Abstract
Steroid hormones, such as progesterone (P), are typically thought of as being primarily secreted by the gonads (albeit adrenals can also be a source) and having their actions through cognate intracellular progestin receptors (PRs). Through its actions in the midbrain Ventral Tegmental Area (VTA), P mediates appetitive (exploratory, anxiety, social approach) and consummatory (social, sexual) aspects of rodents’ mating behavior (Frye et al., 2006a). However, P and its natural metabolites (“progestogens”) are produced in the midbrain VTA independent of peripheral sources and midbrain VTA of adult rodents is devoid of intracellular PRs. One approach we have used to understand P’s effects and mechanisms in the VTA for mating is manipulate P’s actions in the VTA and to examine effects on lordosis (the posture female rodents assume for mating to occur). This review focuses on the effects and mechanisms of progestogens to influence reproduction and related proce sses. Actions of P and its 5α-reduced metabolite and neurosteroid, 5α-pregnan-3α-ol-20-one (3α,5α-THP; allopregnanolone) in the midbrain VTA to facilitate mating is described. The findings that 3α,5α-THP biosynthesis in the midbrain occurs with mating are discussed. Evidence for 3α,5α-THP’s actions in the midbrain VTA via non-traditional steroid targets is summarised. The broader relevance of these actions of 3α,5α-THP, for aspects of reproduction, beyond lordosis, are summarised. Finally, the potential role of the pregnane xenobiotic receptor in mediating 3α,5α-THP biosynthesis in the midbrain is introduced.
Keywords: neurosteroid, progesterone, lordosis, midbrain ventral tegmental area, steroid biosynthesis, steroid metabolism
Introduction
Beyond their well-known effects in the body for maintaining pregnancy, progesterone (P) and its natural products (termed “progestogens”), such as 5α-pregnan-3α-ol-20-one (3α,5α-THP; allopregnanolone), have robust actions at the level of the central nervous system (CNS). In addition to what can be considered these non-traditional effects of progestogens in the CNS, progestogens have sources and mechanisms that cannot be explained by the traditional dogma of what neuroendocrine signaling entails. Of interest is further understanding the non-traditional effects of steroids in general, but a focus in this review will be novel actions of progestogens, which share characteristics with other steroids. Specifically, research from our laboratory is aimed at elucidating the effects, sources, and mechanisms by which progestogens mediate motivated behaviours, such as reproduction and other reproductively-relevant behaviours. A particular emphasis is on actions of progestogens in the midbrain ventral tegmental area (VTA), as described further in this review, to influence such motivated behaviours. To this end, the approach we first used to understand progestogens’ effects and mechanisms in the VTA for mating was to manipulate progestogens’ actions in this region and to examine effects on lordosis, the stereotypical mating posture that female rodents assume under appropriate hormonal and environmental contexts, as a bioassay. These studies have been followed up by investigating whether there are similar effects of progestogens in reproductively-relevant behaviours, such as aggression and affect, which will be discussed in this review. What follows is a review of some of the main results and recent findings regarding the sources and mechanisms of progestogens using this model to understand the role of progestogens for mating behaviour within a broader context of understanding non-tradtional neuroendocrine signaling for other motivated behaviours.
Progestogens as neurosteroids and their functional effects
Neurosteroids are steroids that are synthesised in the CNS, rather than peripheral glands (gonads, adrenals, and/or placenta; 1, 2). Levels of neurosteroids are typically greater in the CNS than in circulation (1, 2). Enzymes involved in peripheral gland steroidogenesis have been identified in the CNS (3, 4). As well, high CNS levels of neurosteroids persist after surgical removal of peripheral glands (gonadectomy-GDX and/or adrenalectomy-ADX; 1, 2, 5–7). Thus, some neurosteroids in the CNS of rodents are produced by local biosynthesis.
De novo production of progestogens in brain
Progestogens can be formed centrally from biosynthesis and/or metabolism. The brain, like the adrenals, gonads, and placenta, is an endocrine organ and can produce neurosteroids independent of peripheral glands to form neuroactive products (neuroactive steroids; 1, 2, 5–8). The BZRP gene mediates the expression of the translocator protein (18 kDa; TSPO, previously known as the peripheral-type benzodiazepine receptor) that is widely expressed throughout the body, particularly in steroidogenic tissues (9, 10). TSPOs import cholesterol into the mitochondria, whereupon it is oxidised to pregnenolone by two proteins that initiate steroidogenesis – the steroidogenic acute regulatory (StAR) protein and cytochrome P450-dependent C27 side chain cleavage enzymes (P450scc), rate-limiting steps in steroid biosynthesis, or production of steroids from the precursor, cholesterol (11–13). The latter involves a nuclear pregnane xenobiotic receptor (PXR) that regulates gene transcription for cytochrome P450 enzymes, which may be necessary for biosynthesis of 3α,5α-THP (14–16). Pregnenolone is converted by 3β-hydroxysteroid dehydrogenase (3β-HSD) to P. P, from central or peripheral sources, is metabolised irreversibly by 5α-reductase (5α-R) to dihydroprogesterone (DHP), followed by actions of 3α-hydroxysteroid dehydrogenease enzymes (3α-HSD), to form 3α,5α-THP (2). P is also a precursor of androstenedione and deoxycorticosterone (2). Levels of brain enzyme expression are highest in midbrain, limbic regions (cortex, hippocampus, basal ganglia, hypothalamus, thalamus), cerebellum, tectum, pons, medulla, spinal cord, and pituitary (3, 4, 17–19).
Progestogens’ organizational and activational effects for reproduction
Hormones, secreted by the gonads, may have both organizational and activational effects. Organizational effects of hormones occur during critical periods of development (typically pre- or peri-natally) and can result in permanent changes in CNS structure (i.e. sexually dimorphic areas of the hypothalamus) and/or function (hypothalamic–pituitary–gonadal (HPG) and/or adrenal (HPA) axes; 21). These changes can alter physiological and/or behavioural processes among females and males. P and its natural metabolites (DHP and 3α,5α-THP) exert profound activational and/or organizational effects. Among adult female rodents, a salient activational effect of progestogens is evidenced when levels are increased around ovulation, and in response to mating-relevant stimuli (22–24). This enhancement of progestogens facilitates mating behaviour and promotes expression of the stereotypical lordosis posture in response to a sexual contact among adult female rodents (8, 22–24). Research from our laboratory is focused on the sources and mechanisms of progestogens in the midbrain VTA. To this end, reproductive responding (lordosis) is utilized as a bioassay to further understand progestogens’ role in the midbrain VTA.
Biosynthesis of progestogens in the midbrain to facilitate mating behaviour
A question is the source of progestogens for reproductive behavior. Whether increased levels of 3α,5α-THP in midbrain are due to central biosynthesis and/or dependent upon ovarian sources was of interest. The midbrain VTA is one brain target of interest given its key role in sexual behavior and other processes mediated by the dopaminergic system and because there is high expression of steroid biosynthesis and metabolism enzymes in this region. To investigate the role of central biosynthesis versus metabolism of ovarian P in the midbrain VTA for mating, pro-oestrous rats were infused with inhibitors of 3α,5α-THP formation to the midbrain VTA, such as PK11195 (an inhibitor of TSPO), indomethacin (an inhibitor of 3α-HSD), and/or vehicle. PK11195 inhibits 3α,5α-THP formation from cholesterol, while indomethacin blocks DHP metabolism to 3α,5α-THP. Infusions of any combination of inhibitor significantly attenuated midbrain 3α,5α-THP levels of pro-oestrous rats concomitant with reductions in sexual behaviour (20). In order to assess whether central 3α,5α-THP is necessary and sufficient for these effects, pro-oestrous rats were infused with 3α,5α-THP subsequent to inhibitor infusions and results indicated a reinstatement of sexual behaviour that was commensurate to that of vehicle-infused controls (20). In a follow-up study, behavioural effects of infusions of a neurosteroid enhancer (FGIN 1,27, a TSPO agonist) following inhibitor infusion was assessed and revealed that enhancement of natural central biosynthesis could overcome effects of 3α,5α-THP inhibitors (8). Thus, these data suggest that central biosynthesis of 3α,5α-THP in the VTA is necessary and sufficient to enhance expression of sexual behaviours in pro-oestrous rats.
Progestogens’ traditional mechanisms of action involving progestin receptors (PRs)
Actions of steroids through nuclear PRs
Nuclear steroid receptors are typically found in the nucleus or cytoplasm of cells. These intracellular receptors are activated when hydrophobic steroids freely diffuse through cell membranes and bind to them. The steroid-receptor complex then translocates to the nucleus, where it binds to hormone response elements on genes, which changes the rates of transcription and translation (25). This traditional “genomic” action of steroids involving transcription of DNA and synthesis of protein is a relatively slow process, taking minimally 10 min, but more often hours to days, to elicit a biological response (26, 27).
PRs are intracellular, cognate, steroid receptors for P that were first characterised nearly 40 years ago (28). As with other steroid receptors in the nuclear receptor superfamily, PRs bind ligands and act as transcription factors to evoke expression of mRNAs leading to changes in protein synthesis, and, thereby, biological actions (29, 30). In their unliganded state, PRs complex with chaperone molecules, such as several heat shock proteins, which is necessary for binding in the presence of P (31, 32). When bound by ligand, there are conformation changes in the PRs, dimerization, and interactions with response elements of PRs (PREs) in the promoter regions of target genes (33–36). However, these PREs are not always required to instate gene-mediated changes in protein synthesis (37–39).
Progestogen’s actions via PRs in the hypothalamus to facilitate initiation of lordosis
Although P can have many behavioural effects, one focus has been on its role in reproductive behaviours, such as lordosis. P can have actions through PRs in the ventromedial hypothalamus (VMH) to facilitate lordosis, and expression of PRs is increased coincident with initiation of sexual receptivity (29). Activation or knockdown of PRs in the hypothalamus using PR antagonists, anti-sense oligodeoxynucleotides (ODNs) to PR mRNA, or PR gene disruption in rodents, demonstrate initiation and attenuation of lordosis, respectively (40–47). These, and other findings, suggest that PRs are critical for initiation of P-facilitated reproductive behaviours, such as lordosis.
Progestogens non-classical actions involving PRs
There are also “non-genomic” mechanisms involving PRs and activation of second messenger signalling molecules (48, 49). Indeed, PRs are expressed in many regions of the CNS (e.g. amygdala, bed nucleus of the stria terminalis, hypothalamus, hippocampus, cortex, preoptic area, the ventromedial and dorsomedial nucleus of the hypothalamus, arcuate nucleus, cerebellum; 50–59), in most cell types. There is some indication that both forms of PR expression (PR-A and PR-B) can vary by brain region or cell type, such as reproductive tissues, as a function of hormonal milieu (60–63). Sex differences and hormonal mediation of PR-A and PR-B expression has been observed in the olfactory bulbs, hippocampus, and cerebellum of rats (54,55). As well, non-genomic actions of progestogens in the hypothalamus may involve factors, such as calmodulin-dependent protein kinase II (64). Thus, it is possible that progestogens can have actions independent of classic mechanisms via PRs.
Actions of progestogens independent of nuclear PRs
Steroid hormones play fundamental roles in the development and/or function of the CNS; however, the effects and mechanisms of action associated with steroid hormones needs to be better understood. Steroids are typically considered to have actions through nuclear steroid receptors. In addition to the relatively slow, genomic actions that have traditionally characterised steroid-mediated effects, steroids can also exert rapid and “non-genomic” actions. Indeed, steroids can act on target tissues faster (from milliseconds to 10 minutes) than the time required for transcriptional activity of nuclear steroid receptors (26, 27). These rapid, “non-genomic” actions of steroids have been investigated over the past few decades and have been demonstrated for all the major classes of steroids (65–68). The effects, substrates, and mechanism of such “non-genomic” steroid actions are of great interest.
Progestogens seem to have actions independent of nuclear PRs in a number of systems. In gametes, there are rapid, non-genomic actions of progestogens, including rapid increases in sperm motility and in the induction of the sperm acrosomal reaction (69–74). Progestogens also have rapid effects on oocyte maturation in fish and amphibian species (75–77). In brain, there are rapid effects of progestogens on gonadotropin-releasing hormones (78–80). In addition, there is a rapid activation of breast cancer cell signaling (81) and signal transduction pathways in human T lymphocytes and Jurkat cells that lack nuclear PRs (82). Thus, there are many instances of progestogens exerting rapid effects independent of nuclear steroid receptors.
Progestogen’s actions via PRs in the midbrain VTA are not required for lordosis
Observations of the relationship between progestogens in the midbrain VTA and lordosis quotient have been conserved across multiple rodent species, including rats, hamsters, and mice (Figure 1). In rodents, the facilitation of lordosis occurs, in part, by oestrogen (E) and P through actions via cognate PRs in the ventral portion of the VMH (83). The duration and intensity of lordosis is mediated by actions of P in the midbrain VTA (17, 18). However, there are very few intracellular PRs localised to the VTA of rodents in adulthood and these are not inducible by E (84–87). Infusion of PR anti-sense ODNs to the VTA does not alter lordosis, while manipulations directed to the VMH reduce lordosis (17, 18, 40, 88). However, intravenous or intra-VTA infusions of P to hamsters or rats, rapidly enhances firing of VTA neurons within 60 s (89–90) and facilitates lordosis within 2 minutes (91, 92). This occurs even when P’s actions are relegated to cell membranes (93, 94). Thus, P’s actions in the VTA to facilitate lordosis do not require classic actions via intracellular PRs.
Figure 1.
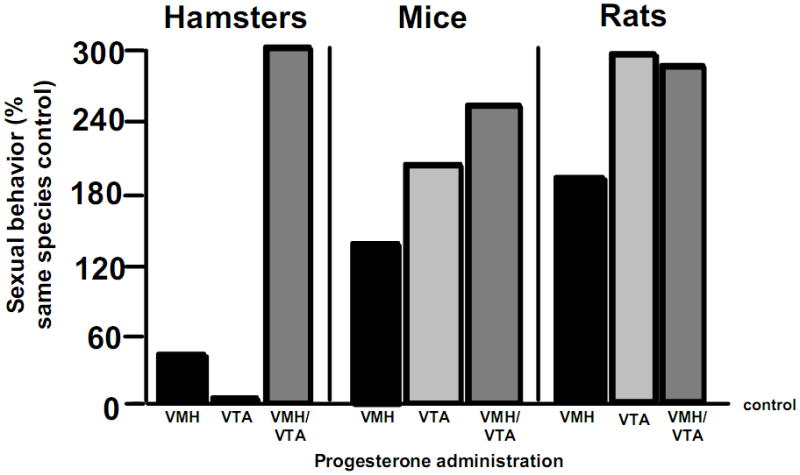
There are species differences in dependence on progesterone (P) for enhancement of lordosis via direct administration to the ventromedial hypothalamus (VMH) and/or ventral tegmental area (VTA) of the midbrain, across rodents. Data shown are percentage of sexual behavior of rodents administered P compared to those administered oestrogen (E) subcutaneously. Rats have greatest response to P to the VTA and/or VMH, mice have a moderate response to P to VTA and/or VMH, and hamsters require P to both the VTA and VMH for sexual behavior.
Actions of progestogens in the midbrain VTA via non-traditional steroid targets to facilitate mating behaviour
Manipulating P’s actions in the VTA of naturally sexually-receptive, or ovariectomised (OVX), E-primed rodents, and observing changes in sexual responding, has elucidated P’s functionally-relevant mechanisms for mating. In the VTA, P’s metabolite, 3α,5α-THP, has actions to facilitate mating (17, 18, 95, 96). 3α,5α-THP is devoid of affinity for cognate, intracellular PRs, but can have agonist-like actions at γ-aminobutyric acid (GABA) receptors, dopamine type 1-like receptors (D1), and antagonist-like actions at N-methyl-D-aspartate type glutamate receptors (NMDARs; 8, 40, 97–100) in the VTA to facilitate mating, as described in the next section.
It is beyond the scope of this review to exhaustively cover the plethora of non-steroidal targets that progestogens have. For example, 3α,5α-THP can have actions through other non-steroidal targets, which can include ligand-gated, ion channels and/or G protein-coupled receptors, such as glycine, glutamate (e.g. kainite, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid- AMPA), norepinephrine, dopamine, serotonin, acetylcholine, oxytocin and nicotinic/muscarinic receptors, as well as sigma type 1 and 2 receptors (101, 102). Indeed, we have investigated the effects of an oxytocin antagonist to the VTA. In this study, hormone-primed, OVX hamsters were administered the oxytocin antagonist, t,d(CH(2))(5),[TYr(ME)(2),Thr(4),Tyr-NH(9,2)], and the time spent in lordosis during a 10-minute test with a sexually-experienced male was determined. The oxytocin antagonist attenuated lordosis (Figure 2), an effect similar to what has been observed among rats (103). Moreover, 3α,5α-THP can alter neuronal function of membrane E receptors (104) and G protein-coupled membrane PRs (105). Although neurosteroids have many targets, our focus has been on actions through GABAA receptors, NMDARs, and D1 receptors, as described as follows.
Figure 2.
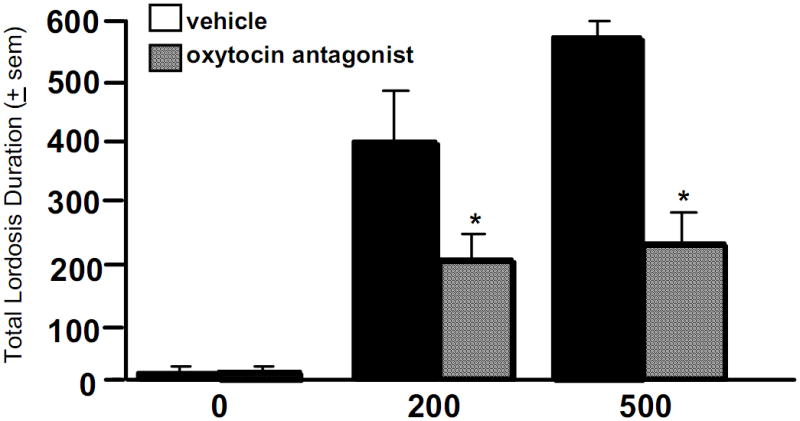
Subcutaneous injections of progesterone (P) dose-dependently (500 > 200 μg) enhances the duration of lordosis among ovariectomised hamsters. This effect is attenuated with subsequent administration of an oxytocin antagonist, t, d(CH(2))(5),[TYr(ME)(2),Thr(4),Tyr-NH(9,2)], via bilateral infusions to the ventral tegmental area (VTA), compared to saline vehicle infusions. * indicates significantly different from vehicle-administered controls, p < 0.05.
Progestogens have actions via GABA to mediate lordosis
Progestogens have actions via the GABAergic system. Anatomical and biochemical studies indicate that the number, density, and affinity of GABAA receptors are modified by hormones. In support, number (106), density (107), and affinity (108) of GABAA receptors are enhanced during pro-oestrous compared to other phases of the oestrous cycle. The number of GABAA receptor binding sites also tended to be greater in the midbrain of hormone-primed, compared to OVX, hamsters (109). Levels of GABA are also increased in the midbrain of sexually receptive, compared to non-receptive, hamsters (17, 18). In a competitive [3H] muscimol (GABA mimetic ligand) binding assay, we observed that the concentration of GABA necessary to displace 50% of [3H] muscimol from its binding site, is significantly less in the midbrain of hormone-primed and mated hamsters compared to OVX hamsters (109). Thus, progestogens can alter GABA levels and GABA receptors.
Through actions via GABAA receptors in the VTA, progestogens mediate appetitive (exploratory, anxiety, social approach) and consummatory (social, sexual) aspects of rodents’ mating behaviour (17, 18, 96). Infusions of muscimol, a GABA mimetic, to the VTA increases lordosis of hamsters or rats administered low dosages of systemic P (94, 110–112). Administration of the metabotropic GABAB agonist, baclofen (which does not activate ionotropic GABAA receptors), to the VTA enhances lordosis of OVX, E and P-primed rats (Figure 3). Pharmacological blockade of GABAA receptors, via infusions of GABAA antagonists, bicuculline or picrotoxin, to the VTA, attenuates P- facilitated lordosis of hamsters or rats compared to vehicle infused controls (40, 109, 111, 112). GABA is synthesised in neurons by the enzyme glutamic acid decarboxylase (GAD), from the precursor, glutamate, and is degraded by GABA-transaminase. Infusions of GAD antisense to the VTA of hormone-primed and pro-oestrous rats or hamsters disrupt lordosis, and reduce GAD immunoreactivity in the midbrain, compared to vehicle or sense infusions (88, 113). Infusion of valproate, a GABA-transaminase inhibitor, to the VTA of hamsters facilitates lordosis (111). Thus, enhancement and blockade of GABAA receptors in the VTA of hormone-primed rodents mediates expression of lordosis, and may be influenced by progestogen actions at GABAA receptors.
Figure 3.
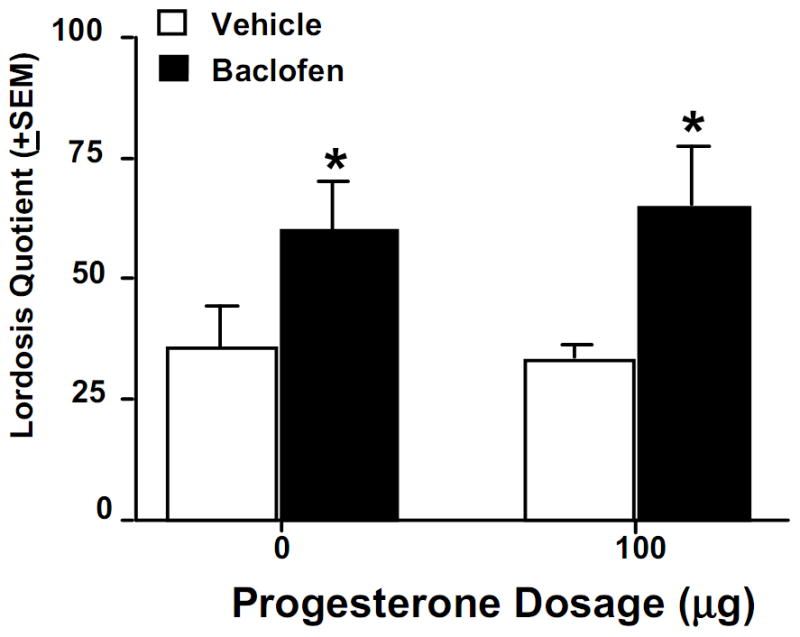
Systemic administration of the GABAB agonist, baclofen, enhances lordosis among ovariectomised, oestrogen (E)- and progesterone (P)-primed, rats compared to vehicle administration. * indicates significantly different from vehicle-administered controls, p < 0.05.
3α,5α-THP is among the most effective ligands for GABAA receptors. Structural requirements associated with progestogens that are the most effective positive modulators of GABAA receptors include A-ring reduction at the 5 position (42), which is characteristic of 3α,53α-THP. At nanomolar concentrations, 3α,5α-THP alters the duration of GABA current (114). In nanomolar concentrations, 3α,5α-THP directly activates GABAA receptors and is a positive, allosteric modulator. 3α,5α-THP increases chloride channel currents and lowers neuronal excitability with 20 and 200- fold higher efficacy than benzodiazepines or barbiturates, respectively (115–121). 3α,5α-THP is approximately 60 times more potent than P at enhancing GABAA receptor function (79). Compared to P, 3α,5α-THP is more effective at rapidly inhibiting binding of the convulsant tert-butylbicyclophosphorothionate to the GABA-operated chloride channel (122), potentiating GABA’s effects on chloride uptake (123), and increasing flunitrazepam binding (79). Infusion of progestogens to the VTA of rats and hamsters, resulting in facilitation of lordosis, corresponds to their ability to enhance GABA- stimulated chloride influx and SR 95531 receptor binding in the VTA (113). Thus, 3α,5α-THP’s lordosis-enhancing effects in the rodent VTA may occur in part via GABAA receptors.
Progestogens have actions via NMDARs and D1 receptors to mediate lordosis
Many of the GABAergic neurons that exist in the midbrain VTA also express NMDARs (124). Indeed, it has been proposed that post-synaptic glutamate receptors, such as NMDARs, AMPA/kainate, and metabotropic glutamate receptors, mediate reinforcing effects via their modulatory activity on DA cell bodies (125). Dopaminergic cell bodies in the VTA have glutamatergic and GABAergic inputs and co-localise NMDARs (126, 127). Given this neuronal circuitry in the midbrain VTA and findings that 3α,5α-THP reduces glutamate’s excitatory effects (1`28), this suggests that progestogens may also have actions via NMDARs and dopamine signaling.
NMDARs may be a substrate for progestogens’ actions for lordosis. Autoradiography studies indicate OVX rats administered P and/or E have reduced NMDA binding in cortex (129, 130). Midbrain levels of glutamate are increased with P administration (17, 18). Antagonising NMDARs via intra-VTA infusions of MK-801 or LY235959 enhances progestogen-facilitated lordosis (99, 100). These data indicate actions of 3α,5α-THP in the VTA may occur, in part, through its antagonist-like actions at NMDARs. Presynaptic actions on the mesocorticolimbic dopamine pathway may be a key mechanism by which dopaminergic drugs and GABAA receptor modulators (drugs or progestogens) achieve their effects on sex behaviour.
Dopaminergic neurons in the midbrain VTA are another substrate through which 3α,5α-THP has actions to facilitate lordosis. Dopamine cell bodies are localised to the VTA, and activity of D1 receptors can regulate their function (131). GABAergic terminals in the VTA, which have D1, synapse on dopaminergic cell bodies that contain GABAA receptors and NMDARs (126, 127). Increased GABA input onto GABAA receptors that are located on GABAergic interneurons in the VTA (131) reduces the inhibitory actions of these cells on dopamine-containing cell bodies and increases somatodendritic dopamine release (133). This may account in part for enhancements in lordosis, produced by muscimol infusions to this region. Progestogens increase D1 density in the striatum and dopamine levels in the midbrain (17, 18, 134). D1 antagonists attenuate, and D1 agonists enhance, progestin-facilitated lordosis when administered to the VTA (135, 136). Moreover, infusions to the VTA of the GABAA receptor antagonist, bicuculline, attenuate the facilitatory effects of the D1 agonist, SKF38393, and/or progestogens for lordosis (98). Another consideration to make is that several lines of evidence point to a role of sigma type 1 receptors in the activity of NMDA and D1 receptors, suggesting a potential additional mechanism for progestogens to have actions through this pathway (137). Thus, progestogens may have effects in the midbrain VTA by modulating GABAergic and/or mesocorticolimbic dopamine function.
The role of signal transduction cascades for progestogens’ actions
Downstream activation of signal transduction factors may underlie some of progestogens’ actions at membrane targets, such as GABAA receptors, NMDARs and/or D1 (138, 139). D1 receptors are well-known metabotropic receptors (140). Emerging evidence suggests that GABAA receptors may also have actions involving signal transduction pathways. 3α,5α-THP’s effects to prolong the opening of GABAA receptor chloride channels in vitro can be inhibited with antagonists of G-proteins, PKC, or cAMP-dependent protein kinase (141). Further, systemic administration of P increases levels of cAMP in the prefrontal cortex in a 3α,5α-THP- and GABAA receptor- dependent manner (17, 18, 95). Blocking activity of adenylyl cyclase, G-proteins, PKA, PLC, or PKC attenuates the enhancing effects of GABAA receptors or D1 agonists, or NMDAR antagonists, for 3α,5α-THP-facilitated lordosis (135, 136, 141–150). Thus, progestogens’ actions in the VTA involve activation of metabotropic pathways.
Dynamic actions of progestogens in the midbrain VTA to mediate expression of reproductive behaviours
In response to mating, 3α,5α-THP levels in midbrain VTA, metabolised from ovarian P or as a result of neurosteroidogenesis, are rapidly increased compared to non-mated naturally-receptive or hormone-primed rodents (17, 18, 95, 151–153). The rapidity of the mating-induced increase in midbrain 3α,5α-THP, and independence of secretion from the ovaries and/or adrenals, suggests that biosynthesis and subsequent metabolism of central, rather than peripheral, progestogens underlie mating-induced increases we have observed among rats, mice, and/or hamsters (17, 18). Mating- induced 3α,5α-THP biosynthesis not only occurs in the midbrain, but also in the hippocampus, cortex, and striatum, areas of the brain involved in cognitive, affective, and motivated processes (151, 153). Thus, the effects and mechanisms underlying dynamic effects of 3α,5α-THP to facilitate, and be increased by, mating are of great interest.
Progestogens may influence the propensity for expression of sexual receptivity in females, rather than instate receptivity behaviours. For example, progestogens do not seem to instate lordosis, but rather make it more likely that such a response will be evoked in the presence of mating-relevant stimuli (i.e. anogenital investigation and mounting by a male). Rodent models that are typically considered to elucidate whether a function may be steroid mediated are co-variation (oestrous cyclicity), surgical removal (OVX), and replacement (hormone-priming) studies. Thus, the following section describes evidence supporting the role of progestogens in the midbrain VTA to facilitate mating behavior, and reproductively-relevant behaviour, of rodents.
Endogenous increases in P and 3α,5α-THP in the midbrain are associated with sexual receptivity
In the VTA, P has effects to facilitate lordosis subsequent to formation of 3α,5α-THP (8, 17, 18). Indeed, there are co-variations between midbrain 3α,5α-THP levels and lordosis. Mating and frequency in lordosis in female rodents coincides with ovulation, during the pro-oestrous phase of the oestrous cycle, when circulating and midbrain levels of progestogens, and other hormones in addition to progestogens, are elevated compared to the dioestrous phase (17, 18; Figure 4). This effect is attenuated among 12–14 month old, mid-aged rats, concomitant with progestogen decline (Figure 4). Higher midbrain 3α,5α-THP levels and lordosis are observed among mid-aged rats that are not yet reproductively-senescent, compared to mid-age counterparts that are reproductively-senescent (154).
Figure 4.
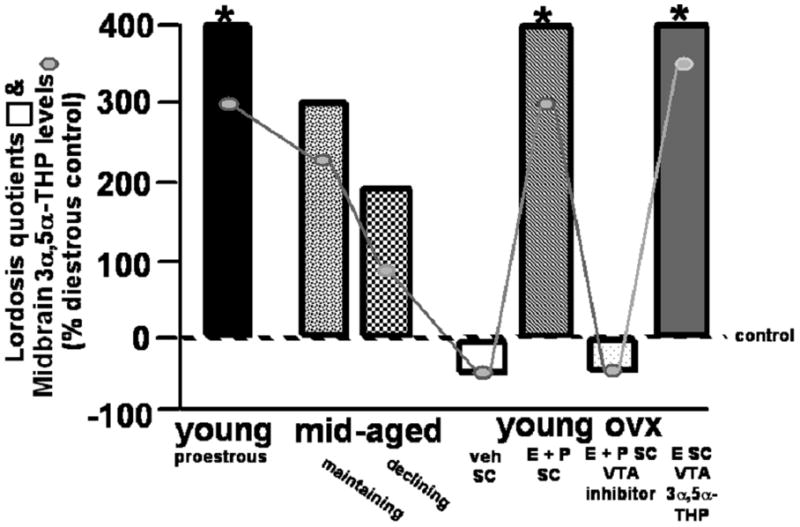
Lordosis quotients (indicated in bars) are enhanced when midbrain concentrations of 3α,5α-THP are increased either cyclically in young rats on pro-oestrus, in mid-age when reproductive status is maintained, when progesterone (P) is administered systemically after ovariectomy (OVX), or when 3α,5α-THP is infused to the midbrain ventral tegmental area (VTA) compared to when midbrain levels of 3α,5α-THP are low (as occurs during dioestrus, decline in reproductive status in mid-age, following OVX, or when P metabolism in the VTA is inhibited). Midbrain levels of 3α,5α-THP of rats in these conditions are indicated by connected circles, as compared to dioestrous controls. * indicates significantly different from dioestrous controls, p < 0.05.
Removal and reinstatement of P and 3α,5α-THP in midbrain VTA and sexual receptivity
The relationship between progestogens and lordosis has also been demonstrated through surgical removal and replacement studies. Lordosis and midbrain levels of 3α,5α-THP are attenuated following OVX, but lordosis can be reinstated following systemic or midbrain VTA administration of P or 3α,5α-THP to OVX, E-primed rats (17, 18; Figure 4). Administration of a 5α-reductase inhibitor to the VTA results in a reduction in P’s conversion to 3α,5α-THP and lordosis is attenuated (similar to that observed with inhibitors of steroid biosynthesis or metabolism; 94, 97, 155–157; Figure 4). These effects may be specific to the VTA. Progestogen administration to sites nearby the VTA (such as the central grey, red nucleus, or substantia nigra) does not evoke facilitation of lordosis (151, 152). As well, application of biosynthesis or metabolism enzyme inhibitors to sites nearby the VTA does not produce decrements in P-facilitated lordosis (156). Thus, formation and/or actions of 3α,5α-THP in the midbrain VTA may play an important role in the facilitation of lordosis.
Progestogens in the midbrain VTA mediate expression of reproductively-relevant behaviours
Given the role 3α,5-THP may play in reproductive behaviours, it is of interest to elucidate the role of 3α,5α-THP for behavioural processes relevant for reproduction, such as social interactions, affect, and/or cognition. As a key brain region in the reward pathway, the VTA is involved in motivated behaviors beyond lordosis, such as aggressive, defensive, and affective behaviors. Indeed, 3α,5α-THP has been implicated in the etiology, expression, and/or therapeutic treatment of anxiety, stress/mood, and cognitive (dys)regulations (8). By investigating behaviours commonly disrupted in neuropsychiatric disorders, such as sexual and social functions, and using an ethologically-relevant model of rodent reproductive behaviour, we hope to provide insight into the functional role of 3α,5α-THP.
3α,5α-THP & aggressive behaviour
Aggressive and defensive behaviours normally exhibited by female rodents toward male rodents must be dampened so that successful mating can occur, which suggests a role of progestogens in the VTA, which are high with mating, for these motivated behaviors. The frequency of aggressive behaviours displayed by pro-oestrous rats are decreased compared to that of dioestrous rats (17, 18, 158), and coincident with increased 3α,5α-THP levels in the midbrain (Figure 5). Social isolation increases aggressive behaviour of isolated rodents toward an intruder and drastically reduces 3α,5α-THP levels (159, 160). Fluoxetine can normalise social interactions and reverses decline in 3α,5α-THP (161). 3α,5α-THP, like other compounds that exert agonist-like effects at GABAA receptors, has biphasic effects on aggressive behaviours. At moderate dosages, 3α,5α-THP increases aggression of mice beyond species-typical, adaptive levels (162). At high levels, 3α,5α-THP decreases aggressive behaviour and produces sedation of mice (162). Moreover, blocking actions of 5α-reductase reverse effects of neurosteroids, such as 3α,5α-THP and 3α-androstanediol, for aggressive behaviors (163–165).
Figure 5.
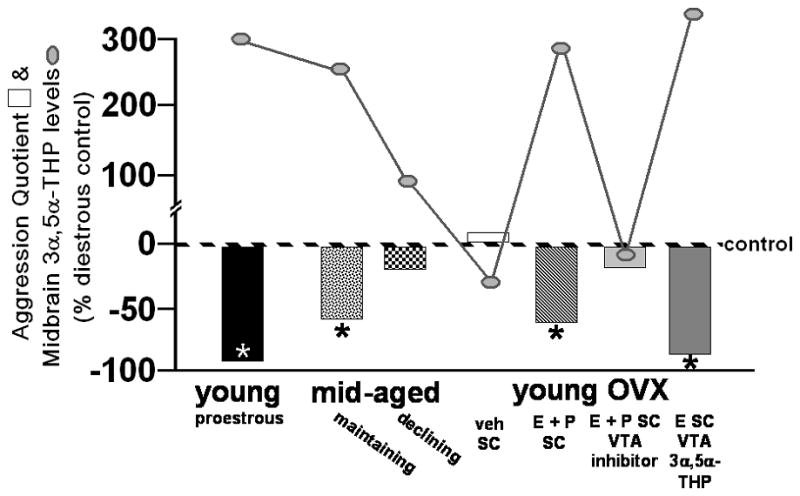
Female aggression, in response to mounting by a male, is reduced when midbrain concentrations of 3α,5α-THP are enhanced either cyclically on pro-oestrous among young rats, in mid-age when reproductive status is maintained, when progesterone (P) is administered systemically after ovariectomy (OVX), or when 3α,5α-THP is infused to the midbrain ventral tegmental area (VTA) compared to when midbrain levels of 3α,5α-THP are low (as occurs during diestrus, decline in reproductive status in mid-age, following OVX, or when P metabolism in the VTA is inhibited). Midbrain levels of 3α,5α-THP of rats in these conditions are indicated by connected circles, as compared to dioestrous controls. * indicates significantly different from dioestrous controls, p < 0.05.
3α,5α-THP & affective behaviour
As with aggressive and defensive behaviours, it is proposed that anxiety and fear behaviours normally exhibited by female rodents are dampened by progestogens so that successful mating can occur. In support, concomitant with enhancement of sexual receptivity, pro-oestrous females have decreased anxiety- and depression-like behaviour compared to their dioestrous counterparts (166, 167), coincident with increased 3α,5α-THP levels in the midbrain (Figure 6). OVX or inhibition of P’s metabolism to 3α,5α-THP via 5α-reductase attenuates these enhancements (166, 168; Figure 6). Enhancing 3α,5α-THP can produce anti-anxiety-like effects (169–171).
Figure 6.
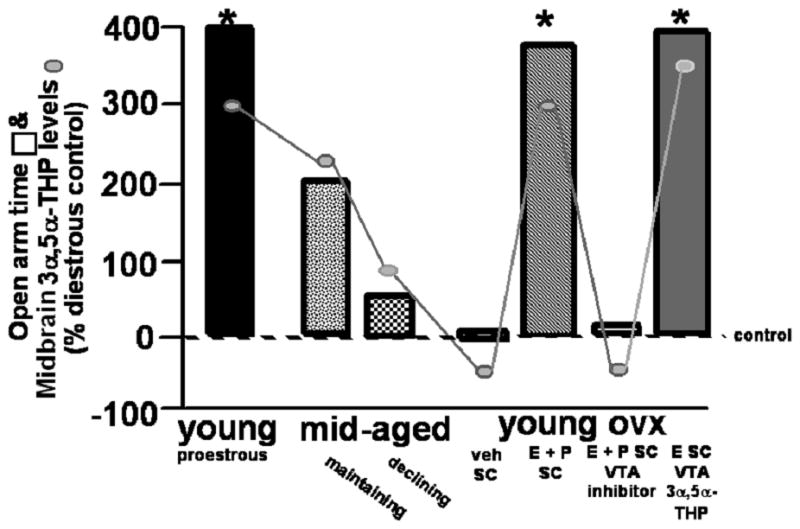
The amount of time spent on the open arms of an elevated plus maze is increased when midbrain concentrations of 3α,5α-THP are enhanced either cyclically on pro-oestrous, in mid-age when reproductive status is maintained, when progesterone is administered systemically after ovariectomy (OVX), or when 3α,5α-THP is infused to the midbrain ventral tegmental area (VTA) compared to when midbrain levels of 3α,5α-THP are low (as occurs during diestrus, decline in reproductive status in mid-age, following OVX, or when P metabolism in the VTA is inhibited). Midbrain levels of 3α,5α-THP of rats in these conditions are indicated by connected circles, as compared to dioestrous controls. * indicates significantly different from dioestrous controls, p < 0.05.
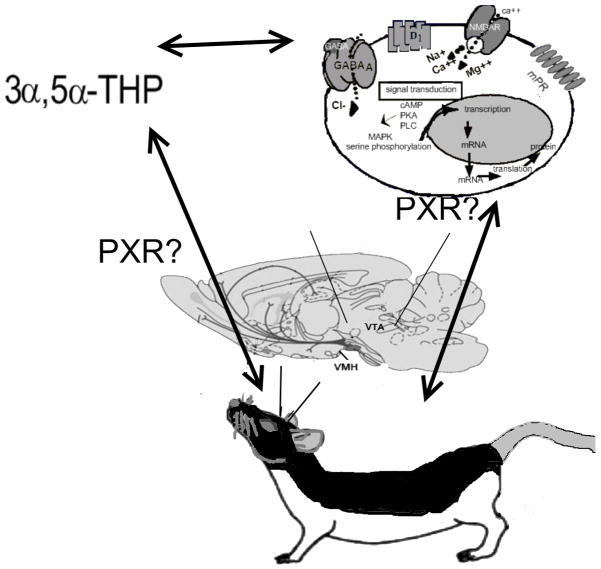
PXR as a source of 3α,5α-THP biosynthesis in the VTA
PXR may be a target in the midbrain for 3α,5α-THP biosynthesis, relevant for motivated behaviours. PXR is a type II nuclear receptor (subfamily 1, group I, member 2; NR1I2) of average size (431 amino acids) that remains in the nucleus regardless of whether or not it is bound, and it must be agonised in order to promote gene transcription (172, 173). PXR is involved in the metabolism of cholesterol to bile acids and steroids (172). When PXR heterodimerises with retinoid X receptor (RXR), it regulates gene transcription in a ligand-dependent fashion. This promotes the production of many proteins, including cytochrome P450 enzymes (CYP enzymes), which are responsible for metabolism of several drugs and steroids (174, 175). PXR is most often considered in the liver, where it exists in relatively high concentrations (176). Indeed, PXR’s specific functions in most parts of the brain are unknown; however, PCR and Western Blotting has revealed PXR in rat brain capillaries (177), and various regions of the human brain (178), and the rabbit cortex, midbrain, and cerebellum (176). In our laboratory, pilot studies have confirmed the expression of the PXR gene, RNA, and protein in the midbrain of rats in proestrus, as per microarray, reverse transcriptase polymerase chain reaction, and Western blots. Thus, we are interested in elucidating the role of PXR in 3α,5α-THP biosynthesis in the midbrain VTA.
PXR ligands enhance consummatory sexual behaviour
We have begun to assess the functional significance of actions at PXR in the VTA for effects on mating. OVX Long-Evans rats were stereotaxically implanted with bilateral guide cannulae aimed at the VTA. Rats were E-primed (10 μg) and, 44 hours later, infused (1 μl) with either a positive PXR positive modulator, 3β,5α-THP (200 ng), 3α,5β-THP (200 ng), 3α,5α-THP (200 ng), RU-486 (135 ng), or β-cyclodextrin vehicle (n=6/grp). Ten minutes later, rats were assessed for sexual receptivity. Preliminary results indicate that lordosis was increased among rats infused with any of the PXR- positive modulators, and was significantly greater among 3α,5α-THP- or RU-486- infused rats compared to vehicle-infused controls (Figure 7).
Figure 7.
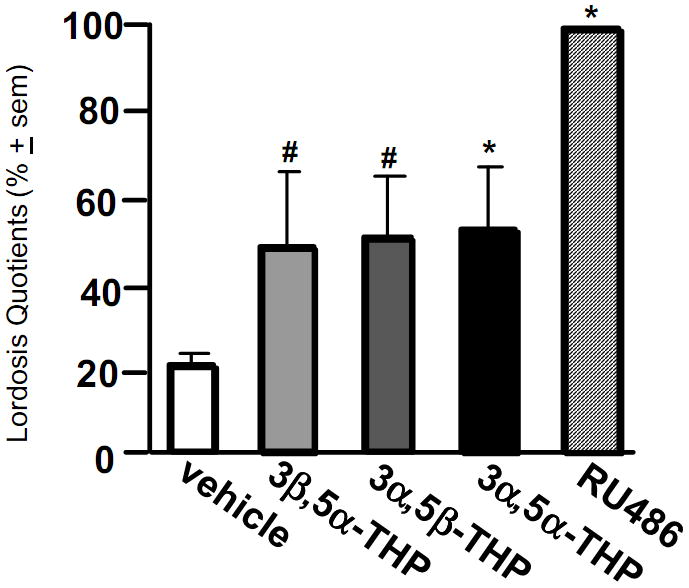
Intra-ventral tegmental area (VTA) infusions of positive pregnane xenobiotic receptor (PXR) modulators enhance lordosis. Infusions of 3β,5α-THP (200 ng) and 3α,5β-THP (200 ng) tended to increase lordosis quotients, and infusions of 3α,5α-THP (200 ng) and RU486 (135 ng) significantly increased lordosis quotients among ovariectomised, oestrogen-primed rats. # indicates tendency to differ from vehicle-infused controls, p < 0.10. * indicates significant difference from vehicle-infused controls, p < 0.05.
Blocking PXR in the VTA attenuates sexual behaviour
Although the preliminary data above imply that activating PXR in the midbrain VTA may facilitate lordosis, the consequence of PXR knockdown should be investigated to further elucidate the potential role of PXR in the midbrain VTA. OVX, E-primed (10μg) rats were infused with either a PXR anti-sense ODN (250 ng; n=15), a scrambled mis-sense ODN (250 ng; n=8) control, or β-cyclodextrin as a vehicle control (n=8) bilaterally to the VTA. ODNs were infused 44h and 24h prior to testing for paced mating. Ten minutes before testing, rats were infused with FGIN1–27 (5 μg), a neurosteroidogenesis enhancer that is an agonist of TSPO. As we have previously observed, infusions of FGIN 1–27 to the VTA significantly enhanced lordosis. Infusions of anti-sense ODNs to the VTA significantly reduced lordosis and attenuated FGIN 1–27’s effects (Figure 8). Thus, PXR may be important for facilitation of neurosteroid-enhanced lordosis.
Figure 8.
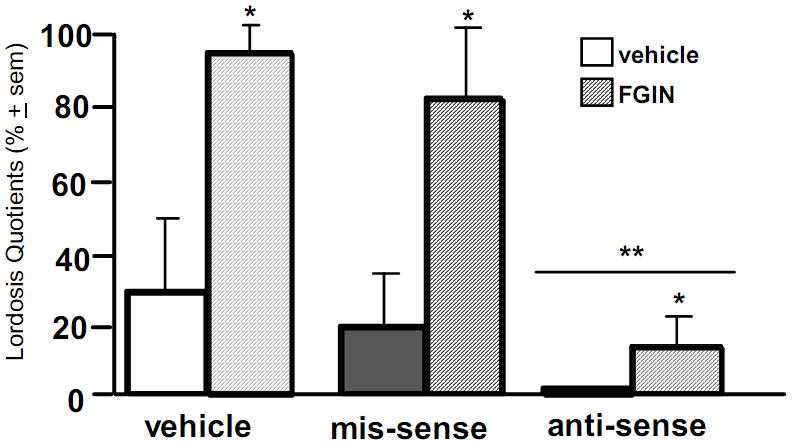
Intra-ventral tegmental area (VTA) infusions of the neurosteroidogenesis enhancer, FGIN 1–27, significantly enhanced lordosis quotients among ovariectomised, hormone-primed, female rats (n=3–10/grp) and infusions of anti-sense oligonucleotides to the VTA blocked this effect. * indicates significant main effect for FGIN 1–27 to enhance lordosis quotients compared to vehicle, p < 0.05. ** indicates significant main effect for anti-sense infusions to reduce lordosis quotients compared to mis-sense or vehicle-infusions, p < 0.05.
Summary
This review focused on emerging findings using lordosis as a behavioural bioassay for progestogens’ actions in the midbrain VTA to challenge traditional dogma in neuroendocrinology and reveal novel substrates for, and sources of, progestogens’ actions. Progestogens, as with other steroid hormones, can act in the CNS via membrane or rapid-signalling actions, which occur within seconds to minutes. Progestogens can produce rapid effects on neuronal excitability and synaptic function that involve direct or indirect modulation of ion-gated, or other neurotransmitter receptors and transporters, rather than classic, nuclear hormone receptors (Figure 9). Further, the sources of these progestogens are not only the gonads, adrenal and placenta, but also the brain, which acts as an endocrine organ that requires coordinated actions of steroidogenic enzymes in neurons and glia in different parts of the CNS to metabolise peripheral steroids to neuroactive products (neuroactive steroids) or to produce steroids de novo in the brain independent of peripheral gland secretion (neurosteroids). A novel target of biosynthesis may be PXR (Figure 9). Through its actions in the midbrain VTA, progestogens mediate appetitive (exploratory, anxiety, social approach) and consummatory (social, sexual) aspects of rodents’ reproductive behavior. Moreover, there is broader relevance for understanding the actions and sources of progestogens, such as 3α,5α-THP, for aspects of reproduction, beyond lordosis, such as for other motived behaviors (aggression, affect, etc). Indeed, understanding these non-traditional actions of pregnane (neuro)steroids has relevance for other steroid hormones that have rapid actions through non-steroidal targets for their broad functional effects (e.g. estrogens, androgens).
Figure 9.
Proposed relationship between pregnane xenobiotic receptor (PXR), 3α,5α-THP, and target substrates for actions to enhance lordosis. PXR may be involved in biosynthesis of 3α,5α-THP in the midbrain ventral tegmental area (VTA) with mating. Moreover, 3α,5α-THP is a ligand of PXR and, thus, PXR, like neurotransmitter targets (γ-aminobutyric acid- GABA receptors, N-methyl-D-aspartate receptors- NMDARs, and dopamine type 1-like receptors- D1) and other membrane receptor targets may be important for actions of 3α,5α-THP in the VTA for lordosis.
Acknowledgments
This research was supported, in part, by funding from the National Science Foundation, and the National Institute of Mental Health. The assistance of Dr. Sandra Petralia, Dr. Madeline Rhodes, Dr. Alicia Walf, and Dr. Jason Paris is appreciated. Editorial work on this manuscript by Danielle Llaneza is also appreciated.
Literature Cited
- 1.Baulieu EE. Steroid hormone receptors. Expos Annu Biochim Med. 1980;34:1–25. [PubMed] [Google Scholar]
- 2.Baulieu EE. Neurosteroids: a new function in the brain. Biol Cell. 1991;71:3–10. doi: 10.1016/0248-4900(91)90045-o. [DOI] [PubMed] [Google Scholar]
- 3.Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa A, Miyatake A, Ohnishi T, Ichikawa Y. Steroidogenic acute regulatory protein (StAR) transcripts constitutively expressed in the adult rat central nervous system: colocalization of StAR, cytochrome P-450SCC (CYP XIA1), and 3β-hydroxysteroid dehydrogenase in the rat brain. J Neurochem. 1998;71:2231–8. doi: 10.1046/j.1471-4159.1998.71062231.x. [DOI] [PubMed] [Google Scholar]
- 5.Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38:379–95. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- 6.Mellon SH. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78:1003–8. doi: 10.1210/jcem.78.5.8175951. [DOI] [PubMed] [Google Scholar]
- 7.Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–22. [PubMed] [Google Scholar]
- 8.Frye CA. Neurosteroids-From Basic Research to Clinical Perspectives. In: Rubin Robert T, Pfaff Donald W., editors. Hormones/Behavior Relations of Clinical Importance. San Diego: Academic Press; 2009. pp. 395–416. [Google Scholar]
- 9.Batarseh A, Papadopoulos V. Regulation of translocator protein 18 kDa (TSPO) expression in health and disease states. Mol Cell Endocrinol. 2010;327:1–12. doi: 10.1016/j.mce.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27(8):402–9. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 11.King SR, Matassa AA, White EK, Walsh LP, Jo Y, Rao RM, Stocco DM, Reyland ME. Oxysterols regulate expression of the steroidogenic acute regulatory protein. J Mol Endocrinol. 2004;32:507–17. doi: 10.1677/jme.0.0320507. [DOI] [PubMed] [Google Scholar]
- 12.Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;3:283–92. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- 13.Papadopoulos V, Lecanu L, Brown RC, Han Z, Yao ZX. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience. 2006;138:749–56. doi: 10.1016/j.neuroscience.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 14.Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Bäckman M, Ohlsson R, Postlind H, Blomquist P, Berkenstam A. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci U S A. 1998;95:12208–13. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterström RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102:1016–23. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frye CA. The role of neurosteroids and nongenomic effects of progestins in the ventral tegmental area in mediating sexual receptivity of rodents. Horm Behav. 2001;40:226–233. doi: 10.1006/hbeh.2001.1674. [DOI] [PubMed] [Google Scholar]
- 18.Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res Brain Res Rev. 2001;37:201–22. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Bertics PJ, Karavolas HJ. Regional distribution of cytosolic and particulate 5α-dihydroprogesterone 3α-hydroxysteroid oxidoreductases in female rat brain. J Steroid Biochem Mol Biol. 1997;60:311–8. doi: 10.1016/s0960-0760(96)00195-1. [DOI] [PubMed] [Google Scholar]
- 20.Frye CA, Paris JJ. Progesterone turnover to its 5α-reduced metabolites in the ventral tegmental area of the midbrain is essential for initiating social and affective behavior and progesterone metabolism in female rats. J Endocrinol Invest. 2011 doi: 10.3275/7334. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melcangi RC, Panzica G. Steroids and the nervous system. Introduction Ann NY Acad Sci. 2003;1007:1–5. doi: 10.1196/annals.1286.040. [DOI] [PubMed] [Google Scholar]
- 22.Beyer C, Hoffman KL, González-Flores O. Neuroendocrine regulation of estrous behavior in the rabbit: similarities and differences with the rat. Horm Behav. 2007;52:2–11. doi: 10.1016/j.yhbeh.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 23.Etgen AM, Chu HP, Fiber JM, Karkanias GB, Morales JM. Hormonal integration of neurochemical and sensory signals governing female reproductive behavior. Behav Brain Res. 1999;105:93–103. doi: 10.1016/s0166-4328(99)00085-6. [DOI] [PubMed] [Google Scholar]
- 24.Pfaff D, Frohlich J, Morgan M. Hormonal and genetic influences on arousal--sexual and otherwise. Trends Neurosci. 2002;25:45–50. doi: 10.1016/s0166-2236(00)02084-1. [DOI] [PubMed] [Google Scholar]
- 25.O’Malley BW. A life-long search for the molecular pathways of steroid hormone action. Mol Endocrinol. 2005;19:1402–11. doi: 10.1210/me.2004-0480. [DOI] [PubMed] [Google Scholar]
- 26.Pfaff DW, Gerlach JL, McEwen BS, Ferin M, Carmel P, Zimmerman EA. Autoradiographic localization of hormone-concentrating cells in the brain of the female rhesus monkey. J Comp Neurol. 1976;170:279–93. doi: 10.1002/cne.901700302. [DOI] [PubMed] [Google Scholar]
- 27.Pfaff DW, McEwen BS. Actions of estrogens and progestins on nerve cells. Science. 1983;219:808–14. doi: 10.1126/science.6297008. [DOI] [PubMed] [Google Scholar]
- 28.O’Malley BW, Sherman MR, Toft DO. Progesterone “receptors” in the cytoplasm and nucleus of chick oviduct target tissue. Proc Natl Acad Sci U S A. 1970;67:501–8. doi: 10.1073/pnas.67.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blaustein JD. Progestin receptors: neuronal integrators of hormonal and environmental stimulation. Ann N Y Acad Sci. 2003;1007:238–50. doi: 10.1196/annals.1286.023. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Lonard DM, O’Malley BW. A contemporary understanding of progesterone receptor function. Mech Ageing Dev. 2004;125:669–78. doi: 10.1016/j.mad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Pratt WB. The hsp90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med. 1998;217:420–34. doi: 10.3181/00379727-217-44252. [DOI] [PubMed] [Google Scholar]
- 32.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med. 2003;228:111–33. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 33.Allan GF, Leng X, Tsai SY, Weigel NL, Edwards DP, Tsai MJ, O’Malley BW. Hormone and antihormone induce distinct conformational changes which are central to steroid receptor activation. J Biol Chem. 1992;267:19513–20. [PubMed] [Google Scholar]
- 34.DeMarzo AM, Beck CA, Onate SA, Edwards DP. Dimerization of mammalian progesterone receptors occurs in the absence of DNA and is related to the release of the 90-kDa heat shock protein. Proc Natl Acad Sci USA. 1991;88:72–6. doi: 10.1073/pnas.88.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards DP, DeMarzo AM, Onate SA, Beck CA, Estes PA, Nordeen SK. Mechanisms controlling steroid receptor binding to specific DNA sequences. Steroids. 1991;56:271–8. doi: 10.1016/0039-128x(91)90046-x. [DOI] [PubMed] [Google Scholar]
- 36.Leonhardt SA, Boonyaratanakornkit V, Edwards DP. Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids. 2003;68:761–70. doi: 10.1016/s0039-128x(03)00129-6. [DOI] [PubMed] [Google Scholar]
- 37.Groshong SD, Owen GI, Grimison B, Schauer IE, Todd MC, Langan TA, Sclafani RA, Lange CA, Horwitz KB. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1) Mol Endocrinol. 1997;11:1593–607. doi: 10.1210/mend.11.11.0006. [DOI] [PubMed] [Google Scholar]
- 38.Richer JK, Lange CA, Manning NG, Owen G, Powell R, Horwitz KB. Convergence of progesterone with growth factor and cytokine signaling in breast cancer. Progesterone receptors regulate signal transducers and activators of transcription expression and activity. J Biol Chem. 1998;273:31317–26. doi: 10.1074/jbc.273.47.31317. [DOI] [PubMed] [Google Scholar]
- 39.Dressing GE, Goldberg JE, Charles NJ, Schwertfeger KL, Lange CA. Membrane progesterone receptor expression in mammalian tissues: a review of regulation and physiological implications. Steroids. 2011;76:11–7. doi: 10.1016/j.steroids.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frye CA, Vongher JM. GABAA, D1, and D5, but not progestin receptor, antagonist and anti-sense oligonucleotide infusions to the ventral tegmental area of cycling rats and hamsters attenuate lordosis. Behav Brain Res. 1999a;103:23–34. doi: 10.1016/s0166-4328(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 41.Frye CA, Vongher JM. Progesterone and 3α,5α-THP enhance sexual receptivity in mice. Behav Neurosci. 2001;115:1118–28. [PubMed] [Google Scholar]
- 42.Glaser JH, Etgen AM, Barfield RJ. Intrahypothalamic effects of progestin agonists on estrous behavior and progestin receptor binding. Physiol Behav. 1985;34:871–7. doi: 10.1016/0031-9384(85)90006-x. [DOI] [PubMed] [Google Scholar]
- 43.Mani SK, Blaustein JD, Allen JM, Law SW, O’Malley BW, Clark JH. Inhibition of rat sexual behavior by antisense oligonucleotides to the progesterone receptor. Endocrinology. 1994;135:1409–14. doi: 10.1210/endo.135.4.7925102. [DOI] [PubMed] [Google Scholar]
- 44.Mani SK, Allen JM, Lydon JP, Mulac-Jericevic B, Blaustein JD, DeMayo FJ, Conneely O, O’Malley BW. Dopamine requires the unoccupied progesterone receptor to induce sexual behavior in mice. Mol Endocrinol. 1996;10:1728–37. doi: 10.1210/mend.10.12.8961281. [DOI] [PubMed] [Google Scholar]
- 45.Olster DH, Blaustein JD. Development of progesterone-facilitated lordosis in female guinea pigs: relationship to neural estrogen and progestin receptors. Brain Res. 1989;484:68–76. doi: 10.1016/0006-8993(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 46.Ogawa S, Olazábal UE, Parhar IS, Pfaff DW. Effects of intrahypothalamic administration of antisense DNA for progesterone receptor mRNA on reproductive behavior and progesterone receptor immunoreactivity in female rat. J Neurosci. 1994;14:1766–74. doi: 10.1523/JNEUROSCI.14-03-01766.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pollio G, Xue P, Zanisi M, Nicolin A, Maggi A. Antisense oligonucleotide blocks progesterone-induced lordosis behavior in ovariectomized rats. Brain Res Mol Brain Res. 1993;19:135–9. doi: 10.1016/0169-328x(93)90158-l. [DOI] [PubMed] [Google Scholar]
- 48.Lange CA, Richer JK, Shen T, Horwitz KB. Convergence of progesterone and epidermal growth factor signaling in breast cancer. Potentiation of mitogen-activated protein kinase pathways. J Biol Chem. 1998;273:31308–16. doi: 10.1074/jbc.273.47.31308. [DOI] [PubMed] [Google Scholar]
- 49.Nilsen J, Brinton RD. Impact of progestins on estradiol potentiation of the glutamate calcium response. Neuroreport. 2002;13:825–30. doi: 10.1097/00001756-200205070-00018. [DOI] [PubMed] [Google Scholar]
- 50.Auger CJ, De Vries GJJ. Progestin receptor immunoreactivity within steroid-responsive vasopressin-immunoreactive cells in the male and female rat brain. Neuroendocrinol. 2002;14:561–67. doi: 10.1046/j.1365-2826.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- 51.Camacho-Arroyo I, Guerra-Araiza C, Cerbon MA. Progesterone receptor isoforms are differentially regulated by sex steroids in the rat forebrain. Neuroreport. 1998;9:3993–6. doi: 10.1097/00001756-199812210-00001. [DOI] [PubMed] [Google Scholar]
- 52.Guerra-Araiza C, Cerbon MA, Morimoto S, Camacho-Arroyo I. Progesterone receptor isoforms expression pattern in the rat brain during the estrous cycle. Life Sci. 2000;66:1743–52. doi: 10.1016/s0024-3205(00)00497-5. [DOI] [PubMed] [Google Scholar]
- 53.Guerra-Araiza C, Reyna-Neyra A, Salazar AM, Cerbon MA, Morimoto S, Camacho-Arroyo I. Progesterone receptor isoforms expression in the prepuberal and adult male rat brain. Brain Res Bull. 2001;54:13–7. doi: 10.1016/s0361-9230(00)00410-x. [DOI] [PubMed] [Google Scholar]
- 54.Guerra-Araiza C, Coyoy-Salgado A, Camacho-Arroyo I. Sex differences in the regulation of progesterone receptor isoforms expression in the rat brain. Brain Res Bull. 2002;59:105–9. doi: 10.1016/s0361-9230(02)00845-6. [DOI] [PubMed] [Google Scholar]
- 55.Guerra-Araiza C, Villamar-Cruz O, Gonzalez-Arenas A, Chavira R, Camacho-Arroyo I. Changes in progesterone receptor isoforms content in the rat brain during the oestrous cycle and after oestradiol and progesterone treatments. J Neuroendocrinol. 2003;15:984–90. doi: 10.1046/j.1365-2826.2003.01088.x. [DOI] [PubMed] [Google Scholar]
- 56.Hagihara K, Hirata S, Osada T, Hirai M, Kato J. Distribution of cells containing progesterone receptor mRNA in the female rat di- and telencephalon: an in situ hybridization study. Brain Res Mol Brain Res. 1992;14:239–49. doi: 10.1016/0169-328x(92)90179-f. [DOI] [PubMed] [Google Scholar]
- 57.Hagihara K, Hirata S, Osada T, Hirai M, Kato J. Expression of progesterone receptor in the neonatal rat brain cortex: detection of its mRNA using reverse transcription-polymerase chain reaction. J Steroid Biochem Mol Biol. 1992;41:637–40. doi: 10.1016/0960-0760(92)90396-z. [DOI] [PubMed] [Google Scholar]
- 58.Kato J, Hirata S, Nozawa A, Mouri N. The ontogeny of gene expression of progestin receptors in the female rat brain. J Steroid Biochem Mol Biol. 1993;47:173–82. doi: 10.1016/0960-0760(93)90072-5. [DOI] [PubMed] [Google Scholar]
- 59.Kato J, Hirata S, Nozawa A, Yamada-Mouri N. Gene expression of progesterone receptor isoforms in the rat brain. Horm Behav. 1994;28:454–63. doi: 10.1006/hbeh.1994.1043. [DOI] [PubMed] [Google Scholar]
- 60.Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29:313–39. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conneely OM, Maxwell BL, Toft DO, Schrader WT, O’Malley BW. The A and B forms of the chicken progesterone receptor arise by alternate initiation of translation of a unique mRNA. Biochem Biophys Res Commun. 1987;149:493–501. doi: 10.1016/0006-291x(87)90395-0. [DOI] [PubMed] [Google Scholar]
- 62.Duffy DM, Wells TR, Haluska GJ, Stouffer RL. The ratio of progesterone receptor isoforms changes in the monkey corpus luteum during the luteal phase of the menstrual cycle. Biol Reprod. 1997;57:693–9. doi: 10.1095/biolreprod57.4.693. [DOI] [PubMed] [Google Scholar]
- 63.Kastner P, Bocquel MT, Turcotte B, Garnier JM, Horwitz KB, Chambon P, Gronemeyer HJ. Transient expression of human and chicken progesterone receptors does not support alternative translational initiation from a single mRNA as the mechanism generating two receptor isoforms. Biol Chem. 1990;265:12163–7. [PubMed] [Google Scholar]
- 64.Balasubramanian B, Portillo W, Reyna A, Chen JZ, Moore AN, Dash PK, Mani SK. Nonclassical mechanisms of progesterone action in the brain: II. Role of calmodulin-dependent protein kinase II in progesterone-mediated signaling in the hypothalamus of female rats. Endocrinology. 2008;149:5518–26. doi: 10.1210/en.2008-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pietras R, Szego C. Endometrial cell calcium and oestrogen action. Nature. 1975;253:357–359. doi: 10.1038/253357a0. [DOI] [PubMed] [Google Scholar]
- 66.Norman A, Mizwicki M, Norman D. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3:27–41. doi: 10.1038/nrd1283. [DOI] [PubMed] [Google Scholar]
- 67.Thomas P. Characteristics of membrane progestin receptor α (mPRα) and progesterone membrane receptor component one (PGMRC1) and their roles in mediating rapid progestin actions. Frontiers in Neuroendocrinology. 2008;29:292–312. doi: 10.1016/j.yfrne.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu Y, Hanna R, Schaaf M, Spaink H, Thomas P. Candidates for membrane progestin receptors--past approaches and future challenges. Comp Biochem Physiol C Toxicol Pharmacol. 2008;148:381–9. doi: 10.1016/j.cbpc.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 69.Baldi E, Luconi M, Bonaccorsi L, Forti G. Nongenomic effects of progesterone on spermatozoa: mechanisms of signal transduction and clinical implications. Front Biosci. 1998;3:D1051–59. doi: 10.2741/a345. [DOI] [PubMed] [Google Scholar]
- 70.Blackmore P, Beebe S, Danforth D, Alexander N. Progesterone and 17α-hydroxyprogesterone. Novel stimulators of calcium influx in human sperm. J Biol Chem. 1990;265:1376–80. [PubMed] [Google Scholar]
- 71.Luconi M, Francavilla F, Porazzi I, Macerola B, Forti G, Baldi E. Human spermatozoa as a model for studying membrane receptors mediating rapid nongenomic effects of progesterone and estrogens. Steroids. 2004;69:553–9. doi: 10.1016/j.steroids.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 72.Maller JL. The elusive progesterone receptor in Xenopus oocytes. Proc Natl Acad Sci U S A. 2001;98:8–10. doi: 10.1073/pnas.98.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sabeur K, Edwards D, Meizel S. Human sperm plasma membrane progesterone receptor(s) and the acrosome reaction. Biol Reprod. 1996;54:993–1001. doi: 10.1095/biolreprod54.5.993. [DOI] [PubMed] [Google Scholar]
- 74.Thomas P, Tubbs C, Detweiler C, Das S, Ford L, Breckenridge-Miller D. Binding characteristics, hormonal regulation and identity of the sperm membrane progestin receptor in Atlantic croaker. Steroids. 2005;70:427–33. doi: 10.1016/j.steroids.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 75.Josefsberg Ben-Yehoshua L, Lewellyn AL, Thomas P, Maller JL. The role of Xenopus membrane progesterone receptor β in mediating the effect of progesterone on oocyte maturation. Mol Endocrinol. 2007;21:664–73. doi: 10.1210/me.2006-0256. [DOI] [PubMed] [Google Scholar]
- 76.Kostellow A, Ziegler D, Morrill G. Regulation of Ca2+ and cyclic AMP during the first meiotic division in amphibian oocytes by progesterone. J Cyclic Nucleotide Res. 1980;6:347–358. [PubMed] [Google Scholar]
- 77.Thomas P, Zhu Y, Pace M. Progestin membrane receptors involved in the meiotic maturation of teleost oocytes: a review with some new findings. Steroids. 2002;67:511–7. doi: 10.1016/s0039-128x(01)00180-5. [DOI] [PubMed] [Google Scholar]
- 78.Calogero A, Palumbo M, Bosboom A, Burrello N, Ferrara E, Palumbo G, Petraglia F, D’Agata R. The neuroactive steroid allopregnanolone suppresses hypothalamic gonadotropin-releasing hormone release through a mechanism mediated by the gamma-aminobutyric acidA receptor. J Endocrinol. 1998;158:121–5. doi: 10.1677/joe.0.1580121. [DOI] [PubMed] [Google Scholar]
- 79.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–7. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 80.Sim J, Skynner M, Herbison A. Direct regulation of postnatal GnRH neurons by the progesterone derivative allopregnanolone in the mouse. Endocrinology. 2001;142:4448–53. doi: 10.1210/endo.142.10.8451. [DOI] [PubMed] [Google Scholar]
- 81.Faivre E, Skildum A, Pierson-Mullany L, Lange CA. Integration of progesterone receptor mediated rapid signaling and nuclear actions in breast cancer cell models: role of mitogen-activated protein kinases and cell cycle regulators. Steroids. 2005;70:418–26. doi: 10.1016/j.steroids.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 82.Dosiou C, Hamilton A, Pang Y, Overgaard M, Tulac S, Dong J, Thomas P, Giudice L. Expression of membrane progesterone receptors on human T lymphocytes and Jurkat cells and activation of G-proteins by progesterone. J Endocrinol. 2008;196:67–77. doi: 10.1677/JOE-07-0317. [DOI] [PubMed] [Google Scholar]
- 83.Schwartz-Giblin S, Pfaff DW. Hypothalamic output controlling reticulospinal and vestibulospinal systems important for emotional behavior. Int J Neurol. 1985–1986;19–20:89–110. [PubMed] [Google Scholar]
- 84.Blaustein JD, Tetel MJ, Ricciardi KH, Delville Y, Turcotte JC. Hypothalamic ovarian steroid hormone-sensitive neurons involved in female sexual behavior. Psychoneuroendocrinology. 1994;19:505–16. doi: 10.1016/0306-4530(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 85.Caldwell JD, Walker CH, Faggin BM, Carr RB, Pedersen CA, Mason GA. Characterization of progesterone-3-[125I-BSA] binding sites in the medial preoptic area and anterior hypothalamus. Brain Res. 1995;693:225–32. doi: 10.1016/0006-8993(95)00727-8. [DOI] [PubMed] [Google Scholar]
- 86.MacLusky NJ, McEwen BS. Progestin receptors in rat brain: distribution and properties of cytoplasmic progestin-binding sites. Endocrinology. 1980;106:192–202. doi: 10.1210/endo-106-1-192. [DOI] [PubMed] [Google Scholar]
- 87.Warembourg M. Radioautographic study of the rat brain, uterus and vagina after [3H]R-5020 injection. Mol Cell Endocrinol. 1978;12:67–79. doi: 10.1016/0303-7207(78)90102-8. [DOI] [PubMed] [Google Scholar]
- 88.Frye CA, Murphy RE, Platek SM. Anti-sense oligonucleotides, for progestin receptors in the VMH and glutamic acid decarboxylase in the VTA, attenuate progesterone-induced lordosis in hamsters and rats. Behav Brain Res. 2000;115:55–64. doi: 10.1016/s0166-4328(00)00242-4. [DOI] [PubMed] [Google Scholar]
- 89.Frye CA, Bayon LE, Vongher JM. Intravenous progesterone elicits a more rapid induction of lordosis in rats than does SKF38393. Psychobiology. 2000b;28:99–109. [Google Scholar]
- 90.Rose JD. Tectoreticular neuronal activity associated with behavior and progesterone action during the induction of lordosis responding in hamsters. Soc Neurosci Abst. 1990;16:924. [Google Scholar]
- 91.DeBold JF, Frye CA. Genomic and non-genomic actions of progesterone in the control of female hamster sexual behavior. Horm Behav. 1994;28:445–53. doi: 10.1006/hbeh.1994.1042. [DOI] [PubMed] [Google Scholar]
- 92.Pleim ET, Baumann J, Barfield RJ. A contributory role for midbrain progesterone in the facilitation of female sexual behavior in rats. Horm Behav. 1991;25:19–28. doi: 10.1016/0018-506x(91)90036-h. [DOI] [PubMed] [Google Scholar]
- 93.Frye CA, Mermelstein PG, DeBold JF. Evidence for a non-genomic action of progestins on sexual receptivity in hamster ventral tegmental area but not hypothalamus. Brain Res. 1992;578:87–93. doi: 10.1016/0006-8993(92)90233-y. [DOI] [PubMed] [Google Scholar]
- 94.Frye CA, Gardiner SG. Progestins can have a membrane-mediated action in rat midbrain for facilitation of sexual receptivity. Horm Behav. 1996;30:682–91. doi: 10.1006/hbeh.1996.0069. [DOI] [PubMed] [Google Scholar]
- 95.Frye CA. Progestins influence motivation, reward, conditioning, stress, and/or response to drugs of abuse. Pharmacol Biochem Behav. 2007;86:209–19. doi: 10.1016/j.pbb.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frye CA, Rhodes ME, Petralia SM, Walf AA, Sumida K, Edinger KL. 3α-hydroxy-5α-pregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience. 2006;138:1007–14. doi: 10.1016/j.neuroscience.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frye CA, Vongher JM. Progesterone has rapid and membrane effects in the facilitation of female mouse sexual behavior. Brain Res. 1999;815:259–69. doi: 10.1016/s0006-8993(98)01132-9. [DOI] [PubMed] [Google Scholar]
- 98.Frye CA, Paris JJ. Effects of neurosteroid actions at N-methyl-D-aspartate and GABA A receptors in the midbrain ventral tegmental area for anxiety-like and mating behavior of female rats. Psychopharmacology. 2011;213:93–103. doi: 10.1007/s00213-010-2016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Petralia SM, DeBold JF, Frye CA. MK-801 infusions to the ventral tegmental area and ventromedial hypothalamus produce opposite effects on lordosis of hormone-primed rats. Pharmacol Biochem Behav. 2007;86:377–85. doi: 10.1016/j.pbb.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frye CA, Marrone J, Walf A. Effects of manipulating progesterone and NMDA receptors in the ventral tegmental area for lordosis of hamsters and rats. Psychopharmacology. 2008;200:71–80. doi: 10.1007/s00213-008-1143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–6. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- 102.Johannessen M, Fontanilla D, Mavlyutov T, Ruoho AE, Jackson MB. Antagonist action of progesterone at σ-receptors in the modulation of voltage-gated sodium channels. Am J Physiol Cell Physiol. 2011;300:C328–37. doi: 10.1152/ajpcell.00383.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frye CA, Walf AA. Oxytocin and/or steroid hormone binding globulin infused into the ventral tegmental area modulates progestogen-mediated lordosis. Neuropharmacology. 2010;58:44–9. doi: 10.1016/j.neuropharm.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–95. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- 105.Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA. 2003;100:2237–42s. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mascó D, Weigel R, Carrer HF. Gamma aminobutyric acid mediates ventromedial hypothalamic mechanisms controlling the execution of lordotic responses in the female rat. Behav Brain Res. 1986;19:153–62. doi: 10.1016/0166-4328(86)90013-6. [DOI] [PubMed] [Google Scholar]
- 107.Westerling P, Lindgren S, Meyerson Functional changes in GABAA receptor stimulation during the oestrous cycle of the rat. B Br J Pharmacol. 1991;103:1580–4. doi: 10.1111/j.1476-5381.1991.tb09830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilson MA. Influences of gender, gonadectomy, and estrous cycle on GABA/BZ receptors and benzodiazepine responses in rats. Brain Res Bull. 1992;29:165–72. doi: 10.1016/0361-9230(92)90022-p. [DOI] [PubMed] [Google Scholar]
- 109.Frye CA. Inhibition of 5α-reductase enzyme or GABA(A) receptors in the VMH and the VTA attenuates progesterone-induced sexual behavior in rats and hamsters. J Endocrinol Invest. 2001c;24:399–407. [PubMed] [Google Scholar]
- 110.Frye CA, DeBold JF. Muscimol facilitates sexual receptivity in hamsters when infused into the ventral tegmentum. Pharmacol Biochem Behav. 1992;42:879–87. doi: 10.1016/0091-3057(92)90044-g. [DOI] [PubMed] [Google Scholar]
- 111.Frye CA, Mermelstein PG, DeBold JF. Bicuculline infused into the hamster ventral tegmentum inhibits, while sodium valproate facilitates, sexual receptivity. Pharmacol Biochem Behav. 1993;46:1–8. doi: 10.1016/0091-3057(93)90308-g. [DOI] [PubMed] [Google Scholar]
- 112.Frye CA, Walf AA, Petralia SM. In the ventral tegmental area, progestins have actions at D1 receptors for lordosis of hamsters and rats that involve GABAA receptors. Horm Behav. 2006;50:332–7. doi: 10.1016/j.yhbeh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 113.Frye CA, Vongher JM. Progestins’ rapid facilitation of lordosis when applied to the ventral tegmentum corresponds to efficacy at enhancing GABAA receptor activity. J Neuroendocrinology. 1999;11:829–37. doi: 10.1046/j.1365-2826.1999.00367.x. [DOI] [PubMed] [Google Scholar]
- 114.Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure-activity relationships for steroid interaction with the gamma-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1987;241:346–53. [PubMed] [Google Scholar]
- 115.Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(a) receptor. Nat Rev Neurosci. 2005;6:565–75. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 116.Brot MD, Akwa Y, Purdy RH, Koob GF, Britton KT. The anxiolytic-like effects of the neurosteroid allopregnanolone: interactions with GABA(A) receptors. Eur J Pharmacol. 1997;325:1–7. doi: 10.1016/s0014-2999(97)00096-4. [DOI] [PubMed] [Google Scholar]
- 117.Gee KW, McCauley LD, Lan NC. A putative receptor for neurosteroids on the GABAA receptor complex: the pharmacological properties and therapeutic potential of epalons. Crit Rev Neurobiol. 1995;9:207–27. [PubMed] [Google Scholar]
- 118.Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 119.Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–5. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- 120.Reddy DS. Pharmacology of catamenial epilepsy. Methods Find Exp Clin Pharmacol. 2004;26:547–61. doi: 10.1358/mf.2004.26.7.863737. [DOI] [PubMed] [Google Scholar]
- 121.Weir CJ, Ling AT, Belelli D, Wildsmith JA, Peters JA, Lambert JJ. The interaction of anaesthetic steroids with recombinant glycine and GABAA receptors. Br J Anaesth. 2004;92:704–11. doi: 10.1093/bja/aeh125. [DOI] [PubMed] [Google Scholar]
- 122.Vincens M, Shu C, Moguilewsky M, Philibert D. A progesterone metabolite enhances the activity of the GABAA receptor complex at the pituitary level. Eur J Pharmacol. 1989;168:15–21. doi: 10.1016/0014-2999(89)90627-4. [DOI] [PubMed] [Google Scholar]
- 123.Im WB, Blakeman DP, Davis JP. Na(+)-dependent GABA transport system scavenges endogenous external GABA and prevents desensitization of GABAA receptors in rat cerebrocortical synaptoneurosomes. Brain Res. 1990;521:143–7. doi: 10.1016/0006-8993(90)91535-o. [DOI] [PubMed] [Google Scholar]
- 124.Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–15. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mansvelder HD, De Rover M, McGehee DS, Brussaard AB. Cholinergic modulation of dopaminergic reward areas: upstream and downstream targets of nicotine addiction. Eur J Pharmacol. 2003;480:117–23. doi: 10.1016/j.ejphar.2003.08.099. [DOI] [PubMed] [Google Scholar]
- 126.Bayer VE, Pickel VM. GABA-labeled terminals form proportionally more synapses with dopaminergic neurons containing low densities of tyrosine hydroxylase immunoreactivity in rat ventral tegmental area. Brain Res. 1991;559:44–55. doi: 10.1016/0006-8993(91)90285-4. [DOI] [PubMed] [Google Scholar]
- 127.Willick ML, Kokkinidis L. The effects of ventral tegmental administration of GABAA, GABAB and NMDA receptor agonists on medial forebrain bundle self-stimulation. Behav Brain Res. 1995;70:31–6. doi: 10.1016/0166-4328(94)00181-e. [DOI] [PubMed] [Google Scholar]
- 128.Park-Chung M, Wu FS, Farb DH. α-Hydroxy-5α-pregnan-20-one sulfate: a negative modulator of the NMDA-induced current in cultured neurons. Mol Pharmacol. 1994;46:146–50. [PubMed] [Google Scholar]
- 129.Cyr M, Ghribi O, Di Paolo T. Regional and selective effects of oestradiol and progesterone on NMDA and AMPA receptors in the rat brain. J Neuroendocrinol. 2000;12:445–52. doi: 10.1046/j.1365-2826.2000.00471.x. [DOI] [PubMed] [Google Scholar]
- 130.Wu FS, Gibbs TT, Farb DH. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol Pharmacol. 1991;40:333–6. [PubMed] [Google Scholar]
- 131.Stoof JC, Kebabian JW. Two dopamine receptors: biochemistry, physiology and pharmacology. Life Sci. 1984;35:2281–96. doi: 10.1016/0024-3205(84)90519-8. [DOI] [PubMed] [Google Scholar]
- 132.Churchill L, Dilts RP, Kalivas PW. Autoradiographic localization of gamma-aminobutyric acidA receptors within the ventral tegmental area. Neurochem Res. 1992;17:101–6. doi: 10.1007/BF00966870. [DOI] [PubMed] [Google Scholar]
- 133.Klitenick MA, DeWitte P, Kalivas PW. Regulation of somatodendritic dopamine release in the ventral tegmental area by opioids and GABA: an in vivo microdialysis study. J Neurosci. 1992;12:2623–32. doi: 10.1523/JNEUROSCI.12-07-02623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lévesque D, Di Paolo T. Effect of the rat estrous cycle at ovariectomy on striatal D-1 dopamine receptors. Brain Res Bull. 1990;24:281–4. doi: 10.1016/0361-9230(90)90216-m. [DOI] [PubMed] [Google Scholar]
- 135.Frye CA, Walf AA, Sumida K. Progestins’ actions in the VTA to facilitate lordosis involve dopamine-like Type 1 and 2 receptors. Pharmacol Biochem Behav. 2004;78:405–18. doi: 10.1016/j.pbb.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 136.Sumida K, Walf AA, Frye CA. Progestin-facilitated lordosis of hamsters may involve dopamine-like type 1 receptors in the ventral tegmental area. Behav Brain Res. 2005;161:1–7. doi: 10.1016/j.bbr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 137.Feng XQ, Dong Y, Fu YM, Zhu YH, Sun JL, Wang Z, Sun FY, Zheng P. Progesterone inhibition of dopamine-induced increase in frequency of spontaneous excitatory postsynaptic currents in rat prelimbic cortical neurons. Neuropharmacology. 2004;46:211–222. doi: 10.1016/j.neuropharm.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 138.Frye CA, Walf AA. Membrane actions of progestins at dopamine type 1-like and GABAA receptors involve downstream signal transduction pathways. Steroids. 2008;73:906–913. doi: 10.1016/j.steroids.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kow LM, Pfaff DW. Mapping of neural and signal transduction pathways for lordosis in the search for estrogen actions on the central nervous system. Behav Brain Res. 1998;92:169–80. doi: 10.1016/s0166-4328(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 140.Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004;61:641–4. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- 141.Fáncsik A, Linn DM, Tasker JG. Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J Neurosci. 2000;20:067–75. doi: 10.1523/JNEUROSCI.20-09-03067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Frye CA, Walf AA, Petralia SM. Progestins’ effects on sexual behaviour of female rats and hamsters involving D1 and GABA(A) receptors in the ventral tegmental area may be G-protein-dependent. Behav Brain Res. 2006;172:286–93. doi: 10.1016/j.bbr.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 143.Frye CA, Walf AA. In the ventral tegmental area, the membrane-mediated actions of progestins for lordosis of hormone-primed hamsters involve phospholipase C and protein kinase C. J Neuroendocrinol. 2007;19:717–24. doi: 10.1111/j.1365-2826.2007.01580.x. [DOI] [PubMed] [Google Scholar]
- 144.Frye CA, Walf AA, Petralia SM. Progestin facilitation of lordosis in rodents involves adenylyl cyclase activity in the ventral tegmental area. Horm Behav. 2006;50:237–44. doi: 10.1016/j.yhbeh.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 145.Frye CA, Walf AA. In the ventral tegmental area, progestogens’ membrane-mediated actions for lordosis of rats involve the second-messenger phospholipase C. Brain Res. 2008;1230:218–23. doi: 10.1016/j.brainres.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Frye CA, Walf AA. Activity of protein kinase C is important for 3α,5α-THP’s actions at dopamine type 1-like and/or GABAA receptors in the ventral tegmental area for lordosis of rats. Brain Res Bull. 2008;77:91–7. doi: 10.1016/j.brainresbull.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Frye CA, Walf AA. Infusions of anti-sense oligonucleotides for DARPP-32 to the ventral tegmental area reduce effects of progesterone- and a dopamine type 1-like receptor agonist to facilitate lordosis. Behav Brain Res. 2010a;206:286–92. doi: 10.1016/j.bbr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Petralia SM, Frye CA. In the ventral tegmental area, cyclic AMP mediates the actions of progesterone at dopamine type 1 receptors for lordosis of rats and hamsters. J Neuroendocrinol. 2006;18:902–14. doi: 10.1111/j.1365-2826.2006.01488.x. [DOI] [PubMed] [Google Scholar]
- 149.Petralia SM, Frye CA. In the ventral tegmental area, G-proteins and cAMP mediate the neurosteroid 3α,5α-THP’s actions at dopamine type 1 receptors for lordosis of rats. Neuroendocrinology. 2004;80:233–43. doi: 10.1159/000082752. [DOI] [PubMed] [Google Scholar]
- 150.Petralia SM, Walf AA, Frye CA. In the ventral tegmental area, progestins’ membrane-mediated actions for lordosis of hamsters and rats involve protein kinase A. Neuroendocrinology. 2006;84:405–14. doi: 10.1159/000100510. [DOI] [PubMed] [Google Scholar]
- 151.Frye CA, Rhodes ME. Progestin concentrations are increased following paced mating in midbrain, hippocampus, diencephalon, and cortex of rats in behavioral estrus, but only in midbrain of diestrous rats. Neuroendocrinology. 2006b;83:336–47. doi: 10.1159/000096051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Frye CA, Sumida K, Zimmerberg B, Brunelli SA. Rats bred for high versus low anxiety responses neonatally demonstrate increases in lordosis, pacing behavior, and midbrain 3α, 5α-THP levels as adults. Behav Neurosci. 2006;120:281–9. doi: 10.1037/0735-7044.120.2.281. [DOI] [PubMed] [Google Scholar]
- 153.Frye CA, Paris JJ, Rhodes ME. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5α-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 2007;133:663–74. doi: 10.1530/rep.1.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Walf AA, Paris JJ, Llaneza DC, Frye CA. I. Levels of 5α-reduced progesterone metabolite in the midbrain account for variability in reproductive behavior of middle-aged female rats. Brain Res. 2011;1379:137–48. doi: 10.1016/j.brainres.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Frye CA, Leadbetter EA. 5α-reduced progesterone metabolites are essential in hamster VTA for sexual receptivity. Life Sci. 1994;54:653–9. doi: 10.1016/0024-3205(94)00548-6. [DOI] [PubMed] [Google Scholar]
- 156.Frye CA, Petralia SM. Lordosis of rats is modified by neurosteroidogenic effects of membrane benzodiazepine receptors in the ventral tegmental area. Neuroendocrinology. 2003;77:71–82. doi: 10.1159/000068338. [DOI] [PubMed] [Google Scholar]
- 157.Petralia SM, Jahagirdar V, Frye CA. Inhibiting biosynthesis and/or metabolism of progestins in the ventral tegmental area attenuates lordosis of rats in behavioural oestrus. J Neuroendocrinol. 2005;17:545–52. doi: 10.1111/j.1365-2826.2005.01342.x. [DOI] [PubMed] [Google Scholar]
- 158.Frye CA, Bayon LE, Pursnani NK, Purdy RH. The neurosteroids, progesterone and 3α,5α-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998;808:72–83. doi: 10.1016/s0006-8993(98)00764-1. [DOI] [PubMed] [Google Scholar]
- 159.Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, Guidotti A. Brain 5α-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci USA. 2001;98:2849–54. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Matsumoto K, Pinna G, Puia G, Guidotti A, Costa E. Social isolation stress-induced aggression in mice: a model to study the pharmacology of neurosteroidogenesis. Stress. 2005;8:85–93. doi: 10.1080/10253890500159022. [DOI] [PubMed] [Google Scholar]
- 161.Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci USA. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Miczek KA, Fish EW, DeBold JF. Neurosteroids, GABAA receptors, and escalated aggressive behavior. Horm Behav. 2003;44:242–57. doi: 10.1016/j.yhbeh.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 163.Fish EW, Faccidomo S, DeBold JF, Miczek KA. Alcohol, allopregnanolone and aggression in mice. Psychopharmacology. 2001;153:473–83. doi: 10.1007/s002130000587. [DOI] [PubMed] [Google Scholar]
- 164.Frye CA, Rhodes ME, Walf A, Harney JP. Testosterone enhances aggression of wild-type mice but not those deficient in type I 5alpha-reductase. Brain Res. 2002;948:165–70. doi: 10.1016/s0006-8993(02)03076-7. [DOI] [PubMed] [Google Scholar]
- 165.Pinna G, Agis-Balboa RC, Pibiri F, Nelson M, Guidotti A, Costa E. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochem Res. 2008;33:1990–2007. doi: 10.1007/s11064-008-9718-5. [DOI] [PubMed] [Google Scholar]
- 166.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3a,5a-THP. Pharmacol Biochem Behav. 2000a;67:587–96. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 167.Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–15. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- 168.Rhodes ME, Frye CA. Inhibiting progesterone metabolism in the hippocampus of rats in behavioral estrus decreases anxiolytic behaviors and enhances exploratory and antinociceptive behaviors. Cogn Affect Behav Neurosci. 2001;1:287–96. doi: 10.3758/cabn.1.3.287. [DOI] [PubMed] [Google Scholar]
- 169.Frye CA, Paris JJ, Rhodes ME. Increasing 3α,5α-THP following inhibition of neurosteroid biosynthesis in the ventral tegmental area reinstates anti-anxiety, social, and sexual behavior of naturally receptive rats. Reproduction. 2009;137(1):119–28. doi: 10.1530/REP-08-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Frye CA, Rhodes ME. Infusions of 5α-pregnan-3α-ol-20-one (3α,5α-THP) to the ventral tegmental area, but not the substantianigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3α,5α-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J Neuroendocrinol. 2006a;18:960–75. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- 171.Frye CA, Rhodes ME. Infusions of 3α,5α-THP to the VTA enhance exploratory, anti-anxiety, social, and sexual behavior and increase levels of 3α,5α-THP in midbrain, hippocampus, diencephalon, and cortex of female rats. Behav Brain Res. 2008;187:88–99. doi: 10.1016/j.bbr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005;28:249–6. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 173.Woods CG, Heuvel JP, Rusyn I. Genomic profiling in nuclear receptor-mediated toxicity. Toxicol Pathol. 2007;35:474–94. doi: 10.1080/01926230701311351. [DOI] [PubMed] [Google Scholar]
- 174.Harmsen S, Meijerman I, Beijnen J, Schellens JH. The role of nuclear receptors in pharmacokinetic drug-drug interactions in oncology. Cancer Treat Rev. 2007;33:369–80. doi: 10.1016/j.ctrv.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 175.Masuyama H, Suwaki N, Tateishi Y, Nakatsukasa H, Segawa T, Hiramatsu Y. The pregnane X receptor regulates gene expression in a ligand- and promoter-selective fashion. Mol Endocrinol. 2005;19:1170–80. doi: 10.1210/me.2004-0434. [DOI] [PubMed] [Google Scholar]
- 176.Marini S, Nannelli A, Sodini D, Dragoni S, Valoti M, Longo V, Gervasi PG. Expression, microsomal and mitochondrial activities of cytochrome P450 enzymes in brain regions from control and phenobarbital-treated rabbits. Life Sci. 2007;13:910–7. doi: 10.1016/j.lfs.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 177.Bauer B, Hartz AM, Fricker G, Miller DS. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol. 2004;66:413–9. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 178.Lamba V, Yasuda K, Lamba JK, Assem M, Davila J, Strom S, Schuetz EG. PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol Appl Pharmacol. 2004;199:251–65. doi: 10.1016/j.taap.2003.12.027. [DOI] [PubMed] [Google Scholar]