INTRODUCTION
Hypertension is common among patients with chronic kidney disease (CKD) and diabetes mellitus. In this population hypertension increases risk for kidney disease onset and progression and cardiovascular (CV) morbidity and mortality. Diabetic nephropathy is the most common cause of CKD in those with diabetes and is the leading attributable cause for incident end stage renal disease (ESRD) in the United States (US). The mechanism of hypertension in diabetic nephropathy is complex, incompletely understood, and includes excess sodium retention, sympathetic nervous system (SNS) and renin-angiotensin-aldosterone system (RAAS) activation, endothelial cell dysfunction (ECD), and increased oxidative stress. Both non-pharmacological and pharmacological interventions, including RAAS antagonists, are critically important in the management of hypertension in diabetic nephropathy. The purpose of this article is to examine the pathophysiology of hypertension in diabetic nephropathy and the clinical trials that support the implementation of strategies aimed at these pathophysiologic mechanisms. Evidence from prior and very recent clinical trials in patients not on dialysis is reviewed. Management of hypertension in patients on dialysis is an important topic that is beyond the scope of this review, but has been well reported previously (1).
DIABETES AND KIDNEY DISEASE-DIABETIC NEPHROPATHY
Epidemiology
Diabetic nephropathy is characterized by hypertension, progressive albuminuria, glomerulosclerosis, and decline in glomerular filtration rate (GFR) leading to ESRD. Hypertension in the setting of diabetes is defined as a systolic blood pressure ≥ 130 mmHg or a diastolic blood pressure ≥ 80 mmHg. Diabetic nephropathy is the leading cause of ESRD in the US with an adjusted incidence rate of 158 per million (2). The risk of CKD is higher in patients with type 1 (DM1) than type 2 diabetes (DM2), but the overall absolute number of patients with DM2 and nephropathy is greater. Self-reported diabetes is associated with a prevalence of CKD of 8.9% (stage I), 12.8% (stage II), 19.4% (stage III), and 2.7% (stage IV and V combined); the overall odds ratio of having CKD for a diabetic patient is 2.51 (CI 2.07-3.05) (3). Diabetic nephropathy is not the only cause of kidney disease in diabetic patients, but certain characteristics strongly support this diagnosis. Renal biopsy, the gold standard for establishing the etiology of kidney disease, is not commonly performed in patients with diabetes; instead it is usually reserved for those in whom a non-diabetic cause is suspected. When diabetic retinopathy coexists with albuminuria, the likelihood of diabetic nephropathy is very high and suggests the presence of the specific pattern of nodular glomerulosclerosis, the so called Kimmelstiel-Wilson lesion (4). Guidelines state that CKD can be attributed to diabetes in the presence of macroalbuminuria (>300 mg/24 hr) or the presence of microalbuminuria (30-300 mg/24 hr) in the context of diabetic retinopathy or a history of diabetes exceeding 10 years (5). Lack of retinopathy, lack of autonomic neuropathy, and presence of albuminuria at the time of the diagnosis of diabetes all suggest a non-diabetic etiology for persistent albuminuria in diabetic patients (6).
DIABETIC NEPHROPATHY AND HYPERTENSION
Epidemiology
Hypertension is approximately twice as prevalent in patients with diabetes compared to the general population (7). In DM1, hypertension typically occurs in patients with microalbuminuria or overt nephropathy (8). Estimates of the prevalence of hypertension in normoalbuminuric patients with DM1 are varied; older studies using the definition of hypertension as 160/95 mmHg showed a prevalence of 19% (9). One larger Danish cross sectional study including over 1700 diabetics and 10,000 controls showed that in patients with DM1 and without micro or macroalbuminuria, the prevalence of hypertension (again defined as 160/95 mmHg) was similar to that of the general population (3.9% vs. 4.4%) (8). Of note, subjects with DM1 in the latter study were younger on average than those in the former, which may explain the lower prevalence of hypertension. However, a “non-dipping” nocturnal blood pressure pattern in normoalbuminuric DM1 patients predicts future microalbuminuria, possibly identifying high risk patients before the onset of kidney disease(10). In the visit before microalbuminuria occurred, elevated daytime systolic blood pressure (either office or ambulatory) was still not present. Genetic factors also play a role in the association of hypertension with microalbuminuria based on blood pressure analysis of family members of diabetic patients with microalbuminuria (11).
In DM2, hypertension commonly exists prior to kidney disease. The common risk factors for glucose intolerance and hypertension (i.e. obesity) may explain this association. In one study, 58% of patients with newly diagnosed DM2 (without proteinuria) already had hypertension, with other studies showing as high as 70% (12,13). Diabetes duration does not increase the incidence of hypertension, although the presence of impaired kidney function does. Hypertension leads to further progression of kidney disease and contributes to the increased incidence of CV disease in this population.
The above studies overall suggest that microalbuminuria precedes hypertension more commonly in DM1 than DM2. In either scenario, worsening renal function further contributes to elevated BP. The prevalence of hypertension in diabetic nephropathy increases at each stage of CKD, approaching 90% for ESRD patients (14). Individual susceptibility to renal disease and hypertension likely involves the combination of metabolic and hemodynamic disturbances that are commonly shared by most diabetics, as well as genetic determinants that further dictate each patient’s vulnerability. Some genes may increase susceptibility, while others may be renoprotective. It is not clear whether these genes determine the incidence of diabetic nephropathy specifically or just the vulnerability of renal disease in general in the context of an additional risk factor such as diabetes. The reader is directed to other reviews for further details on this topic (15,16).
Etiology
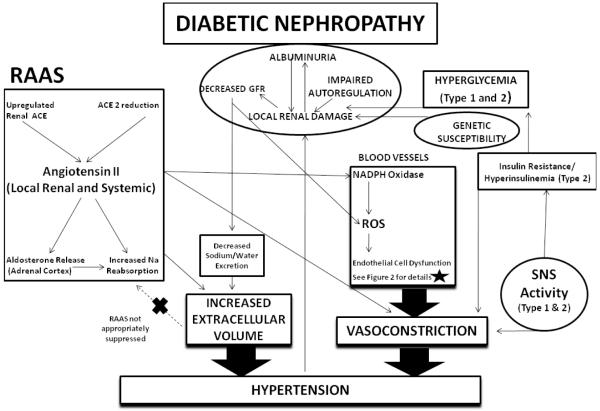
Multiple factors contribute to increases in blood pressure and hypertension in patients with diabetes and nephropathy. The major causes of hypertension in both DM1 and DM2 include volume expansion owing to increased renal sodium reabsorption and peripheral vasoconstriction owing to dysregulation of factors that regulate peripheral vascular resistance (Figure 1). Activation of the RAAS, upregulation of endothelin1 (ET-1), upregulation of reactive oxygen species, and downregulation of nitric oxide (NO) conspire to produce hypertension in this setting. Importantly, many of these pathogenic factors have local non-hemodynamic effects that can accelerate kidney disease and CV disease among patients with diabetes and kidney disease.
Figure 1.
The mechanisms of hypertension in diabetes and kidney disease are mutlifactorial and largely mediated by increased vasoconstriction and increased extracellular volume.
The initial disturbances of diabetic nephropathy involve local renal damage mediated by metabolic effects from uncontrolled hyperglycemia seen in both Type 1 and Type 2 diabetics. This is compounded by hemodynamic effects from increased RAAS and impaired afferent arteriolar autoregulation. An individual’s susceptibility to renal disease is likely determined in part by genetic make-up, as well. Over time, this local renal damage results in decreased GFR and albuminuria. As GFR is reduced, there is further impairment in sodium excretion resulting in increased extracellular volume. Additionally, oxidative stress is increased in CKD patients, leading to endothelial cell dysfunction and vasoconstriction.
There is elevated renal RAAS activity and Ang II, with possible contributions from decreased ACE 2 and increased ACE activity. Ang II increases renal sodium reabsorption via direct actions on the kidney and indirectly via aldosterone release. Systemic RAAS is not appropriately suppressed despite the increased extracellular volume. Ang II increases vasoconstriction via increases in oxidative stress through induction of NADPH oxidase and directly via binding to VSMC.
The presence of increased SNS activity seen in both DM1 and DM2 as a form of autonomic neuropathy increases vasoconstriction, as well as contributes to impaired glucose tolerance/insulin resistance (DM2), which itself is associated with ECD.
Renin-Angiotensin-Aldosterone System
Angiotensin II (Ang II) is responsible for many of the actions of the RAAS. Ang II binds to angiotensin type 1 (AT1) receptors in many tissues causing vasoconstriction in vascular smooth muscle cells (VSMC), increased sodium reabsorption at the renal proximal tubule, and stimulation of aldosterone release from the adrenal cortex. These actions of Ang II in addition to aldosterone-stimulated sodium reabsorption in the collecting duct serve to increase vasoconstriction, sodium reabsorption, and hence blood pressure. In addition to these effects, Ang II also increases production of superoxide by activation of NADPH oxidase in the systemic vasculature, heart and kidney (17).
Although levels of plasma renin activity (PRA) are suppressed or similar in diabetics compared to controls (18,19), there is evidence that intrarenal RAAS activity is increased in diabetes. Increased Angiotensin Converting Enzyme (ACE) is expressed in the glomerulus and renal vasculature of rats with streptozocin induced diabetes (20). The enhanced presence of glomerular ACE has also been noted in humans with DM2 and nephropathy (21,22). Although baseline PRA is not elevated in patients with diabetes, RAAS activity is poorly suppressed with sodium loading and is considered to be inappropriately elevated given the volume expanded state that occurs in diabetes (19). Pharmacologic interventions that inhibit production of Ang II or block AT1 receptor that target the RAAS are a cornerstone in the treatment of hypertension in patients with diabetic nephropathy (see Management section below).
A recent development in the association of RAAS with diabetic nephropathy has been the discovery of ACE2, a homologue to the ACE. ACE2 binds Ang I, converts it to Ang 1-9 (instead of Ang II), and converts Ang II to Ang 1-7. Unlike Ang II, Ang 1-7 has vasodilatory, antifibrotic, and natriuretic effects (23). Differences in renal ACE2 levels have been detected in hypertensive humans compared to controls. Furthermore, animals with streptozocin induced diabetic nephropathy have decreased renal expression of ACE2 (24). As more is learned about the association of ACE2 with hypertension and diabetic nephropathy, it may become possible to earlier identify diabetic patients at high risk of developing kidney disease.
Sodium Balance
Sodium balance undoubtedly also plays a large role in the hypertension seen in diabetics with renal disease. Patients with diabetes have increased total exchangeable body sodium even in the absence of increased systemic RAAS activity (25). In early diabetes, the increased renal RAAS activity may enable increased sodium reabsorption independent of GFR. It is hypothesized that this is a response to the osmotic diuretic effects of tubular hyperglycemia, and that the typical increase in GFR seen in early diabetes defends against this sodium avid state (26). Sodium loading does not appropriately suppress systemic RAAS in DM2, enabling hypertension to occur in the context of a high sodium diet (19). Sodium sensitivity occurs in patients with DM2 (27) and is associated with albuminuria (28). Insulin reduces renal sodium excretion independent of serum glucose levels (29). This may further explain the increased prevalence of hypertension prior to onset of DM2, rather than DM1. As GFR progressively declines, the kidney’s ability to excrete sodium and water further diminishes. Volume sensitivity persists as a mechanism that facilitates further sodium excretion at the expense of higher blood pressure in the context of a sodium load (30). Continued exposure to sodium may also induce changes in the VSMC that allow hypertension to persist (31).
Sympathetic Nervous System Activity
Increased SNS activity is an important mechanism contributing to the pathogenesis of hypertension in patients with diabetic nephropathy. For example, the nocturnal blood pressure elevations which precede microalbuminuria are believed to be manifestations of autonomic neuropathy, another microvascular complication related to hyperglycemia (32,33). A pattern of sustained adrenergic activity associated with high nocturnal blood pressure also occurs in patients with DM2 and nephropathy (34). Nocturnal heart rate patterns are abnormal in hyperinsulinemic non-diabetic individuals, suggesting insulin resistance as a possible link between SNS activation and hypertension (35).
Endothelial Cell Dysfunction
The actions of the RAAS are complemented and counterbalanced by substances released from vascular endothelial cells. Endothelial cell dysfunction involves imbalances in the production or function of endogenous vasoconstrictors and vasodilators. Endothelial cells contain nitric oxide synthase (NOS), which produces NO from L-arginine. NO induces VSMC relaxation and vasodilatation. ET-1 is also released from the endothelium, and induces vasoconstriction by binding to endothelin A receptors on VSMC. The presence of asymetric dimethylarginine (ADMA), a competitive inhibitor of NO production, reduces the amount of NO available to cause vasodilatation. Reactive oxygen species also impair the vasodilatory action of NO. The net effect of the imbalance of these systems in diabetic nephropathy is sustained vasoconstriction.
Asymmetric dimethyl argininine. (ADMA)
In a study of healthy human subjects, ADMA infusion increased both blood pressure and total peripheral resistance (36). This study also confirmed that ADMA is metabolized via dimethylarginine dimethylaminohydrolase (DDAH). Increased ADMA levels are seen in patients with DM2 (37), patients with CKD, and those with ESRD (38). Elevated levels are associated with CV events in DM1 patients with nephropathy and ESRD patients (39,40). Animal studies in partially nephrectomized rats show that elevated ADMA levels correlate with systolic blood pressure and suggest the responsible mechanism is decreased DDAH activity (41). Although decreased renal clearance could explain ADMA accumulation in patients with ESRD, it is not clear why DDAH is downregulated in CKD.
Endothelin-1
ET-1 is a potent vasoconstrictor produced in the vascular endothelium that induces vasoconstriction upon binding to ETA receptors in VSMC. Insulin can increase endothelial production of both NO and ET-1 through separate pathways. The insulin resistance in DM2 results in a selective increase in ET-1, but decreased NO production (42). Elevated ET-1 levels are found in DM2 patients, as well as in patients with CKD (43,44). In CKD, administration of endothelin receptor antagonists (particularly ETA) result in blood pressure reductions, implicating its role in clinical hypertension (45). A discussion of a trial involving one of these drugs in diabetic nephropathy is included later.
Nitric Oxide
The exact role of NO in the pathogenesis of hypertension in diabetic nephropathy is less well defined. Intrarenal levels of NO and various isoforms of NOS are increased in the early hyperfiltration stages of kidney disease in diabetes, but decrease as disease progresses to overt diabetic nephropathy (46). Rats rendered diabetic by streptozotocin demonstrate an aggravated blood pressure response to chronic L-NAME, an eNOS inhibitor, infusion as compared to non-diabetic rats (47), suggesting that in early stages of experimental diabetes, blood pressure control is highly dependent on NO.
Oxidative Stress
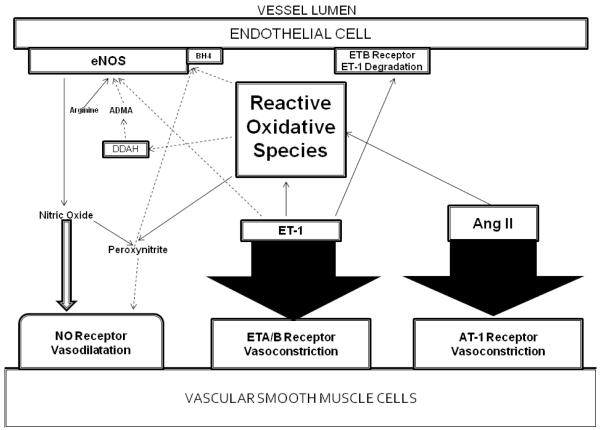
Hyperglycemia is believed to be one of the causes of increased oxidative stress in diabetes (48). CKD itself is also a state of high oxidative stress associated with increased oxidative species and decreased antioxidant defenses (49,50). Partial nephrectomy in animals results in increased levels of oxidative stress markers and decreased excretion of NO, but reductions in blood pressure occur after administration of antioxidants (51). In experimental animal models of DM2 with histologic evidence of early nephropathy, urinary markers of oxidative stress were seen along with increased peroxynitrite (52). Oxidative stress is influenced by and affects the mediators of both RAAS and ECD to further contribute to hypertension enhanced vasoconstriction (Figure 2).
Figure 2.
Under normal circumstances, NO is produced by eNOS on the endothelial cell using L-arginine as a substrate and tetrahydrobiopterin (BH4) as a cofactor. eNOS is inhibited by asymmetrical dimethylarginine, which itself is inhibited by DDAH. The vasodilating effects of NO is balanced by the vasoconstricting effects of Angiotensin II and Endothelin 1 on the Vascular Smooth Muscle Cells. Under circumstances of oxidative stress, ROS interact with 1) BH4, resulting in decreased activity of eNOS and decreased NO production; 2) DDAH, resulting in accumulation of ADMA and inhibition of NO production by eNOS; 3) NO itself, resulting in production of the peroxynitrite radical and further ROS. ET-1 and Ang-II increase ROS production via NADPH oxidase. The cumulative effect favors vasoconstriction.
When exposed to Ang II, cultured VSMC demonstrate increased activation of NADH/NADPH oxidase (53). Chronic Ang II infusion also resulted in increased superoxide production via NADH/NADPH oxidase during in vivo experiments in rats (54). Reductions in oxidative stress markers correlated with albuminuria reductions in diabetic patients treated with an angiotensin receptor blocker but not with a thiazide diuretic (55). With this accumulation of evidence of the impact of Ang II on oxidative stress, including in humans, RAAS inhibiting agents are a promising tool to alleviate the effects of oxidative stress.
Endothelial Cell Dysfunction
NO synthesis is impaired when BH4, a cofactor for eNOS, interacts with ROS. Even when NO can be produced, NO-ROS interactions result in peroxynitrite production. The formation of this radical decreases NO availability and propagates more oxidative stress through production of other ROS. Furthermore, the activity of DDAH is impaired by oxidative stress. ET-1 can increase the production of ROS through an NADPH oxidase pathway in DOCA-salt hypertensive rats, establishing another relationship between oxidative stress and ECD (56).
Autoregulatory Impairment
In healthy kidneys, the autoregulatory functions of the afferent arteriole via a myogenic reflex and the juxtaglomerular apparatus via tubuloglomerular feedback serve to maintain constant glomerular pressures despite variations in systemic blood pressure. When these mechanisms are impaired, such as in diabetic nephropathy, elevated systemic blood pressure are directly transmitted to the renal microvasculature and glomeruli (57). The ultimate consequences are glomerular hypertension and activation of local mediators that induce inflammation, fibrosis, and further injury. This hemodynamic stress is further accentuated in diabetic nephropathy by inducing up regulation of renal mesangial GLUT-1 transporters that allow intracellular hyperglycemia and its complications to persist (58).
MANAGEMENT OF HYPERTENSION IN DIABETIC NEPHROPATHY
GUIDELINES
The essential goals of therapy in the management of diabetic nephropathy are treatment of hypertension and reduction of albuminuria. The presence of microalbuminuria is the first clinical manifestation of renal disease in patients with diabetes. In DM1, once microalbuminuria is established as a persistent feature, it is expected that without treatment 80% of these patients will progress to develop overt nephropathy with macroalbuminuria. Of this group, 50-75% will progress to ESRD at variable rates in the next 10-20 years (left untreated) [59]. Conversely, for untreated DM2 with microalbuminuria, 40% are expected to progress to overt nephropathy, of which 20% will progress to ESRD within the following 20 years.
Hypertension leads to progression of kidney disease and increases CV risk in these patients. In spite of the high incidence of overt nephropathy and ESRD without treatment, pharmacologic lowering of blood pressure was shown to slow progression of kidney failure in patients with DM1 more than 20 years ago (60-62). In these studies the addition of antihypertensives decreased the rate of decline in GFR from an average of 1.23 (in 5 out of 6 subjects), 0.94, and 0.91 ml/min/month to 0.49, 0.1-0.29, and 0.39 ml/min/month respectively. More recently, several studies demonstrated the critical role of using drugs that block the RAAS in further slowing progression of diabetic nephropathy (63-66). Using stricter BP goals than in earlier studies, DM1 patients on placebo had an average decline in creatinine clearance of 17 mL/min/yr compared to 11 mL/min/yr for patients on captopril (63). For DM2 patients, irbesartan use resulted in an average decline in Cr clearance of −5.5 mL/min/yr compared to −6.5 and −6.8 mL/min/yr in placebo and amlodipine_groups (65). These studies demonstrated that the benefit of such therapy could not be explained solely on the basis of blood pressure lowering. Hence, angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB) have become standard of care in patients with diabetic nephropathy. We will first review the evidence supporting the effects of these classes of agents on renal outcomes and then discuss the appropriate target blood pressure for patients with diabetic nephropathy.
INHIBITORS OF THE RENIN ANGIOTENSIN ALDOSTERONE SYSTEM
ACE Inhibitors or Angiotensin Receptor Blockers as Monotherapy
In addition to lowering blood pressure, current recommendations include using either an ACEi or ARB as first line treatment of hypertension in patients with diabetic nephropathy. ACEi slow the decline in GFR and prevent increases in albuminuria in DM1 patients with diabetic nephropathy (63,64). The Irbesartan in Diabetic Nephropathy Trial (IDNT) and Reduction in Endpoints in NIDDM with the Angiotensin Antagonist Losartan (RENAAL) studies were seminal studies establishing the efficacy of ARBs in patients with DM2 and nephropathy (65,66). These studies specified the composite of doubling serum creatinine (Cr), ESRD, and death as their primary outcome, but most benefit came from renal outcomes. IDNT randomized 1715 patients with nephropathy to either irbesartan 300 mg or amlodipine 10 mg, or placebo once daily. The number of composite outcome events was significantly lower in irbesartan compared to amlodipine and placebo. There were significantly less subjects with doubled serum Cr and significantly slower increases in serum Cr in the irbesartan group compared to both amlodipine and placebo, but unadjusted rates of ESRD or death were not different. The final achieved blood pressures in this study were 140/77 mmHg, 141/77 mmHg, and 144/80 mmHg for irbesartan, amlodipine, and placebo. The RENAAL trial studied 1513 patients with DM2 and nephropathy with the same primary composite outcome as IDNT. Subjects were randomized to either losartan (50-100 mg daily) or placebo, and losartan was associated with a lower occurrence of the primary outcome in both the unadjusted and adjusted analyses. Similar to IDNT, there was a significant reduction in the individual end point of doubling of serum Cr, but not death. Contrary to IDNT, the risk for ESRD was significantly lower during treatment with losartan; and in a prespecified secondary outcome hospitalization for heart failure was also reduced by losartan. There was no benefit from losartan regarding composite CV events (detailed analysis included below). The mean arterial pressures (MAP) were not different between groups at baseline or the end of the study, although it was lower in the losartan following a 1-year interim analysis. The final blood pressures in RENAAL were 140/74 and 142/74 mmHg for losartan and placebo.
The IRbesartan in MicroAlbuminuria (IRMA) study evaluated 590 patients with hypertension, DM2 and microalbuminuria and demonstrated that progression to macroalbuminuria was significantly reduced by administration of 300 mg irbesartan compared to placebo (67). Although the MAP difference was statistically lower in those assigned to irbesartan 300 mg daily as compared to placebo, the study still supported the role of ARB even prior to the onset of overt nephropathy.
Results from trials studying the primary prevention of microalbuminuria have been mixed. Phase A of the Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) (68) randomized hypertensive patients with DM2 and normoalbuminuria to receive either trandolapril, long-acting verapamil (Verapamil SR), combination therapy, or placebo. There was significant reduction in blood pressure in both the trandolapril and combination group compared to placebo, but not for verapamil as compared to placebo. The incidence of microalbuminuria was 10% for placebo, 6.0% for trandolapril, 5.7% for combination, and 11.9% for verapamil. Even after adjustment for systolic blood pressure and diastolic blood pressure, the acceleration factor for progression was significantly lower in the trandolapril and combination group vs. placebo, but not for verapamil vs. placebo. The underlying conclusion was that ACEi based therapy could delay onset of microalbuminuria in hypertensive DM2 patients.
The Randomized Olmesartan And Diabetes MicroAlbuminuria Protection (ROADMAP) study recently assessed the effect of olmesartan, a long-acting ARB, on development of microalbuminuria in hypertensive patients with DM2 and normoalbuminuria. This trial randomized 4,447 patients to olmesartan 40 mg or placebo with a target systolic blood pressure <130/80 mmHg, and it demonstrated reduced risk for microalbuminuria onset among those assigned to olmesartan (69). More subjects assigned to olmesartan achieved systolic blood pressure goal than placebo (80% vs. 71%), raising the possibility that the observed effect was explained by blood pressure lowering alone. In analyses adjusting for blood pressure control, the risk reduction for new onset microalbuminuria observed with olmesartan persisted.
In contrast to these studies, no benefit of ACEi or ARB therapy could be detected in normotensive normoalbuminuric patients with DM1. This study was unique in that the primary outcome variable was glomerular mesangial volume. The study compared renal biopsy specimens and albuminuria at baseline and after approximately 5 years of administration of losartan, enalapril, or placebo. There was no difference in the mesangial fractional volume per glomerulus amongst the treatment groups. Interestingly, there was a higher incidence of microalbuminuria in the losartan group compared to placebo (70). Hypertension was more common and blood pressure was higher in the placebo group during the study, while glycemic control was not different. Taken together these studies indicate that ACE inhibitor or ARB administration for slowing onset of microalbuminuria is effective in hypertensive patients with type DM2 but not in normotensive patients with DM1. The Diabetics Exposed to Telmisartan and Enalapril (DETAIL) study (71) demonstrated that ARBs are non-inferior to ACE inhibitors in patients with DM2 and microalbuminuria based on the outcome of GFR decline (non inferiority margin −10 mL/min over 5 years). However, there was a significant amount of missing data when the results were analyzed.
Integrating all of these results has promoted ACEi and ARB to first line therapy options for hypertension in patients with diabetic nephropathy without any evidence suggesting superior efficacy of active drugs to one another. The mean systolic blood pressure in many groups in these trials was >140 mmHg (Table 1), indicating that, even in the research setting, further blood pressure reduction is possible. A separate discussion of specific blood pressure targets is included later in this review.
TABLE 1.
Characteristics and Achieved Blood Pressure of Trials Using Single Agent RAAS Blockade in Diabetes (ACE Inhibitor or ARB)
| Author | Population/P roteinuria |
Treatment | Achieved BP (mmHg) |
|---|---|---|---|
|
Lewis 1993
(63) |
DM1 500mg/24h |
Captopril Placebo |
96+/− 8 (MAP) 100 +/− 8 (MAP) p=.16 if prior HTN; p<.001 if no prior HTN |
|
Parving
2001 (64) |
DM1 300mg/24h |
Captopril Standard Therapy |
129/77 137/84 p<0.02 |
|
Lewis 2001
IDNT (65) |
DM2 900 mg/24h |
Irbesartan Amlodipine Placebo |
140/77 141/77 144/80 p<.001 for MAP in placebo vs other 2 groups |
|
Brenner
2001 RENAAL (66) |
DM2 500 mg/24h |
Losartan Placebo |
140/74, MAP 95.9
142/74, MAP 96.8 (p=.59) |
|
Parving
2001 IRMA II (67) |
DM2 20-200 μg/min |
Irbesartan 300 mg Irbesartan 150 mg Placebo |
141/83, MAP 102 143/83, MAP 103 144/83, MAP 103 SBP: p=.004 vs combined ARB groups |
|
Barnett 2004
DETAIL (71) |
DM2 11-999 μg/min |
Telmisartan Enalapril |
6.9 mmHg reduction in SBP 2.9 mmHg reduction in SBP CI (−8.5, 0.5) |
|
Ruggennenti
2004 BENEDICT (68) |
DM2 <20 μg/min Verapamil T+ V |
Placebo Trandolapril |
142/83 139/81 (p<.002 v. placebo) 141/82 (NS vs placebo) 139/80 (p<.002 v. placebo) |
DM1(or 2)=Diabetes Mellitus Type 1 or Diabetes Mellitus Type 2 HTN=Hypertension, MAP=Mean Arterial Pressure
ACE Inhibitors and Angiotensin Receptor Blockers as Dual Therapy
Since ACEi and ARBs individually are renoprotective, questions arose regarding the utility of combination therapy with both an ACE inhibitor and ARB. The principle behind this strategy was more complete inhibition of Ang II, which can be produced through non-ACE pathways. Most older trials studying combination ACE inhibitor + ARB in diabetic nephropathy have had small sample sizes and treatment durations, although the effects were promising. Combination therapy in patients with DM1 and DM2 with nephropathy yielded significant reductions in albuminuria and/or blood pressure (72-74) and was generally well tolerated. The Candesartan and Lisinoril Microalbuminuria (CALM II) study was a slightly larger and longer study comparing the effects of adding candesartan vs. additional lisinopril 20 mg onto a regimen already including lisinopril 20 mg daily (75). Combination therapy was not different than maximization therapy regarding ambulatory blood pressure, clinic blood pressure, or albuminuria after 12 months (Table 2). Of note, not all patients had albuminuria at baseline, and renal function preservation was not different in the 2 groups.
TABLE 2.
Characteristics and Achieved Blood Pressure in Trials Using Combination of ACE Inhibitor and ARB in Diabetes
| Author | Population/Proteinuria | Treatment | Achieved BP (mmHg) |
|---|---|---|---|
|
Andersen
2005 CALM II (75) |
DM1 or 2 | L 20 mg + L 20 mg L 20 mg + C 16 mg |
128/74 123/73 p=.16 for ASBP |
|
ON
TARGET (76) |
DM 1 or 2; or Vascular Disease |
Ramipril (R) Telmisartan (T) R+T |
−0.9/0.6 from baseline − 2.4/1.4 mmHg from baseline |
|
VA-
NEPHRON (78) |
DM2 300 mg/g |
Losartan+Lisinopril Losartan+Placebo |
ONGOING |
DM 1 or 2=Diabetes Mellitus Type 1 or Type 2; ASBP=Ambulatory Systolic Blood Pressure; L=Lisinopril; C=Candesartan
Concerns about this strategy arose with The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) (76). This study tested the hypothesis that among patients at high risk for a CV event, the combination of an ACEi and an ARB would be superior to either agent alone. Importantly 38% of study subjects had DM2, and 13% had microalbuminuria. Overall, there were no differences in the primary end point consisting of stroke, myocardial infarction and sudden cardiac death between groups. However, those randomized to combination therapy had a higher rates of renal impairment and hyperkalemia (serum potassium >5.5 mmol/L). Further analysis of the renal outcomes indicated a more rapid decline in estimated GFR and a higher rate of need for dialysis for acute renal failure episodes during the trial (77). However, there was no increase in the incidence of the need for chronic dialysis in those treated with the combination. When acute dialysis episodes were excluded from the analysis, combination therapy still increased the risk for the primary outcome (doubling serum Cr, dialysis, or death), but not the secondary outcome (doubling serum Cr or dialysis). Furthermore, specific sub group analysis demonstrated that those considered high risk for renal outcomes (diabetes, albuminuria, hypertension) did not receive benefit or harm from combination therapy. Conversely those considered at low risk for renal outcomes had higher incidences of the individual components and composite secondary outcome on combination therapy. Moreover the rate of increase in albuminuria was lower in those assigned to the combination as compared to monotherapy. The authors concluded that combination therapy should not be offered to low risk patients, while the long term effects in patients with diabetic nephropathy require larger studies.
Currently, there are no results from large-scale, multicenter randomized trials powered to study renal or CV endpoints in patients with diabetic nephropathy treated with combinations of an ACEi + ARB. The VA-NEPHRON study is a multi-center prospective, randomized parallel group trial testing the efficacy and safety of ACEi (lisinopril) +ARB (losartan) vs. ARB on the composite endpoint of reduction in GFR> 30 mL/min (if GFR > 60 mL/min), reduction in GFR by 50% (if GFR <60 mL/min), ESRD or death in patients with DM2 and nephropathy (78). The results are expected in 2013/2014.
Direct Renin Inhibitors in Diabetic Nephropathy
Renin levels are elevated with ACEi or ARB use and may produce Ang II through non-ACE pathways (79). Aliskiren is a direct renin inhibitor that lowers blood pressure and albuminuria in patients with diabetic nephropathy (80). Although this treatment would unlikely replace the current standard of care, its utility as add on therapy requires investigation. When compared to losartan + placebo, the combination of losartan + aliskiren reduced albuminuria in patients with DM2 and nephropathy (81). Consistent with other studies in similar populations, less than 50% of the subjects achieved the goal systolic blood pressure of <130 mmHg. A similar study compared the effects of aliskiren + irbesartan vs. each medication as monotherapy or placebo (82). Again there was greater reduction in albuminuria with combination therapy compared to monotherapy in the absence of significant blood pressure differences between combination and irbesartan therapy. These apparent blood pressure-independent reductions in albuminuria are of interest as it remains unclear how these effects will translate into long term renal or CV outcome benefits. The effects on blood pressure of these trials are summarized in Table 3. Currently the Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints (ALTITUDE) study is underway investigating the effects of Aliskiren 300 mg vs. placebo in DM2 patients with CV comorbidities (83). The primary outcome in this study is a composite of CV death, non-fatal MI, non-fatal stroke, ESRD, and doubling serum Cr. Recruitment is ongoing and expected to close in 2012 with results available in 2013/2014.
TABLE 3.
Characteristics and Achieved Blood Pressure in Trials Using Direct Renin Inhibitors as Add-On Therapy in Diabetes
| Author | Population/Proteinuria | Treatment | Achieved BP (mmHg) |
|---|---|---|---|
|
Persson
2009 (82) |
DM2 100mg/24h |
Irbesartan (I) Aliskiren (A) I+A Placebo |
12/5 reduction (p<.001/p=.002) 3/4 reduction (DBP p<.01) 10/6 mmHg reduction (p<.001/p<.001) P values vs placebo |
|
Parving
2008 (81) |
DM2 300 mg/g Cr |
Losartan + Aliskiren Losartan + Placebo |
P=0.07, 0.08 for SBP, DBP (specific values not included) |
|
ALTITUDE
(83) |
DM2 High CV Risk |
Aliskiren Placebo (Already on ACEi or ARB) |
ONGOING |
DM2=Diabetes Mellitus Type 2; SBP=Systolic Blood Pressure; DBP=Diastolic Blood Pressure; ACEi=Angiotensin Coverting Enzyme Inhibitor; ARB=Angiotensin Receptor Blocker
Mineralocorticoid Receptor Antagonists in Diabetic Nephropathy
With both ACEi and ARBs, plasma aldosterone levels are expected to decrease. In some patients on maintenance therapy with these drugs, aldosterone levels increase to pre-treatment levels via the phenomenon of “aldosterone escape.” Aldosterone escape occurs in up to 40% of patients on ACEi or ARBs and may contribute to local renal damage, albuminuria increases, and possibly systemic hypertenson (84,85). This occurs irrespective of which agent or dose is used (86). Interest in using mineralocorticoid receptor antagonists (MRA) has been justified by their ability to decrease proteinuria in diabetic nephropathy when used with other RAAS blockers. A small randomized crossover study in DM1 patients with diabetic nephropathy already taking an ACEi or ARB demonstrated a 30% reduction in albuminuria (p<0.001) with 25 mg daily of spironolactone vs. placebo (87). A significant difference in the 24-hour ambulatory blood pressure was not found, although the daytime ambulatory blood pressure was lower with spironolactone (p<0.02). Another small study including patients with DM1 and DM2 with nephrotic-range proteinuria showed a similar reduction in albuminuria (88). In this study 24 hour and daytime systolic and diastolic blood pressure were significantly lower with MRA, although nocturnal blood pressure was not different. A larger randomized parallel design study compared the effects of spironolactone vs. losartan vs. placebo as add on therapy to patients with diabetic nephropathy already taking Lisinopril 80 mg daily (89). There was a 34% decrease in albuminuria with spironolactone compared to placebo and a 17% decrease with losartan compared to placebo. Neither ambulatory nor clinic systolic blood pressure were significantly different between groups. In all these studies, effects on albuminuria were determined to be blood pressure independent. The effects on blood pressure from these trials are summarized in Table 4. An increased risk for hyperkalemia exists when combining an MRA with other RAAS blocking agents or with combination of ACE/ARB. This risk appears to be related to baseline potassium levels, baseline GFR, and change in GFR during study (90). Prudent monitoring of patients on multiple agents is recommended to reduce the risk of adverse events.
TABLE 4.
Characteristics and Achieved Blood Pressures in Diabetic Nephropathy Studies Using Mineralocorticoid Receptor Antagonists
| Population/Proteinuria | Treatment | Achieved BP (mmHg) |
|
|---|---|---|---|
|
Schjoedt
2005 (87) |
DM1; 300mg/d (already on ACEi or ARB) |
Spironolactone Placebo |
136/69 144/72 24 hr SBP p=.082, 24 hr DBP p=.067 |
|
Schjoedt
2006 (88) |
DM1 or 2; 2.5g/d (already on ACEi or ARB) |
Spironolactone Placebo |
137/77 143/81 24 hr SBP p=.004, 24 hr DBP p=.001 |
|
Mehdi 2009
(89) |
DM1 or 2; 300 mg/g Cr | Spironolactone Losartan Placebo (already on Lisinopril 80 mg) |
132 134 136 (NS between groups) |
DM 1 (or 2): Diabetes Mellitus Type 1 or 2; SBP=Systolic Blood Pressure; DBP=Diastolic Blood Pressure; ACEi=Angiotensin Converting Enzyme Inhibitor; ARB=Angiotensin Receptor Blocker
Additional Experimental Studies
Although optimization of RAAS inhibition is a widely studied strategy for the treatment of diabetic nephropathy, investigators continue to explore the role of inhibiting other systems. The recently published ASCEND study was designed to study the effects of an ET-1 antagonist Avosentan in DM2 patients with nephropathy already taking ACEi or ARB (91). Using a composite primary outcome of doubling serum Cr, ESRD, or death, the study was terminated prematurely because of increased CV events with avosentan. Pulmonary edema and CHF exacerbations were large contributors to these outcomes. Other strategies involve pharmacologic agents that target pathways that lead to diabetic nephropathy progression that may not involve concurrent changes in blood pressure. These pathways occur locally within the kidney and contribute to fibrosis, inflammation, and other forms of damage.
TARGET BLOOD PRESSURE IN DIABETIC NEPHROPATHY
Follow-up retrospective studies of some of the above trials have allowed for more detailed analyses addressing specific blood pressure effects on renal and CV endpoints. Current KDOQI guidelines recommend a blood pressure of 130/80 mmHg in patients with diabetic nephropathy. Despite these recommendations, achieving this goal remains difficult. Recent survey data of responding diabetic patients indicate that 51.4% had hypertension, 85.2% were taking antihypertensives, but only 35.8% had blood pressure controlled to < 130/80 mmHg (92). Studies in patients with diabetic nephropathy have also demonstrated difficulties, particularly with meeting systolic blood pressure goal, even with regular visits to specialized clinics (93).
The optimal blood pressure level for preserving kidney function and preventing CV catastrophes in patients with diabetic nephropathy has not been determined because there are no adequately powered long-term trials designed to make such determination. Still, some helpful information can be gleaned from post-hoc analyses of completed trials. In the IDNT study, there were no between-group differences in CV or all cause mortality. The target blood pressure was 135/85 mmHg, with 30% achieving the systolic goal and 81% achieving the diastolic goal. Retrospective analysis (94) showed that when divided into quartiles, lower baseline systolic blood pressure (lowest quartile <145 mmHg) and lower follow up systolic blood pressure (lowest quartile <134 mmHg) were associated with fewer renal endpoints. Conversely, in those with systolic blood pressure < 120 mmHg, overall mortality risk increased. Though the number of subjects with systolic blood pressure <120 mmHg was small, the increased risk for CV death compared to those with systolic blood pressure >120 mmHg persisted even after adjustment for baseline comorbidities. Among those with systolic blood pressure >120 mmHg, CV death risk increased with each 10 mmHg systolic blood pressure increase, but this was not so for stroke or nonfatal MI. On the other hand, diastolic blood pressure <85 mmHg was associated with an increased CV mortality risk with each 10 mmHg reduction. Myocardial infarction events accounted for much of this effect, while there was decreased risk for stroke. The overall conclusion was that the target blood pressure of 120/85 mmHg offered the most protection for both renal and CV endpoints.
These results differ from findings in the recently published blood pressure portion of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study (95). In this study, subjects with DM2 at high risk for CV events were randomized to either intensive blood pressure management (systolic blood pressure <120 mmHg) or standard blood pressure management (systolic blood pressure <140 mmHg). There was no difference in the number of CV events between groups, although the actual event rate in the standard treatment group was less than half of that planned in the study design. Subjects with serum Cr >1.5 mg/dL and proteinuria >1g/day were excluded from the study, which limits its generalizability for those with diabetic nephropathy and may explain the lower than expected outcome rate. Despite these negative results, the risk for non-fatal and total stroke was reduced in the intensive management group.
Retrospective analysis of RENAAL provides additional perspective for establishing a goal blood pressure (96). When the composite primary outcome, the risk of ESRD, and the risk of ESRD or death were analyzed using systolic blood pressure <130 mmHg as a reference, baseline systolic blood pressure >160 mmHg and follow up systolic blood pressure >140 mmHg had significantly higher hazard ratios for all outcomes. Baseline diastolic blood pressure did not affect these outcomes, but follow up diastolic blood pressure >90 mmHg (compared to reference <70 mmHg) carried a higher risk for all outcomes. Baseline and follow up pulse pressure (PP) >70 mmHg carried a higher risk for all outcomes, while follow up PP >60 mmHg carried a higher risk for the composite outcomes (doubling Cr/ESRD/death or ESRD/death), but not ESRD alone. In contrast to post-hoc analysis from IDNT, this analysis did not report individual CV endpoints . Therefore, it is difficult to glean the optimal blood pressure level for preservation of renal function and minimization of CV events among patients with DM2 and nephropathy from this analysis.
The Action in Diabetes and Vascular Disease: preterAx and diamicroN-mr –Controlled Evaluation (ADVANCE) study included 11,140 patients with DM2 and other CV risk factors (including 26% with microalbuminuria) randomized to a combination of an ACEi/thiazide diuretic or placebo. The incidence of new onset microalbuminuria, macroalbuminuria, doubling serum Cr, or ESRD when controlled for treatment group was reduced as systolic blood pressure decreased, even down to a systolic blood pressure of 106 mmHg (97). The primary outcome of the main study (composite of incidence of macrovascular and microvascular disease) occurred in significantly less subjects in the intervention arm (not significant as individual endpoints), however a similar analysis based on achieved blood pressure was not reported. The results of this study should be considered in the context of a broadly heterogeneous group of type 2 diabetics (most without nephropathy), but suggest that no lower limit for systolic blood pressure exists with regard to renal outcomes.
ALBUMINURIA REDUCTION IN DIABETIC NEPHROPATHY
Reducing the amount of urinary albumin excretion is another goal in the management of diabetic nephropathy. Regardless of the antihypertensive agent used, lowering blood pressure results in a decrease in albuminuria. As mentioned above, RAAS blockers are first line therapy because of their ability to reduce albuminuria independent of blood pressure reduction compared to other antihypertensives. As more is learned about the intrarenal mechanisms that contribute to disease progression via glomerular hemodynamic disturbances and generation of fibrotic/inflammatory mediators, effective therapies that lack antihypertensive effects are expected to emerge. Albuminuria remains a surrogate end point for progression of diabetic nephropathy linked to blood presssure control. Retrospective analyses associate albuminuria with CV outcomes in the general population and in diabetic nephropathy. At present, we do not have a study that randomizes subjects to specific albuminuria targets and has CV or all cause mortality as a primary endpoint. In the future the optimal blood pressure goals with therapies targeting local renal factors associated with albuminuria will need to be clarified. These are important considerations, but remain beyond the focus of this review.
GENERAL RECOMMENDATIONS
Improved blood pressure control in diabetic nephropathy often requires multiple antihypertensive agents. Starting with an ACEi or ARB incorporates an evidence-based strategy addressing both blood pressure lowering and albuminuria by targeting the effects of Ang II. Using either loop or thiazide diuretics (loop diuretics administered at least twice daily are preferred for those with GFR <50 mL/min) not only addresses volume sensitivity, but also minimizes the risk for hyperkalemia with RAAS blocking drugs and enhances their antiproteinuric effects. Adherence to a low sodium diet is also recommended based on this principle. We know of no outcomes trials focused on dietary sodium intake or diuretics or their combinations on the progression of CKD in diabetics. The emphasis on salt retention is based on the fact that salt retention contributes to hypertension that may in turn accelerate decline in kidney function.
Other non-RAAS inhibiting drugs do not offer any additional renal benefits beyond overall blood pressure control in diabetic nephropathy, and the decision of which additional agents to use should be based on the individual patient’s comorbidities. The Avoiding Cardiovascular Events Through Combination Therapy in Patients Living With Systolic Hypertension (ACCOMPLISH) Trial demonstrated that a combination of ACE inhibitor + CCB (amlodipine) resulted in slower progression of CKD in patients with high CV risk compared to ACE inhibitor + thiazide diuretic, but there was no difference when patients with pre-existing diabetic nephropathy were analyzed (98). Beta blockers should be used when coronary artery disease or compensated congestive heart failure are present. Calcium channel blockers may be utilized prior to beta blockers if no significant CV disease is present. Additional agents including clonidine, vasodilators (hydralazine and minoxodil), and alpha-blockers remain options in those with persistent hypertension. The concept that thiazide diuretics may complicate glycemic control should be considered, although recent evidence suggests that this association is related to the treatable hypokalemia associated with thiazide use (99). Using the above recommendations including maximum dose Lisinopril resulted in achieving a systolic blood pressure <130 mmHg in over 70% of subjects (composed predominantly of African American and Hispanic subjects) during the run in period of a recent clinical trial (100).
We recommend for those with BP under 130/85 and persistent macroalbuminuria careful attention to optimize glycemic control, dietary sodium intake < 2000 mg daily, and 0.8 g/kg/d protein diet while on single agent RAAS inhibition. Also, addition of an ARB or an MRA may be valuable for lowering proteinuria based on above mentioned studies and a meta-analysis (101) but has not been proven to prevent kidney disease progression and cannot be recommended at this time. Moreover, such combinations increase the risk for hyperkalemia. The results of the ALTITUDE (ACEi or ARB + DRI) and VA-NEPHRON (ACEi + ARB) will be met with great anticipation. It will be of paramount importance to identify whether the same findings that were found in retrospective analysis of IDNT (94) regarding the trade-off of improved renal outcomes with increased CV outcomes in the group with the lowest SBP will remain when dual RAAS inhibition (and likely further albuminuria reduction) is used in VA-NEPHRON and ALTITUDE. If these regimens do fall into favor, their utility will likely be focused on the antiproteinuric effects based on previously reviewed evidence using these combinations suggesting only modest reductions in BP (75,82,87,89).
CONCLUSION
Diabetic nephropathy is the most common cause of kidney disease in patients with diabetes. Hypertension is highly prevalent in patients with type 1 and type 2 diabetes and nephropathy. Mechanisms of hypertension in diabetic nephropathy include activation of local (renal) RAAS, increased or abnormal SNS activity, ECD, oxidative stress and abnormal NO metabolism. These mechanisms are responsible for the onset and worsening of hypertension in this population and contribute to the increased risk for adverse CV outcomes. The current management of hypertension in diabetic nephropathy should include therapies that block the angiotensin production or action and blood pressure treatment goals with these drugs should be aimed at a blood pressure <130/80 mmHg. Importantly achieving this goal will require both non-pharmacologic intervention combined with multiple antihypertensive drugs. Current studies are investigating the use of combination therapies that utilize more than one RAAS blocking drug, and formal recommendations await their results.
Footnotes
There are no financial interests or conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Horl M, Horl W. Hemodialysis associated hypertension: Pathophysiology and therapy. Am J Kidney Dis. 2002;39:227–244. doi: 10.1053/ajkd.2002.30542. [DOI] [PubMed] [Google Scholar]
- 2.Excerpts From the United States Renal Data System-2009 Annual Data Report: Atlas of Chronic Kidney Disease and End Stage Renal Disease in the United States. Incidence and Prevalence. Am J Kidney Dis. 2010;55(S1):S231–240. [Google Scholar]
- 3.Excerpts From the United States Renal Data System-2009 Annual Data Report: Atlas of Chronic Kidney Disease and End Stage Renal Disease in the United States. Chronic Kidney Disease in the NHANES Population. Am J Kidney Dis. 2010;55(S1):S35–48. [Google Scholar]
- 4.Schwartz Melvin M, et al. Renal Pathology Patterns in type II diabetes mellitus: relationship with retinopathy. Nephrol Dial Transplantation. 1998:2547–2552. doi: 10.1093/ndt/13.10.2547. [DOI] [PubMed] [Google Scholar]
- 5.National Kidney Foundation KDOQI™ Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49(suppl 2):S1–S180. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Parving HH, et al. Prevalence and causes of albuminuria in non insulin-dependent diabetic patients. Kidney International. 1992;4:758–762. doi: 10.1038/ki.1992.118. [DOI] [PubMed] [Google Scholar]
- 7.Epstein M, Sowers J. Diabetes Mellitus and Hypertension. Hypertension. 1992;19:403–418. doi: 10.1161/01.hyp.19.5.403. [DOI] [PubMed] [Google Scholar]
- 8.Norgaard K, Feldt-Rasmussen B, Johnsen K, Saelan H, Deckert T. Prevalence of hypertension in Type 1 (insulin dependent) diabetes mellitus. Diabetologia. 1990;33:407–410. doi: 10.1007/BF00404089. [DOI] [PubMed] [Google Scholar]
- 9.Parving H, Hommel E, Mathiesen E, et al. Prevalence of microalbuminuria, arterial hypertension, retinopathy, and neuropathy in patients with insulin dependent diabetes. BMJ. 1988;296:156–160. doi: 10.1136/bmj.296.6616.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lurbe E, Redon J, Kesani A. Increase in Nocturnal Blood Pressure and Progression to Microalbuminuria in Type 1 Diabetes. NEJM. 2002;347:797–805. doi: 10.1056/NEJMoa013410. [DOI] [PubMed] [Google Scholar]
- 11.Fagerudd JA, et al. Predisposition to essential hypertension and development of diabetic nephropathy in IDDM patients. Diabetes. 1998;47:439–444. doi: 10.2337/diabetes.47.3.439. [DOI] [PubMed] [Google Scholar]
- 12.Ismail N, Becker B, Strzelczyk P, Ritz E. Renal disease and hypertension in non-insulin dependent diabetes mellitus. Kidney Int. 1999;55:1–28. doi: 10.1046/j.1523-1755.1999.00232.x. [DOI] [PubMed] [Google Scholar]
- 13.Keller C, Bergis K, Filser D, Ritz E. Renal Findings in Patients with Short-Term Type 2 Diabetes. J Am Soc Nephrol. 1996;7:2627–2635. doi: 10.1681/ASN.V7122627. [DOI] [PubMed] [Google Scholar]
- 14.Bakris G, Williams M, Dworkin L, et al. Preserving Renal Function in Adults with Hypertension and Diabetes: A Consensus Approach. Am J Kidney Dis. 2000;36:646–661. doi: 10.1053/ajkd.2000.16225. [DOI] [PubMed] [Google Scholar]
- 15.Freedman B, Bostrom M, Daeihagh P, Boweden D. Genetic Factors in Diabetic Nephropathy. Clin J Am Soc Nephrol. 2007;2:1306–1316. doi: 10.2215/CJN.02560607. [DOI] [PubMed] [Google Scholar]
- 16.Iynegar S, Freedman B, Sedor J. Mining the genome for susceptibility to diabetic nephropathy: the role of large-scale studies and consortia. Semin Nephrol. 2007;27:208–222. doi: 10.1016/j.semnephrol.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Rajagopalan S, Kurz S, Munzel T, et al. Angiotensin II-mediated Hypertension in the Rat Increases Vascular Superoxide Production via Membrane NADH/NADPH Oxidase Activation. J Clinical Investigation. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price D, Porter L, Gordon M, et al. The Paradox of the Low Renin State in Diabetic Nephropathy. J Am Soc Nephrol. 1999;10:2382. doi: 10.1681/ASN.V10112382. [DOI] [PubMed] [Google Scholar]
- 19.Price D, De’Oliveira J, Fisher N, Williams G, Hollenberg N. The State and Responsiveness of the Renin-Angiotensin-Aldosterone System in Patients With Type II Diabetes Mellitus. Am J HTN. 1999;12:348. [PubMed] [Google Scholar]
- 20.Anderson F, Jung F, Ingelfinger JR. Renal rennin-angiotensin system in diabetes: functional, immunohistochemical, and molecular biological correlations. Am J Renal Phys. 1993;265:F477–F486. doi: 10.1152/ajprenal.1993.265.4.F477. [DOI] [PubMed] [Google Scholar]
- 21.Mizuiri S, Yoshikawa H, Tanegashima M, et al. Renal ACE Immunohistochemical Localization in NIDDM Patients with Nephropathy. Am J Kidney Dis. 1998;31:301–307. doi: 10.1053/ajkd.1998.v31.pm9469501. [DOI] [PubMed] [Google Scholar]
- 22.Konoshita T, Wakahara S, Mizuno, et al. Tissue gene expression of rennin-angiotensin system in human type 2 diabetic nephropathy. Diabetes Care. 2006;29:848–852. doi: 10.2337/diacare.29.04.06.dc05-1873. [DOI] [PubMed] [Google Scholar]
- 23.Danilcyzk U, Penninger J. Angiotensin-Converting Enzyme II in the Heart and Kidney. Circulation Research. 2006;98:463–471. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- 24.Tikellis C, Johnston, Forbes J, Burns W, et al. Characterization of Renal Angiotensin-Converting Enzyme 2 in Diabetic Nephropathy. HTN. 2003;41:392–397. doi: 10.1161/01.HYP.0000060689.38912.CB. [DOI] [PubMed] [Google Scholar]
- 25.De Chatel R, Weidmann P, Flammer J, et al. Sodium, rennin, aldosterone, catecholamines, and blood pressure in diabetes mellitus. KI. 1977;12:412–421. doi: 10.1038/ki.1977.132. [DOI] [PubMed] [Google Scholar]
- 26.Brands M, Labazi H. Role of Glomerular Filtration Rate in Controlling Blood Pressure Early in Diabetes. HTN. 2008;52:188–194. doi: 10.1161/HYPERTENSIONAHA.107.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuck M, Corry D, Trujillo A. Salt sensitive blood pressure and exaggerated vascular reactivity in the hypertension of diabetes mellitus. Am J Med. 1990;88:210–216. doi: 10.1016/0002-9343(90)90144-3. [DOI] [PubMed] [Google Scholar]
- 28.Imanishi M, Yoshioka K, Okumura M, et al. Sodium Sensitivity Related to Albuminuria Appearing Before Hypertension in Type 2 Diabetic Patients. Diabetes Care. 2001;24:111–116. doi: 10.2337/diacare.24.1.111. [DOI] [PubMed] [Google Scholar]
- 29.DeFronzo R. The Effect of Insulin on Renal Sodium Metabolism. Diabetologia. 1981;21:165–171. doi: 10.1007/BF00252649. [DOI] [PubMed] [Google Scholar]
- 30.Koomans H, Roos J, Mees E Dourhout, Delawi I. Soidum Balance in Renal Failure: A comparison of patients with normal subjects under extremes of sodium intake. HTN. 1985;7:714–721. doi: 10.1161/01.hyp.7.5.714. [DOI] [PubMed] [Google Scholar]
- 31.Gu JW, Anand V, Shek E, et al. Sodium Induces Hypertrophy of Cultured Myocardial Myoblasts and Vascular Smooth Muscle Cells. HTN. 1998;31:1083–1087. doi: 10.1161/01.hyp.31.5.1083. [DOI] [PubMed] [Google Scholar]
- 32.Spallone V, Gambardella S, Maiello M, et al. Relationship between autonomic neuropathy, 24-h blood pressure profile, and nephropathy in normotensive IDDM patients. Diabetes Care. 1994;17:578–584. doi: 10.2337/diacare.17.6.578. [DOI] [PubMed] [Google Scholar]
- 33.Liniger C, Favre L, Assal J. Twenty-four Hour Blood Pressure and Heart Rate Profiles of Diabetic Patients with Abnormal Cardiovascular Reflexes. Diabetic Medicine. 1991;8:420–427. doi: 10.1111/j.1464-5491.1991.tb01625.x. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen F, Hansen H, Jacobsen P. Increased sympathetic activity during sleep and nocturnal hypertension in Type 2 diabetic patients with diabetic nephropathy. Diabetic Medicine. 1999;16:555–562. doi: 10.1046/j.1464-5491.1999.00127.x. [DOI] [PubMed] [Google Scholar]
- 35.Facchini F, Stoohs R, Reaven G. Enhanced sympathetic nervous system activity: The linchpin between insulin resistance, hyperinsulinemia, and heart rate. Am J HTN. 1996;9:1013–1017. doi: 10.1016/0895-7061(96)87747-8. [DOI] [PubMed] [Google Scholar]
- 36.Achan V, Broadhead M, Malaki M, et al. Asymetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethyl dimethylaminohydrolase. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:1455–1459. doi: 10.1161/01.ATV.0000081742.92006.59. [DOI] [PubMed] [Google Scholar]
- 37.Abbasi F, Asagmi T, Cooke J, et al. Plasma Concentrations of Asymetric Dimethylarginine Are Increased in Patients with Type 2 Diabetes Mellitus. American Journal of Cardiology. 2001;88:1201–1203. doi: 10.1016/s0002-9149(01)02063-x. [DOI] [PubMed] [Google Scholar]
- 38.Kielstein J, Boger R, Bode-Boger S. Asymetric Dimethylarginine Concentrations Differ in Patients with End Stage Renal Disease: Relationship to Treatment Method and Atherosclerotic Disease. J Am Soc Nephrol. 1999;10:594–600. doi: 10.1681/ASN.V103594. [DOI] [PubMed] [Google Scholar]
- 39.Zoccali C, Bode-Boger S, Mallamaci F, et al. Plasma concentrations of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–2117. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- 40.Tarnow L, Hovind P, Teerlink T, Stehouwer C, Parving HH. Elevated Plasma Asymetric Dimethylarginine as a Marker of Cardiovascular Morbidity in Early Diabetic Nephropathy in Type 1 Diabetes. Diabetes Care. 2004;27:765–769. doi: 10.2337/diacare.27.3.765. [DOI] [PubMed] [Google Scholar]
- 41.Matsuguma K, Ueda S, Yamagashi S, et al. Molecular Mechanism for Elevation of Asymetric Dimethylarginine and Its Role for Hypertension in Chronic Kidney Disease. J Am Soc Neph. 2006;17:2176–2183. doi: 10.1681/ASN.2005121379. [DOI] [PubMed] [Google Scholar]
- 42.Muniyappa R, Quon M. Insulin Action and insulin resistance in vascular endothelium. Curr Op Nutr Metab Care. 2007;10:523. doi: 10.1097/MCO.0b013e32819f8ecd. [DOI] [PubMed] [Google Scholar]
- 43.Donatelli M, Colletti I, Bucalo M, Russo V, Verga S. Plasma endothelin levels in NIDDM patients with macroangiopathy. Diabetes Research. 1994;25:159–164. [PubMed] [Google Scholar]
- 44.Koyama H, Tabata T, Nishzawa T, et al. Plasma endothelin levels in patients with uremia. Lancet. 1989;1:991–992. doi: 10.1016/s0140-6736(89)92631-7. [DOI] [PubMed] [Google Scholar]
- 45.Dhaun N, Ferro C, Davenport A, et al. Hemodynamic and renal effects of endothelin receptor antagonism in patients with chronic kidney disease. NDT. 2007;22:3228. doi: 10.1093/ndt/gfm364. [DOI] [PubMed] [Google Scholar]
- 46.Sharma Prabhakar. Role of Nitric Oxide in Diabetic Nephropathy. Seminars in Nephrology. 2004;24:333–334. doi: 10.1016/j.semnephrol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Fitzgerald SM, Brands MW. Nitric Oxide may be required to prevent hypertension at the onset of diabetes. Am J Physiol. 2000;279:E762–768. doi: 10.1152/ajpendo.2000.279.4.E762. [DOI] [PubMed] [Google Scholar]
- 48.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 49.Spittle M, Hoenrich N, Handelman G, et al. Oxidative stress and Inflammation in Hemodialysis Patients. AMJKD. 2001;38:1408–1413. doi: 10.1053/ajkd.2001.29280. [DOI] [PubMed] [Google Scholar]
- 50.Ceballos-Picot I, Witko-Sarsat V, Merad-Boudia M, et al. Glutathione Antioxidant System as a Marker of Oxidative Stress in Chronic Renal Failure. Free Radical Biology and Medicine. 1996;21:845–853. doi: 10.1016/0891-5849(96)00233-x. [DOI] [PubMed] [Google Scholar]
- 51.Vaziri N, Oveisi F, Ding Y. Role of increased oxygen free radical activity in the pathogenesis of uremic hypertension. KI. 1998;53:1748. doi: 10.1046/j.1523-1755.1998.00947.x. [DOI] [PubMed] [Google Scholar]
- 52.Prabhakar S, Starnes J, Shi S, Lonis B, Tran R. Diabetic Nephropathy is Associated with Oxidative Stress and Decreased Renal Nitric Oxide Production. J Am Soc Neph. 2007;18:2945–2952. doi: 10.1681/ASN.2006080895. [DOI] [PubMed] [Google Scholar]
- 53.Griendling K, Ollerenshaw J, Minieri C, Alexander R. Angiotensin II stimulates NADH and NADPH activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 54.Rajagopalan S, Kurz S, Munzel T, et al. Angiotensin II-mediated Hypertension in the Rat Increases Vascular Superoxide Production via Membrane NADH/NADPH Oxidase Activation. J Clinical Investigation. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogawa S, Mori T, Nako, et al. Angiotensin II Type 1 Receptor Blockers Reduce Urinary Oxidative Stress Markers in Hypertensive Diabetic Nephropathy. HTN. 2006;47:699–705. doi: 10.1161/01.HYP.0000203826.15076.4b. [DOI] [PubMed] [Google Scholar]
- 56.Li L, Fink G, Watts S, et al. Endothelin 1 Increases Vascular Superoxide via Endothelin A-NADPH Oxidase Pathway in Low Renin Hypertension. Circulation. 2003;107:1053–1058. doi: 10.1161/01.cir.0000051459.74466.46. [DOI] [PubMed] [Google Scholar]
- 57.Palmer B. Impaired autoregulation: implications for the genesis of hypertension and hypertension induced renal injury. American Journal of Medical Sciences. 2001;321:388–400. doi: 10.1097/00000441-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Gnudi L, Viberti G, Raij L, et al. GLUT-1 Overexpression: Link Between Hemodynamic and Metabolic Factors in Glomerular Injury. HTN. 2003;42:19–24. doi: 10.1161/01.HYP.0000075949.19968.EF. [DOI] [PubMed] [Google Scholar]
- 59.American Diabetes Association Diabetic Nephropathy. Diabetes Care. 2002;25:S82–S89. [Google Scholar]
- 60.Mogensen CE. Long-term antihypertensive treatment inhibiting progression of diabetic nephropathy. BMJ. 1982;285:685–688. doi: 10.1136/bmj.285.6343.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parving HH, Andersen AR, Smidt UM, et al. Effect of antihypertensive treatment on kidney function in diabetic nephropathy. BMJ. 1987;294:1443–1447. doi: 10.1136/bmj.294.6585.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parving HH, Smidt UM, Andersen AR, Svendsen P. Early Aggressive Antihypertensive Treatment Reduces Rate of Decline in Kidney Function in Diabetic Nephropathy. Lancet. 1983;321:1175–1179. doi: 10.1016/s0140-6736(83)92462-5. [DOI] [PubMed] [Google Scholar]
- 63.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 1993;329:1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 64.Parving HH, Hommel E, Jensen BR, Hansen HP. Long-term beneficial effect of ACE inhibition on diabetic nephropathy in normotensive type 1 diabetic patients. Kidney Int. 2001;60:228–34. doi: 10.1046/j.1523-1755.2001.00790.x. [DOI] [PubMed] [Google Scholar]
- 65.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001;345:851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 66.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001;345:861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 67.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N. Engl. J. Med. 2001;345:870–8. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 68.Ruggenenti P, Fassi A, Ilieva A, et al. Preventing Microalbuminuria in Type 2 Diabetes. N Engl J Med. 2004;351:1941–1951. doi: 10.1056/NEJMoa042167. [DOI] [PubMed] [Google Scholar]
- 69.Haller H, Ito S, Janusszewicz A, et al. Prevention of Microalbuminuria in Type 2 Diabetes (Roadmap Trial) J HTN. 2010;28:e323. [Google Scholar]
- 70.Mauer M, Zinman B, Gardiner R, et al. Renal and Retinal Effects of Enalapril and Losartan in Type 1 Diabetes. N Engl J Med. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barnett A, Bain S, Bouter P, et al. Angiotensin Receptor Blockade versus Converting-Enzyme Inhibition in Type 2 Diabetes and Nephropathy. NEJM. 2004;351:1952–1962. doi: 10.1056/NEJMoa042274. [DOI] [PubMed] [Google Scholar]
- 72.Rossing K, Jacobsen P, Pietraszek L, Parving H. Renoprotective effects of adding angiotensin II receptor blocker to a maximal recommended doses of ACE inhibitor in diabetic nephropathy: A randomized double blind crossover trial. Diabetes Care. 2003;26:2268–2274. doi: 10.2337/diacare.26.8.2268. [DOI] [PubMed] [Google Scholar]
- 73.Jacobsen P, Andersen S, Jensen B, Parving HH. Additive Effect of ACE Inhibition and Angiotensin II Receptor Blockade in Type 1 Diabetic Patients with Diabetic Nephropathy. J Am Soc Neph. 2003;14:992–999. doi: 10.1097/01.asn.0000054495.96193.bf. [DOI] [PubMed] [Google Scholar]
- 74.Jacobsen P, Andersen S, Rossing K, Jensen B, Parving HH. Dual blockade of the rennin-angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney International. 2003;63:1874–1880. doi: 10.1046/j.1523-1755.2003.00940.x. [DOI] [PubMed] [Google Scholar]
- 75.Andersen NH, Poulsen PL, Knudsen ST, et al. Long-term dual blockade with candesartan and lisinopril in hypertensive patients with diabetes: the CALM II study. Diabetes Care. 2005;28:273–277. doi: 10.2337/diacare.28.2.273. [DOI] [PubMed] [Google Scholar]
- 76.ON TARGET investigators Telmisartan, Ramipril, or Both in Patients at High Risk for Vascular Events. NEJM. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 77.Mann J, Schmieder R, McQueen, et al. Renal outcomes with telmisartan, ramipril, or both in people at high vascular risk (the ONTARGET study): a multicentre, randomized, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 78. ClinicalTrials.gov: NCT00555217.
- 79.Urata H, Strobel F, Ganten D. Widespread distribution of human chymase. J HTN. 1994;12:S17–22. [PubMed] [Google Scholar]
- 80.Persson F, Rossing P, Schjoedt K, et al. Time course of the antiproteinuric and antihypertensive effects of the direct rennin inhibition in type 2 diabetes. Kidney Internation. 2008;73:1419–1425. doi: 10.1038/ki.2008.68. [DOI] [PubMed] [Google Scholar]
- 81.Parving HH, Persson F, Lewis J, et al. Aliskiren Combined with Losartan in Type 2 Diabetes and Nephropathy. NEJM. 2008;358:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 82.Persson F, Rossing P, Reinhard H. Renal Effects of Aliskiren Compared With and in Combination with Irbesartan in patients with Type 2 Diabetes, Hypertension and Albuminuria. Diabetes Care. 2009;32:1873–1879. doi: 10.2337/dc09-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. ClinicalTrials.gov: NCT00549757.
- 84.Sato A, Hayashi K, Naruse M, Saruta T. Effectiveness of Aldosterone Blockade in Patients with Diabetic Nephropathy. HTN. 2003;41:64–68. doi: 10.1161/01.hyp.0000044937.95080.e9. [DOI] [PubMed] [Google Scholar]
- 85.Schjoedt KJ, Andersen S, Rossing P, et al. Aldosterone escape during blockade of the rennin angiotensin-aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia. 2004;47:1936–1939. doi: 10.1007/s00125-004-1542-0. [DOI] [PubMed] [Google Scholar]
- 86.Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nature Clinical Practice Nephrology. 2007;3:486–492. doi: 10.1038/ncpneph0575. [DOI] [PubMed] [Google Scholar]
- 87.Schjoedt K, Rossing K, Juhl T, et al. Beneficial impact of spironolactone in diabetic nephropathy. KI. 2005;68:2829–2836. doi: 10.1111/j.1523-1755.2005.00756.x. [DOI] [PubMed] [Google Scholar]
- 88.Schjoedt K, Rossing K, Juhl T, et al. Beneficial impact of spironolactone on nephritic range albuminuria in diabetic nephropathy. Kidney International. 2006;70:S36–S42. doi: 10.1038/sj.ki.5001580. [DOI] [PubMed] [Google Scholar]
- 89.Mehdi U, Adams-Huet B, et al. Addition of Angiotensin Receptor Blocker or Mineralocorticoid Antagonism to Maximal Angiotensin-Converting Enzyme Inhibition in Diabetic Nephropathy. JASN. 2009;20:2641–2650. doi: 10.1681/ASN.2009070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khosla N, Kalaitzidis R, Bakris G. Predictors of Hyperkalemia Risk following Hypertension Control with Aldosterone Blockade. Am J Nephrol. 2009;30:418–424. doi: 10.1159/000237742. [DOI] [PubMed] [Google Scholar]
- 91.Mann J, Green D, Jamerson K, et al. Avosentan for Overt Diabetic Nephropathy. J Am Soc Nephrol. 2010;21:527–535. doi: 10.1681/ASN.2009060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–42. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 93.Joss F, Ferguson C, Brown C, Deighan CJ, Paterson KR, Boulton-Jones JM. Intensified treatment of patients with type 2 diabetes mellitus and overt nephropathy. QJM. 2004;97:219–27. doi: 10.1093/qjmed/hch039. [DOI] [PubMed] [Google Scholar]
- 94.Berl T, Hunsicker L, Lewis J, et al. Impact of Achieved Blood Pressure on Cardiovascular Outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol. 2005;16:2170–2179. doi: 10.1681/ASN.2004090763. [DOI] [PubMed] [Google Scholar]
- 95.The ACCORD Study Group Effects of Intensive Blood Pressure Control in type 2 Diabetes Mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bakris G, Weir M, Shanifar S, et al. Effects of Blood Pressure Level on Progression of Diabetic Nephropathy. Arch Int Med. 2003;163:1555–1565. doi: 10.1001/archinte.163.13.1555. [DOI] [PubMed] [Google Scholar]
- 97.De Galan B, Perkovic V, Ninomiya T. Lowering Blood Pressure Reduces Renal Events in Type 2 Diabetes. J Am Soc Neph. 2009;20:883–892. doi: 10.1681/ASN.2008070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bakris G, Sarafidis P, Weir M, et al. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomized controlled trial. Lancet. 2010;375:1173–1181. doi: 10.1016/S0140-6736(09)62100-0. [DOI] [PubMed] [Google Scholar]
- 99.Zillich A, Garg J, Basu S, Bakris G, Carter B. Thiazide Diuretics, Potassium, and the Development of Diabetes: a Quantitative Review. HTN. 2006;48:219–224. doi: 10.1161/01.HYP.0000231552.10054.aa. [DOI] [PubMed] [Google Scholar]
- 100.Van Buren PN, Huet-Adams B, Toto R. Effective Antihypertensive Therapies in High Risk Patients with Diabetic Nephropathy. J Investigative Med. 2010 doi: 10.231/JIM.0b013e3181ff46a5. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kunz R, Friedrich C, Wolbers M, Mann J. Meta-analysis: Effect of Monotherapy and Combination Therapy with Inhibitors of the Renin-Angiotensin System on Proteinuria in Renal Disease. Ann of Int Med. 2008;148:30–48. doi: 10.7326/0003-4819-148-1-200801010-00190. [DOI] [PubMed] [Google Scholar]