiotype antibodies against Ab1, termed Ab2. Some of the anti-idiotypic antibodies (Ab2β) express the internal image of the antigen recognized by the Ab1 antibody and can be used as surrogate antigens [44]. Immunization with Ab2β (the anti-idiotype antibody) can cause the production of anti-anti-id antibodies (termed Ab3) that recognize the corresponding original antigen identified by Ab1. Ab3 antibodies are also denoted Ab1′ to indicate that they may differ in their other epitopes compared with Ab1 [45]. The relationship of these antibodies to each other is illustrated in Figure 1 (Investigators Brochure, Menarini, 2006).
Figure 1. Relationship of Ab1 and Ab2 to CA-125.
(A) The injection of a tumor-associated antigen-binding antibody (Ab1) leads to an immune response containing antibodies (Ab2) mimicking the structure of the original antigen. (B) Vaccination with a tumor-associated antigen-mimicking antibody (Ab2) leads to an immune response directed to the original antigen.
Modified from investigator brochure, with permission.
Animal studies have demonstrated the characteristics of the immune response from immunization with anti-idiotypic vaccines. Both the murine anti-id mAb 3H1 (targeting Carcino Embryonic Antigen [CEA]) in C57Bl/6 (H-2b) sygeneic mice and anti-id hybridomas, targeting L210 leukemia in DBA/A2 mice, have been shown to inhibit tumor growth in leukemia and colorectal cancer cell lines, respectively [46,47].
A variety of older, and generally nonrandomized, clinical studies including patients with colon cancer, melanoma, small-cell lung cancer, lymphoma and neuroblastoma administered anti-idiotypic vaccines. They have demonstrated the induction of both antitumoral, humoral and cellular immune responses, and some have shown an association with disease-free survival and a selected immune end point. The interpretation of clinical benefit is confounded by the lack of randomization, and the heterogeneity of enrolled patients and prospective studies were needed [48–51].
The putative immune mechanisms are not well described. The anti-id antibodies are exogenous proteins and are thought to be taken into antigen-presenting cells by endocytosis and degraded to smaller peptides. These are subsequently presented by MHC class II antigens to activate CD4 T-helper cells. These activated T cells secrete IL-4 and IL-10, which in turn stimulate B cells that are now primarily activated by the anti-id (Ab2β) to produce Ab1′. Ab1′ binds to the original antigen on the tumor cells. An additional pathway is represented by the induction of HLA class I antigen restricted TAA-specific cytotoxic T lymphocytes (CTLs). Peptides containing HLA class I antigen binding motifs can be presented in the context of MHC class I antigens to activate CD8 cytotoxic T cells, which have also been stimulated by IL-2, secreted by the CD4 helper T cells. While the details may not be complete, the ability of anti-id antibody vaccines to break immune tolerance and develop both humoral and cellular responses, albeit of unknown clinical benefit, has been demonstrated in multiple studies [26,52].
Chemistry & pharmacodynamics
Abagovomab is a murine monoclonal anti-idiotypic antibody (MW: 165–175 kDa), produced by a mouse hybridoma and generated against OC125, which serves to functionally imitate the human TAA, CA-125. It (Ab2) shows high affinity for the paratope of Ab1 (binding affinity 2 × 109 l/mol), and binding of Ab2 to Ab1 is almost completely inhibited (93%) by the nominal antigen. The administration of F (Ab')2 fragments of abagovomab to rats produces anti-CA-125 immunity with the production of IgG and IgM anti-anti-idiotypic antibodies (Ab3) that bind both to abagovomab and CA-125. Non-MHC restricted cell-mediated cytotoxicity for NIH OVCAR3 cell lines is also demonstrated [53].
Analysis by SDS-PAGE in nonreducing conditions shows a MW between 164–kDa, consistent with this type of product. The apparent isoelectic points (pI) are between 8.8–9.2. The drug product for subcutaneous injection is a white suspension containing 2 mg/ml of the anti-idiotypic antibody, abagovomab absorbed onto aluminum hydroxide and suspended in a buffered and isotonic saline solution to adjust the pH to 7.5. Stability study results indicate that the drug substance is stable for at least 24 months at -20°C and at +5°C (Investogators Brochure, Menarini Richerche, 2006). The drug substance was produced in the facilities of Goodwin Biotechnology, Inc. (FL, USA) under the supervision of Menarini Ricerche and Menarini Biotech, according to GMP standards in 2006.
Clinical efficacy
Abagovomab has been evaluated in three clinical studies, beginning with a Phase Ib/II study to primarily demonstrate the safety of abagovomab and its ability to stimulate a specific immunologic response. This was followed by two Phase I trials that evaluated the effect of different doses, routes of administration and durations of treatment on the safety and immune responses prior to selecting the optimal dosage and schedule for the pivotal Phase III trial. The Phase Ib/II study showed a strong association with the production of Ab3 and overall survival. The results of the first three studies have been reported, while the pivotal Phase III trial Monoclonal antibody Immunotherapy for Malignancies of Ovary by Subcutaneous Abagovomab (MIMOSA) has completed accrual and efficacy results are expected by the second quarter of 2011.
Phase I/II studies
The initial Phase Ib/II study reported on the first 42 patients available in 2001 and the entire 119-patient intention-to-treat population in 2004 [54,55]. Patients with ovarian cancer, fallopian tube cancer or peritoneal carcinoma, as well as a history of a debulking surgery and prior platinum-based chemotherapy were included. Patients were administered vaccinations of 2 mg of alum-coupled anti-id ACA-125, which was injected intramuscularly into the gluteal muscle. Doses were administered every 2 weeks for a total of four doses during the induction phase, and this was followed by monthly doses until the time of disease progression.
Patients received a median of 4.9 months of abagovomab treatment (range: 0.5–54.8 months). The intramuscular injections were well tolerated; 93.5% of adverse events were grade I and there were no severe adverse events. The most frequently reported adverse events included local injection site reactions (n = 25), nausea (n = 5), fever (n = 3), vomiting (n = 3), chills (n = 2) and leucopenia (n = 2). No evidence for autoimmunity was observed. The study population was heterogeneous with regards to disease bulk as well as treatment received, prior to, and postenrollment.
A specific Ab3 response directed against the anti-id vaccine developed in 68.1% (n = 81) of patients. An antibody-dependent cell-mediated cytotoxicity response was observed in the serum of 26.9% (n = 32) of patients. The median survival of all patients was 19.4 months (range: 0.5–56.1 months). Notably, the group of patients with an Ab3 response displayed a significantly longer survival compared with patients without an immune response (median: 23.5 vs 4.9 months; p <0.001). This association was hypothesis generating, but the clinical significance could not be determined from this nonrandomized study. Data in this non-randomized study suggests that both Ab3 and Ab1′ titers could potentially serve as a predictive biomarkers for clinical responses, and both should be correlated with outcome in the randomized trial.
Two additional Phase I studies of abagovomab were performed in order to determine the optimal dose, schedule and route of administration. The first included 36 patients with recurrent ovarian, fallopian tube or peritoneal cancer, who received either a short-arm or a long-arm vaccination schedule in order to compare safety and immunologic response between the arms [56]. Patients in the short arm received a subcutaneous injection of 2 mg of abagovomab every 2 weeks for four doses during the induction phase, followed by two additional monthly subcutaneous injections. Those in the long arm received the same dose of abagovomab subcutaneously every 2 weeks for four doses, followed by five additional monthly injections during the consolidation phase.
Eligible patients had received initial surgery and platinum-based chemotherapy, followed by relapse treated with a course of chemotherapy within 6 weeks prior to enrollment. The primary end point was dropout rate owing to toxicity, and secondary end points were humoral immune responses in terms of Ab3, HAMA induction and cellular immune responses with regards to CA-125-specific cytotoxicity of CD8+ and CD4+ T cells.
The complete vaccination schedule was completed in 16 patients (89%), treated on the short arm and eight patients (44%), treated on the long arm. The main cause for premature termination was disease progression. Toxicity was not a cause for premature termination in either arm, and adverse events were primarily grade 1–2, most often hematologic toxicities, injection site reactions, nausea, fatigue and abdominal pain.
In total, three patients were not evaluated for an immune response as a result of premature termination from disease progression, or death, prior to Ab3 assessment. Ab3 assessment occurred after five vaccinations and only those patients who received five or more vaccinations were able to be evaluated. Of the 33 patients who did receive five or more vaccinations, all patients showed an increase in Ab3 titer (defined as >1000 ng/ml). Ab3 titer increased during the course of treatment in both groups and persisted in postvaccination follow-up. The median Ab3 titer at 6 weeks' follow-up from the last injection tended to be higher in the long arm (359.6 μg/ml [range: 98.9–988.7]) than in the short arm (209.6 μg/ml [range: 8.6–618.9]). However, this difference was not statistically significantly different (p = 0.0556). HAMA was evaluable in 16 (88.8%) and 17 patients (94.4%) in the long-arm and short-arm groups, respectively. No significant difference was observed in HAMA titers between the arms. CA-125-specific antibodies (Ab1′) were detected in 13 patients (short arm) and six patients (long arm), providing no evidence that a longer treatment duration enhanced this effect. A greater than twofold increase of IFN-γ expressing CA-125-specific CD8+ T cells was observed in nine patients in the long-arm group (75%) and three patients in the short-arm group (17.6%; p = 0.006). A greater than twofold increase in CD4+ CA-125-specific T cells was observed in seven patients in the long-arm group (58.3%) and five patients in the short-arm group (29.4%; p = 0.148). No reliable correlation between the induction of Ab3 and the frequency of CA-125-specific CTLs and helper cells were seen in any group. Overall, this trial demonstrated the tolerability of repeated administration of abagovomab and provided supporting evidence for the induction of an active immune response using subcutaneous administration of the drug.
The second Phase I trial examined the safety and efficacy of two different doses (2 mg or 0.2 mg) of abagovomab, administered either by the intramuscular or subcutaneous route [57]. This multicenter trial evaluated patients with stage II, III or IV ovarian, fallopian tube or primary peritoneal epithelial carcinoma. All patients had relapsed and completed a course of chemotherapy for recurrence prior to enrollment. Patients were allowed to have small-volume residual disease (<2 cm), elevated CA-125 level or complete clinical remission. Four cohorts were examined in order to evaluate safety and efficacy of both dosage and route of administration: 0.2 mg intramuscularly; 2 mg intramuscularly; 0.2 mg subcutaneously; or 2 mg subcutaneously. Patients received an injection every 2 weeks for a total of four injections during the induction phase, and then monthly for a total of two injections during the maintenance phase.
A total of 42 patients received at least one injection with abagovomab, and 66.7% of patients received all six scheduled vaccines (range: two to six injections). All 42 patients were included in the safety analysis. Abagovomab was well tolerated. The most common adverse events included grade I fatigue, fever, myalgia, headache or injection-site reaction. Grade I leucopenia was observed in 10% of patients, and grade II anemia was observed in 12% of patients. No clinical or laboratory evidence of autoimmunity was seen. There were five serious adverse events (intestinal obstruction: 3, abdominal pain: 1, venous thrombosis: 1), all of which were considered unlikely to be related to abagovomab.
A total of 33 patients received four or more injections and were evaluated for immunologic response. In all treatment cohorts, evaluated Ab3 titer was positive at the first evaluation point (week 10) and further increased in subsequent follow-up samples (100% response rate). Neither the route of administration nor the dose led to statistically significant variability in the maximum post-baseline Ab3 titer reached. In a subset of 25 patients with sufficient sera, IFN-γ and IL-10 were measured pre- and 16 weeks post-treatment in order to evaluate for induction of cellular immunity. The difference between pre- and post-levels of IFN-γ and IL-10 was found to be statistically significant from 0 (p < 0.0001 and p = 0.0097, respectively) based on a signed-rank test. The results of this study confirmed the induction of a cellular and humoral response in all evaluable patients, and a good tolerability of the 2-mg dose of abagovomab was demonstrated. The study showed no difference between the immunogenicity induced by the subcutaneous versus intramuscular route of administration, and the subcutaneous route was selected for future study given the ease of administration.
Phase III study
The efficacy of abagovomab is currently being evaluated in a pivotal international Phase III, randomized, double-blind, placebo-controlled study at approximately 120 locations. Eligible patients have a histological and CA-125-confirmed (> 35 U/ml) diagnosis of stage III-IV epithelial ovarian, fallopian tube or primary peritoneal cancer and have had a complete remission following debulking surgery and six to eight cycles of standard platinum/taxane-based intravenous or intraperiteonal chemotherapy. Patients receive a subcutaneous injection of either 2-mg/ml abagovomab or placebo every 2 weeks, for a total of four doses (induction phase), and then monthly as maintenance therapy until relapse or 45 months of therapy are completed. The primary outcome to be evaluated is recurrence-free survival; overall survival, safety and time course of immune response will be evaluated as secondary outcomes. Enrollment was completed in December 2008. The double-blind period was completed in December 2010 and efficacy data is expected to follow by the second quarter of 2011. Preliminary immunogenicity results were recently reported, with 888 patients ultimately enrolled in the study. Blinded immunologic results (data for weeks 10 and 22) showed that 68 and 69% of overall patients were positive for Ab3 (median: 62.000 and 337.000 ng/ml, respectively) while 53 and 63% of patients were positive for HAMA (median: 510 and 644 ng/ml, respectively). This study has confirmed an immunologic response similar to those observed in previous trials [58]. The incidence of discontinuation for toxicity is low and is comparable to the Phase I/II studies, confirming good tolerability. The blinded nature of the data does not permit a preliminary analysis of efficacy.
Safety & tolerability
As is typical of other anti-idiotype vaccines, abagovomab has been well tolerated in prior clinical trials [48–51]. The most common drug-related adverse events were self-limited and consisted of grade I fatigue, fever, myalgia or localized injection site reaction. Anemia and leucopenia were mild and likely partially attributable to pretreatment effect [55–57]. Grade III or higher toxicities were rare and are listed in Table 1. The preliminary safety results of the ongoing Phase III randomized trial were recently reported and confirm a similar tolerability profile. Of 888 enrolled patients, 20 potentially treatment-related adverse events were reported (blinded), and 9.3% of patients withdrew owing to an adverse event. The most common adverse events were injection site reaction and fatigue, and were reported in 76.6 and 16.2% of patients, respectively [58].
Table 1. Phase I/II clinical trials with abagovomab.
| Study | Dose (mg) | Route | Dosing schedule | Prior therapy | n | Results | Toxicity | Ref. |
|---|---|---|---|---|---|---|---|---|
| Wagner et al. (2001) and Reinartz et al. (2004) | 2 | IM | Q 2 weeks × 4 doses then monthly until progression | Surgical debulking and platinum-based chemotherapy | 119 | Ab3 induced in 68% of patients. Ab3+ patients had improved survival | 93.5% of events grade I. No severe adverse events | [54,55] |
| Sabbatini et al. (2006) | 2 vs 0.2 | IM vs SC | Q 2 weeks × 4 then monthly × 2 | Relapsed disease followed by chemotherapy | 42 for SA 33 for AA | No difference in Ab3 response with either dose or administration. Persistent rise in Ab3 titers in all tested patients | Most common Grade I or II: fever, fatigue, HA, myalgia, ISR, leukemia, anemia. 5 serious adverse events felt to be unrelated | [59] |
| Pfsterer et al. (2006) | 2 | SC | Q 2 weeks × 4 then monthly × 2 short arm or monthly × 5 long arm | Relapsed diease followed by platinum-based chemotherapy | 36 | All patients evaluated showed increase in Ab3 titers | Most common Grade I or II: anemia, leukemia, ISR, fatigue, nausea, Abd pain. No treatment-limiting toxicity in either arm | [56] |
AA: Ab3 analysis; Abd: Abdominal; HA: Headache; IM: Intramuscular; ISR: Injection site reaction; Pts: Patients; Q: Every; SA: Saftey analysis; SC: Subcutaneous.
Regulatory affairs
Based on the Phase Ib/II data presented, a pivotal randomized, double-blind Phase III trial (MIMOSA), with efficacy as the end point, completed accrual in December 2008. Follow-up is ongoing, with results expected by the second quarter of 2011. Abagovomab is currently only available for clinical use through enrollment in a clinical trial.
Conclusion
Ovarian cancer remains the fifth most common cancer in women and the most lethal gynecologic malignancy. Most patients will respond to initial debulking surgery and platinum-based chemotherapy, with a clinical complete remission, but eventually they will relapse and subsequently develop chemotherapy resistance. An effective strategy, to be applied in the clinical remission setting to prolong the disease-free interval or prevent relapse, is needed. Abagovomab, an anti-idiotype antibody directed against the TAA CA-125, has been shown to be well tolerated in initial Phase I/II studies and in a preliminary report of the randomized Phase III MIMOSA trial. It induces a specific Ab3 response in 68.1–100% of treated patients, as well as induction of cellular immunity in some patients, as characterized by an increase in IFN-γ and IL-10 titers [55,59]. Furthermore, the initial Phase Ib/II trial demonstrated improved survival (median: 23.4 vs median 4.9 months; p < 0.001) in those patients exhibiting an immune response to abagovomab treatment compared with those who did not. While initial Phase I/II trials have demonstrated an immune effect and have confirmed the safety and tolerability of abagovomab administered intramuscularly or subcutaneously at a 2-mg or 0.2-mg dose, the results of the ongoing pivotal Phase III, randomized trial expected by the second quarter of 2011 will be necessary to determine efficacy.
Future perspective
The remission population of patients with epithelial ovarian, fallopian tube, or peritoneal cancer is an ideal one in which to evaluate immune-targeted strategies. This population is at high risk for recurrence and improving outcome in this patient group is needed. Immunotherapy conceptually offers a promising treatment modality for eradication of microscopic or minimal residual disease with a low rate of associated side effects. If efficacy is observed in the ongoing pivotal Phase III study of abagovomab, with no unacceptable toxicity, its administration would likely become a part of standard maintenance therapy following initial surgical debulking and chemotherapy in all patients with advanced disease. It would then be logical to evaluate the administration of abagovoamb in patients who have relapsed and returned to a second or subsequent remission, as well as the coadministration with bevacizumab or another VEGF-targeted approach if they are shown to have meaningful benefit for women with ovarian cancer.
Executive summary.
-
▪
Patients with ovarian cancer in remission represent an unmet need with regards to the development of novel, nontoxic strategies to prolong remission or prevent recurrence.
-
▪
Abagovomab is a murine, monoclonal, anti-idiotypic antibody (molecular weight: 165–175 kDa) that functionally imitates the tumor associated antigen, CA-125.
-
▪
Prior studies have demonstrated the safety, tolerability and immunogenicity of immunization with abagovomab, and have associated survival with immune response.
-
▪
An ongoing, double-blind, placebo-controlled, multicenter Phase III trial (MIMOSA) is comparing abagovomab maintenance therapy with placebo, and will definitively determine the efficacy of this immunotherapeutic approach in patients with ovarian cancer in first remission.
Acknowledgments
US enrollment was supported by The Cooperative Ovarian Cancer Group for Immunotherapy (COGI), the US cooperative group around which the accruing sites were organized.
Footnotes
Financial & competing interests disclosure: Jonathan Berek, Jacobus Pfisterer and Paul Sabbatini served as the principal or coprincipal investigators of the MIMOSA study. No authors received direct support funding. Support was provided to the institutions in order to conduct the research study. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Markman M, Markman J, Webster K, et al. Duration of response to second-line, platinum-based chemotherapy for ovarian cancer: implications for patient management and clinical trial design. J Clin Oncol. 2004;22(15):3120–3125. doi: 10.1200/JCO.2004.05.195. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DK, Bundy B, Wenzel L, et al. Gynecologic Oncology Group: Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 4.Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 5.Markman M, Liu PY, Wilczynski S, et al. Southwest Oncology Group; Gynecologic Oncology Group: Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a Southwest Oncology Group and Gynecologic Oncology Group trial. J Clin Oncol. 2003;21(13):2460–2465. doi: 10.1200/JCO.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 6▪.Markman M, Liu PY, Moon J, et al. Impact on survival of 12 versus 3 monthly cycles of paclitaxel (175 mg/m2) administered to patients with advanced ovarian cancer who attained a complete response to primary platinum-paclitaxel: follow-up of a Southwest Oncology Group and Gynecologic Oncology Group Phase III trial. Gynecol Oncol. 2009;114(2):195–198. doi: 10.1016/j.ygyno.2009.04.012. The only randomized study suggesting a benefit (albeit progression-free survival only) for consolidation therapy to date. It has not been adopted given toxicity and lack of survival benefit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall GD, Brown JM, Coleman RE, et al. Maintenance treatment with interferon for advanced ovarian cancer: results of the Northern and Yorkshire Gynaecology Group randomised Phase III study. Br J Cancer. 2004;91(4):621–626. doi: 10.1038/sj.bjc.6602037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberts DS, Hannigan EV, Liu PY, et al. Randomized trial of adjuvant intraperitoneal α-interferon in stage III ovarian cancer patients who have no evidence of disease after primary surgery and chemotherapy: an intergroup study. Gynecol Oncol. 2006;100(1):133–138. doi: 10.1016/j.ygyno.2005.07.117. [DOI] [PubMed] [Google Scholar]
- 9.Schilder RJ, Brady MF, Spriggs D, Shea T. Pilot evaluation of high-dose carboplatin and paclitaxel followed by high-dose melphalan supported by peripheral blood stem cells in previously untreated advanced ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2003;88(1):3–8. doi: 10.1006/gyno.2003.6882. [DOI] [PubMed] [Google Scholar]
- 10.Lambert HE, Rustin GJ, Gregory WM, Nelstrop AE. A randomized trial comparing single-agent carboplatin with carboplatin followed by radiotherapy for advanced ovarian cancer: a North Thames Ovary Group study. J Clin Oncol. 1993;11(3):440–448. doi: 10.1200/JCO.1993.11.3.440. [DOI] [PubMed] [Google Scholar]
- 11.Sorbe B. Consolidation treatment of advanced ovarian carcinoma with radiotherapy after induction chemotherapy. Int J Gynecol Cancer. 2003;13(Suppl. 2):192–195. doi: 10.1111/j.1525-1438.2003.13359.x. [DOI] [PubMed] [Google Scholar]
- 12.Varia MA, Stehman FB, Bundy BN, et al. Gynecologic Oncology Group: Intraperitoneal radioactive phosphorus (32P) versus observation after negative second-look laparotomy for stage III ovarian carcinoma: a randomized trial of the Gynecologic Oncology Group. J Clin Oncol. 2003;21(15):2849–2855. doi: 10.1200/JCO.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Nicoletto MO, Tumolo S, Falci C, et al. A randomized study of epithelial ovarian cancer: is chemotherapy useful after complete remission? Int J Med Sci. 2004;1(2):116–125. doi: 10.7150/ijms.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Placido S, Scambia G, Di Vagno G, et al. Topotecan compared with no therapy after response to surgery and carboplatin/paclitaxel in patients with ovarian cancer: Multicenter Italian Trials in Ovarian Cancer (MITO-1) randomized study. J Clin Oncol. 2004;22(13):2635–2642. doi: 10.1200/JCO.2004.09.088. [DOI] [PubMed] [Google Scholar]
- 15▪.Berek JS, Taylor PT, Gordon A, et al. Randomized, placebo-controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer. J Clin Oncol. 2004;22(17):3507–3516. doi: 10.1200/JCO.2004.09.016. Randomized study of the murine, monoclonal oregovomab, directed at CA-125, which unfortunately showed no benefit. [DOI] [PubMed] [Google Scholar]
- 16.Verheijen RH, Massuger LF, Benigno BB, et al. Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission. J Clin Oncol. 2006;24(4):571–578. doi: 10.1200/JCO.2005.02.5973. [DOI] [PubMed] [Google Scholar]
- 17▪.Sabbatini P. Consolidation therapy in ovarian cancer: a clinical update. Int J Gynecol Cancer. 2009;19(Suppl. 2):S35–39. doi: 10.1111/IGC.0b013e3181c14007. This review summarizes consolidation strategies to date. [DOI] [PubMed] [Google Scholar]
- 18.Dizon DS, Hensley ML, Poynor EA, et al. Retrospective analysis of carboplatin and paclitaxel as initial second-line therapy for recurrent epithelial ovarian carcinoma: application toward a dynamic disease state model of ovarian Cancer. J Clin Oncol. 2002;20(5):1238–1247. doi: 10.1200/JCO.2002.20.5.1238. [DOI] [PubMed] [Google Scholar]
- 19.Muggia FM, Braly PS, Brady MF, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2000;18(1):106–115. doi: 10.1200/JCO.2000.18.1.106. [DOI] [PubMed] [Google Scholar]
- 20.Slavin S, Ackerstein A, Or R, et al. Immunotherapy in high-risk chemotherapy-resistant patients with metastatic solid tumors and hematological malignancies using intentionally mismatched donor lymphocytes activated with rIL-2: a Phase I study. Cancer Immunol Immunother. 2010;59(10):1511–1519. doi: 10.1007/s00262-010-0878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez SA, von Hofe E, Kallinteris NL, et al. A new era in anticancer peptide vaccines. Cancer. 2010;116(9):2071–2080. doi: 10.1002/cncr.24988. [DOI] [PubMed] [Google Scholar]
- 22.Burger RA, Brady MF, Bookman MA, et al. Phase III trial of bevacizumab in the primary treatment of advanced epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer: a Gynecology Oncology Group Study. J Clin Oncol. 2010;28 doi: 10.1016/j.ygyno.2013.07.100. Abstract LBA1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23▪.Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373(9668):1033–1040. doi: 10.1016/S0140-6736(09)60251-8. Summarizes monoclonal antibodies used for cancer immunotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 25.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6(10):715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharya-Chatterjee M, Chatterjee S, Foon K. Anti-idiotype antibody vaccine therapy for Cancer. Expert Opin Biol Ther. 2002;2(8):869–881. doi: 10.1517/14712598.2.8.869. The rationale and supporting evidence for anti-idiotype vaccine theraepy is presented. [DOI] [PubMed] [Google Scholar]
- 27.Juretzka MM, Barakat RR, Chi DS, et al. CA125 level as a predictor of progression-free survival and overall survival in ovarian cancer patients with surgically defined disease status prior to the initiation of intraperitoneal consolidation therapy. Gynecol Oncol. 2007;104(1):176–180. doi: 10.1016/j.ygyno.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 28.Berek JS, Taylor PT, Nicodemus CF. CA125 velocity at relapse is a highly significant predictor of survival post relapse: results of a 5-year follow-up survey to a randomized placebo-controlled study of maintenance oregovomab immunotherapy in advanced ovarian Cancer. J Immunother. 2008;31(2):207–214. doi: 10.1097/CJI.0b013e31816060ce. [DOI] [PubMed] [Google Scholar]
- 29.Duffy MJ, Bonfrer JM, Kulpa J, et al. CA125 in ovarian cancer: European Group on Tumor Markers guidelines for clinical use. Int J Gynecol Cancer. 2005;15(5):679–691. doi: 10.1111/j.1525-1438.2005.00130.x. [DOI] [PubMed] [Google Scholar]
- 30.Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29(20):2893–2904. doi: 10.1038/onc.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gubbels JA, Belisle J, Onda M, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5(1):50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leffers N, Fehrmann RS, Gooden MJ, et al. Identification of genes and pathways associated with cytotoxic T-lymphocyte infiltration of serous ovarian cancer. Br J Cancer. 2010;103(5):685–692. doi: 10.1038/sj.bjc.6605820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Brien TJ, Beard JB, Underwood LJ, Dennis RA, Santin AD, York L. The CA125 gene: an extracellular superstructure dominated by repeat sequences. Tumour Biol. 2001;22(6):348–366. doi: 10.1159/000050638. [DOI] [PubMed] [Google Scholar]
- 34▪.Yin BW, Dnistrian A, Lloyd KO. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. Int J Cancer. 2002;98(5):737–740. doi: 10.1002/ijc.10250. Preliminary information regarding the complexity of the mucin encoding CA125 is described. [DOI] [PubMed] [Google Scholar]
- 35.Lloyd KO, Yin BW, Kudryashov V. Isolation and characterization of ovarian cancer antigen CA-125 using a new monoclonal antibody (VK-8): identification as a mucin-type molecule. Int J Cancer. 1997;71(5):842–850. doi: 10.1002/(sici)1097-0215(19970529)71:5<842::aid-ijc24>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 36.Yin BW, Lloyd KO. Molecular cloning of the CA-125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276(29):27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 37.Noujaim AA, Schultes BC, Baum RP, Madiyalakan R. Induction of CA-125-specific B- and T-cell responses in patients injected with MAb-B43.13 – evidence for antibody-mediated antigen-processing and presentation of CA125 in vivo. Cancer Biother Radiopharm. 2001;16(3):187–203. doi: 10.1089/10849780152389384. [DOI] [PubMed] [Google Scholar]
- 38.Berek JS, Schultes BC, Nicodemus CF. Biologic and immunologic therapies for ovarian cancer. J Clin Oncol. 2003;21(Suppl. 10):168–174. doi: 10.1200/JCO.2003.01.517. [DOI] [PubMed] [Google Scholar]
- 39.Baum RP, Noujaim AA, Nanci A, et al. Clinical course of ovarian cancer patients under repeated stimulation of HAMA using MAb OC125 and B43.13. Hybridoma. 1993;12(5):583–589. doi: 10.1089/hyb.1993.12.583. [DOI] [PubMed] [Google Scholar]
- 40.Ehlen TG, Hoskins PJ, Miller D, et al. A pilot Phase II study of oregovomab murine monoclonal antibody to CA125 as an immunotherapeutic agent for recurrent ovarian cancer. Int J Gynecol Cancer. 2005;15(6):1023–1034. doi: 10.1111/j.1525-1438.2005.00483.x. [DOI] [PubMed] [Google Scholar]
- 41.Möbus VJ, Baum RP, Bolle M, et al. Immune responses to murine monoclonal antibody-B43.13 correlate with prolonged survival of women with recurrent ovarian cancer. Am J Obstet Gynecol. 2003;189(1):28–36. doi: 10.1067/mob.2003.347. [DOI] [PubMed] [Google Scholar]
- 42.Berek J, Taylor P, McGuire W, Smith LM, Schultes B, Nicodemus CF. Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer. J Clin Oncol. 2009;27(3):418–425. doi: 10.1200/JCO.2008.17.8400. [DOI] [PubMed] [Google Scholar]
- 43.Jerne NK. Towards a network theory of the immune system. Ann Immunol. 1974;125:373–389. [PubMed] [Google Scholar]
- 44.Herlyn D, Ross AH, Koprowski H. Anti-idiotypic antibodies bear the internal image of a human tumor antigen. Science. 1986;232(4746):100–102. doi: 10.1126/science.3952496. [DOI] [PubMed] [Google Scholar]
- 45.Herlyn D, Harris D, Zaloudik J, et al. Immunomodulatory activity of monoclonal anti-idiotypic antibody to anti-colorectal carcinoma antibody CO17–1A in animals and patients. J Immunother Emphasis Tumor Immunol. 1994;15(4):303–311. doi: 10.1097/00002371-199405000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Raychaudhuri S, Kang CY, Kaveri SV, Kieber-Emmons T, Köhler H. Tumor idiotype vaccines. VII. Analysis and correlation of structural, idiotypic and biologic properties of protective and nonprotective Ab2. J Immunol. 1990;145(2):760–767. [PubMed] [Google Scholar]
- 47.Ruiz PJ, Wolkowicz R, Waisman A, et al. Idiotypic immunization induces immunity to mutated p53 and tumor rejection. Nat Med. 1998;4(6):710–712. doi: 10.1038/nm0698-710. [DOI] [PubMed] [Google Scholar]
- 48.Yáñez R, Barrios Y, Ruiz E, Cabrera R, Díaz-Espada F. Anti-idiotypic immunotherapy in follicular lymphoma patients: results of a long follow-up study. J Immunother. 2008;31(3):310–312. doi: 10.1097/CJI.0b013e31816a8116. [DOI] [PubMed] [Google Scholar]
- 49.Neninger E, Díaz RM, de la Torre A, et al. Active immunotherapy with 1E10 anti-idiotype vaccine in patients with small-cell-lung cancer: report of a Phase I trial. Cancer Biol Ther. 2007;6(2):145–150. doi: 10.4161/cbt.6.2.3574. [DOI] [PubMed] [Google Scholar]
- 50.Saleh MN, LoBuglio AF, Wheeler RH, et al. A Phase II trial of murine monoclonal antibody 17–1A and interferon-γ: clinical and immunological data. Cancer Immunol Immunother. 1990;32(3):185–190. doi: 10.1007/BF01771455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Posner MC, Niedzwiecki D, Venook AP, et al. A Phase II prospective multi-institutional trial of adjuvant active specific immunotherapy following curative resection of colorectal cancer hepatic metastases: Cancer and Leukemia Group B study 89903. Ann Surg Oncol. 2008;15(1):158–164. doi: 10.1245/s10434-007-9654-7. [DOI] [PubMed] [Google Scholar]
- 52▪.Foon KA, Bhattacharya-Chatterjee M. Are solid tumor anti-idiotype vaccines ready for prime time? Commentary re: Wagner U et al.: Immunological consolidation of ovarian carcinoma recurrences with monoclonal anti-idiotype antibody ACA125: immune responses and survival in palliative treatment. Clin Cancer Res. 2001;7:1154–1162. Clin. Cancer Res. 7(5), 1112–1115 (2001) Summarizes strategies for utilizing an anti-idotypic approach to cancer immunization. [PubMed] [Google Scholar]
- 53.Schlebusch H, Wagner U, Grünn U, Schultes B. A monoclonal anti-idiotypic antibody ACA 125 mimicking the tumor- associated antigen CA125 for immunotherapy of ovarian cancer. Hybridoma. 1995;14(2):167–174. doi: 10.1089/hyb.1995.14.167. [DOI] [PubMed] [Google Scholar]
- 54.Wagner U, Köhler S, Reinartz S, et al. Immunological consolidation of ovarian carcinoma recurrences with monoclonal anti-idiotype antibody ACA 125: immune responses and survival in palliative treatment. See: The biology behind: Foon KA & Bhattacharya-Chatterjee M, Are solid tumor anti-idiotype vaccines ready for prime time? Clin Cancer Res. 2001;7:1112–1115. Clin. Cancer Res. 7(5), 1154–1162 (2001) [PubMed] [Google Scholar]
- 55▪.Reinartz S, Köhler S, Schlebusch H, et al. Vaccination of patients with advanced ovarian carcinoma with the anti-idiotype ACA125: immunological response and survival (Phase Ib/II) Clin Cancer Res. 2004;10(5):1580–1587. doi: 10.1158/1078-0432.ccr-03-0056. First clinical report of abagovomab that describes an association between immune response and clinical outcome. [DOI] [PubMed] [Google Scholar]
- 56.Pfisterer J, du Bois A, Sehouli J, et al. The anti-idiotypic antibody abagovomab in patients with recurrent ovarian cancer. A Phase I trial of the AGO-OVAR. Ann Oncol. 2006;17(10):1568–1577. doi: 10.1093/annonc/mdl357. [DOI] [PubMed] [Google Scholar]
- 57.Sabbatini P, Spriggs DR. Consolidation for ovarian cancer in remission. J Clin Oncol. 2006;24(4):537–539. doi: 10.1200/JCO.2005.04.5138. [DOI] [PubMed] [Google Scholar]
- 58.Sabbatini P, Berek JS, Casado A, et al. Abagovomab maintenance therapy in patients with epithelial ovarian cancer after complete response (CR) post-first-line chemotherapy (FLCT): preliminary results of the randomized, double-blind, placebo-controlled, multicenter MIMOSA trial. J Clin Oncol. 2010;28 Abstract 5036. Summarizes safety and immunologic data available to date, from the ongoing, blinded Phase III trial. [Google Scholar]
- 59.Sabbatini P, Dupont J, Aghajanian C, et al. Phase I study of abagovomab in patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer. Clin Cancer Res. 2006;12(18):5503–5510. doi: 10.1158/1078-0432.CCR-05-2670. [DOI] [PubMed] [Google Scholar]