Abstract
Background
Visfatin is an adipokine produced by visceral adipose tissue and has insulin-mimicking effects. Fetuin-A is a hepatic secretory protein that binds the insulin receptor and inhibits insulin action both in vivo and in vitro. The authors of the present study aimed to investigate the levels of serum visfatin and fetuin-A and their correlation with hemoglobin A1c (HbA1c) and urine albumin levels in patients with type 2 diabetes mellitus (T2DM).
Methods
A total of 40 obese patients with T2DM (11 males and 29 females; age, 54.47±10.83 years and 23 obese nondiabetic controls (8 males and 15 females; age, 53.04±11.33 years) were included in the study. Age, sex, and body mass index were similar in the 2 groups. Serum visfatin and fetuin-A levels were measured by enzyme-linked immunosorbent assay. HbA1c and urine albumin levels were measured by high performance liquid chromatography and nephelometric method, respectively.
Results
Serum levels of visfatin in patients with T2DM (4.03±2.44 ng/mL) were similar to the control group (3.65±3.02 ng/mL). Serum fetuin-A levels were significantly lower in patients with T2DM than the controls (298.75±78.86 and 430.73±94.46 µg/mL, respectively). HbA1c levels were significantly higher in the T2DM group compared with controls (7.33±1.32 and 5.44±0.84%, respectively). Correlations between visfatin, fetuin-A and HbA1c levels were not observed.
Conclusion
The present study suggests fetuin-A may play a role in the pathogenesis of T2DM.
Keywords: Albuminuria; Diabetes mellitus, type 2; Fetuin-A; Hemoglobin A1c; Visfatin
INTRODUCTION
Adipose tissue has been shown to secrete a variety of bioactive compounds into the circulation that can have profound effects on metabolism [1]. Visfatin is one of the bioactive compounds that influence insulin receptors by binding to a different place than insulin [2]. There are several studies having conflicting results regarding the correlation of visfatin with insulin. Some studies suggested visfatin may be correlated with insulin resistance [3-5] and another study reported visfatin may be correlated with insulin sensitivity [6]. Chen et al. [7] reported visfatin is associated with abdominal obesity and type 2 diabetes mellitus (T2DM) in the Taiwanese population. Hammarstedt et al. [8] showed higher circulating visfatin levels as well as adipose tissue visfatin mRNA expression in T2DM compared to body mass index (BMI)-unmatched controls.
Fetuin-A is a 60 kDa protein synthesized in hepatocytes. Fetuin-A binds to insulin receptors in adipose and muscular tissue and inhibits insulin receptor tyrosine kinase activity as well as insulin receptor autophosphorylation in vivo and in vitro [9]. Fetuin-A has been suggested to potentially cause insulin resistance and/or metabolic syndrome [9]. In terms of treatment or prevention of insulin resistance, fetuin-A may be considered as a new therapeutic target [10].
Even if visfatin and fetuin-A levels have been studied in T2DM patients [7-13], none of the studies included the relationship of these bioactive compounds to HbA1c and albuminuria. Therefore, the goal of the present study was to investigate the serum visfatin and fetuin-A levels and determine whether there were any serum visfatin and fetuin-A correlations with hemoglobin A1c (HbA1c) and urine albumin levels in obese T2DM patients, as well as contributing to the clarification of the role of visfatin and fetuin-A in glucose metabolism.
METHODS
The present study was performed in Istanbul Okmeydani Educational and Research Hospital Clinical Biochemistry Laboratory with 40 T2DM and 23 healthy control subjects. The patients were followed up in diabetes outpatient clinics. BMI of all individuals were calculated. In both groups, subjects with a BMI ≥25 kg/m2 were included. Attention was given to the similarity between control and T2DM patient groups in terms of BMI, age and gender. The study was approved by the ethics commitee of our hospital. All individuals were informed regarding the tests and their clinical meanings before the study and written consent was obtained from all study participants.
For all laboratory tests in both control and T2DM groups, from each patient 10 mL of blood was collected into a vacuum tube with gel and 3 mL collected into a tube with K3EDTA. In order to determine urine albumin levels, a 24 hour urine specimen was collected into clean, dry plastic bottles. Patients were asked to avoid exercise at least 24 hours before specimen collection.
Thr blood collected in the tube with gel was centrifuged at 2,000×g for 10 minutes after clot formation and the serum separated. Routine biochemical parameters, HbA1c and urine albumin level were measured the same day. Undiluted serum specimen was divided into 2 aliquots and stored at -80℃ for 3 months for visfatin and fetuin-A measurements. Repetitive freezing and thawing were not performed.
Glucose and urea were analyzed in an Olympus AU2700 chemistry analyzer (Beckman Coulter Inc., Fullerton, CA, USA); total cholesterol (TC), creatinine, triglycerides (TG), high density lipoprotein cholesterol (HDL-C) and low density lipoprotein cholesterol (LDL-C) were analyzed in a Modular V2 chemistry analyzer (Roche Diagnostics, Mannheim, Germany) by photometric method; HbA1c was analyzed in an ADAMS A1c HA-8160 analyzer (Arkray Inc., Kyoto, Japan) by high performance liquid chromatography (HPLC); urine albumin was analyzed in a Delta Nephelometry analyzer (Radim Diagnostics, Rome, Italy) by nephelometric method; visfatin (Alpco Diagnostics, Salem, NH, USA) and fetuin-A (AssayMax Human alpha-2-HS-Glycoprotein-AHSG-ELISA Kit; Assaypro, St. Charles, MO, USA) were analyzed by the enzyme-linked immunosorbent assay (ELISA) method.
Performance characterization
Intra-assay and inter-assay coefficients of variation for fetuin-A and visfatin were 4.9% and 7.1%; 5.5% and 6.24%, respectively.
Data processing methods
Statistical analyses were performed by the statistical program SPSS for Windows, version 11.5 (SPSS Inc., Chicago, IL, USA). Jarque-Bera normality test was applied to data; P<0.05 indicated that normal distribution was not achieved. Therefore, to test differences between groups, non-parametric (non-requiring normal distribution) Mann-Whitney U test for 2 independent groups were applied. Kendall's tau_b correlation coefficient was used to evaluate correlations.
RESULTS
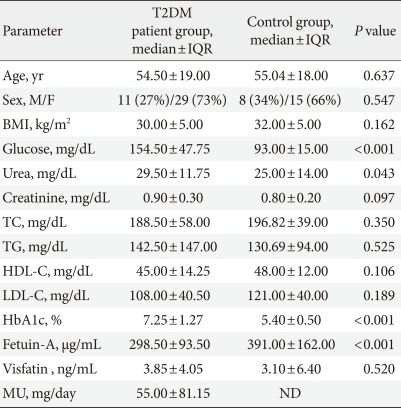
The present study was performed on 63 individuals consisting of 40 T2DM patients and 23 healthy controls. Among the individuals, 44 (70%) were females and 19 (30%) were males. There were no significant differences between T2DM patient and control groups in terms of age, gender, and BMI (P>0.05) (Table 1).
Table 1.
Comparisons of demographic and laboratory data between T2DM patient and control groups
T2DM, type 2 diabetes mellitus; IQR, interquartile range; BMI, body mass index; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; HbA1c, hemoglobin A1c; MU, microalbuminuria; ND, not determined.
A statistically significant difference between groups in terms of fetuin-A was detected (P<0.05). When rank values were controlled in order to determine the source of difference, lower levels were detected in the T2DM patient group in comparison to the control group (P<0.01). HbA1c, glucose and urea were found in higher levels in the T2DM patient group when compared to control group (P<0.001, P<0.001, and P<0.05, respectively). There were no differences between T2DM patient and control groups in terms of creatinine, TC, TG, HDL-C, LDL-C, and visfatin (P>0.05) (Table 1).
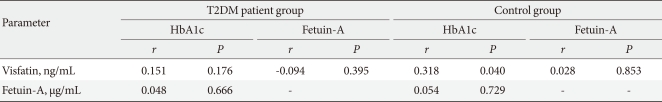
In the second stage of the study, the correlation of fetuin-A, visfatin and HbA1c variables was analyzed. Since the data did not exhibit normal distribution, Kendall's tau_b correlation coefficient was used. For the T2DM patient group, a significant correlation was not observed between the parameters (P>0.05) (Table 2).
Table 2.
Correlations between HbA1c, fetuin-A, and visfatin levels in the control and T2DM patient groups
HbA1c, hemoglobin A1c; T2DM, type 2 diabetes mellitus.
In the control group, a weak, but significant correlation was observed only between the visfatin and HbA1c variables (r=0.318, P<0.05) (Table 2).
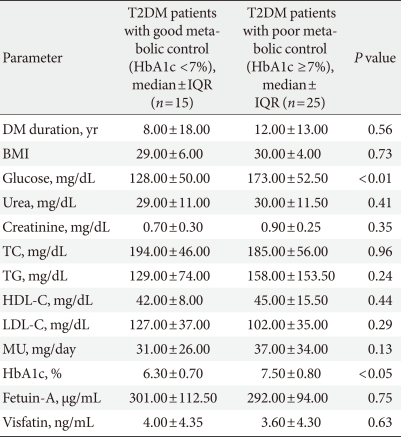
In the T2DM patient group, when 2 subgroups (1 group having HbA1c ≥7% and poor metabolic control and the other group HbA1c <7% and good metabolic control) were compared in terms of demographic and biochemical parameters, only glucose and HbA1c values manifested statistically significant differences (P<0.01 and P<0.05, respectively) (Table 3).
Table 3.
Comparisons of T2DM patients' demographic and laboratory data between HbA1c ≥7% and HbA1c <7% subgroups
T2DM, type 2 diabetes mellitus; HbA1c, hemoglobin A1c; IQR, interquartile range; DM, diabetes mellitus; BMI, body mass index; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; MU, microalbuminuria.
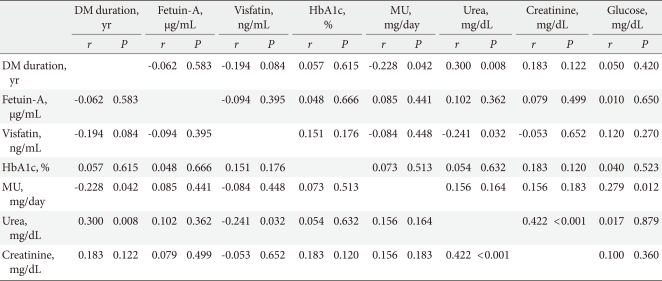
When the correlations between variables in the T2DM patient group were analyzed, microalbuminuria had a weak but significant negative correlation with DM duration, and a positive correlation with glucose; urea had a positive correlation with DM duration and creatinin, and a negative correlation with visfatin (Table 4).
Table 4.
Correlations between HbA1c, fetuin-A, and visfatin levels in the control and T2DM patient groups
T2DM, type 2 diabetes mellitus; DM, diabetes mellitus; HbA1c, hemoglobin A1c; MU, microalbuminuria.
The T2DM patient group was further divided into 2 subgroups having microalbuminuria (mg/day) ≥30 (n=24) and <30 (n=16). When the 2 groups were compared in terms of duration of DM, BMI, glucose, urea, creatinine, TC, TG, HDL-C, LDL-C, HbA1c, visfatin, and fetuin-A parameters, there were no statistically significant differences between the groups.
DISCUSSION
In the present study, demographic and anthropometric parameters such as age, gender, BMI, and serum levels of creatinine, TC, TG, HDL-C, LDL-C did not manifest statistically significant differences between the T2DM patient and control groups. Since both study groups had the same confounding factors for insulin sensitivity, an opportunity to exclude variations originating from these parameters existed.
HbA1c, glucose and urea levels were detected higher in the T2DM patient than control group. Elevation of HbA1c and glucose levels were compatible with DM. The mean microalbuminuria levels of the T2DM patient group were >30 mg/day indicating the presence of microalbuminuria. Elevated serum urea and urine albumin levels indicated the existence of nephropathy in the T2DM group.
In the present study, there were no differences between the T2DM patient and control groups in terms of visfatin levels. Fukuhara et al. [6] reported that visfatin is related to insulin sensitivity [6]. Chen et al. [7] evaluated plasma visfatin levels in 61 T2DM patients and 59 healthy controls with similarity in terms of gender and age. Visfatin levels were higher in T2DM patients and the authors stated visfatin can play a role in pathogenesis of T2DM. Yilmaz et al. [11] evaluated visfatin and fetuin-A levels in 85 T2DM patients having early stage diabetic nephropathy and 38 healthy controls; higher visfatin levels were observed in the T2DM patient group compared to the healthy control group. The visfatin result in the present study is in contradiction with Yilmaz et al. [11]. In the present study, smoking and alcohol drinking habits of the patients were not evaluated. Therefore, the T2DM patient group in the present study showed differences from Yilmaz et al. [11] whose patient group was composed of patients who had DM and had a new diagnosis of nephropathy, excluding patients with smoking and alcohol drinking habits. The reason for different visfatin levels in the T2DM patient group of the present study and Yilmaz's group [11] may be due to differences in demographic characteristics and exclusion criteria.
Dogru et al. [12] evaluated plasma visfatin levels in 22 T2DM patients who were newly diagnosed and did not receive treatment, 22 individuals who had impaired glucose tolerance, and 40 healthy individuals (control group) with similarity in terms of age, BMI and gender and detected higher visfatin levels in the diabetic group. The authors claimed hyperglycemia increases plasma visfatin levels and this increase makes the impairment in glucose tolerance more prominent.
Sandeep et al. [13] evaluated plasma visfatin levels in 150 T2DM patients and 150 individuals having normal glucose tolerance, and observed higher visfatin levels in the diabetic group compared to the normal glucose tolerance group.
Hammarstedt et al. [8] showed higher circulating visfatin levels as well as adipose tissue visfatin mRNA expression in T2DM compared to BMI-unmatched controls. The difference between the BMIs in the 2 groups was a limitation of Hammarstedt et al.'s study.
There are also several studies indicating no association between serum visfatin levels with insulin sensitivity or resistance [4,8,14]. The visfatin results in the present study are compatible with these studies [4,8,14] and are not compatible with other studies [6,7,11-13]. Individuals in the present study's T2DM patient group were treated for both diabetes and microalbuminuria and the treatments had several differences. The reason for finding no differences in terms of visfatin levels between groups may be due to interaction of visfatin with other drugs.
In the present study, lower fetuin-A levels were observed in the T2DM patient group compared to the control group.
Ix et al. [10] followed for 6 years 406 healthy and non-diabetic individuals between 70 and 79 years of age regarding diabetes status; T2DM developed in 135 individuals during the study period. The authors measured fetuin-A levels and observed higher fetuin-A levels in individuals who developed diabetes and stated fetuin-A levels were correlated with developing diabetes in elder people, being independent of other indicators of insulin resistance. The authors claimed that if further studies are performed in middle aged groups having the highest rate of diabetes incidence and if their results were confirmed, fetuin-A can offer an useful therapeutic target and can provide new insights for glucose metabolism in humans [10].
Takata et al. [15] investigated the effect of high glucose level on the fetuin-A gene in human hepatoma cell line HepG2 and observed fetuin-A expression in the cells. The authors claimed that increase in expression of AHGS (fetuin-A) in the liver may be a reason for glucose toxicity in diabetic conditions.
Mori et al. [16] evaluated serum fetuin-A levels in 162 type 2 diabetic and 160 non-diabetic individuals and did not find any difference between groups in terms of fetuin-A and any significant correlation between insulin resistance and fetuin-A. The authors hypothesized the reason may be the presence of stronger determinants similar to protein modifications such as non-enzymatic glycation masking the effect of fetuin-A on insulin resistance and having excessive glucose toxicity under diabetic conditions or influence of pharmacologic treatment of diabetic individuals on fetuin-A levels.
In another study, Mori et al. [9] investigated the effects of insulin sensitizing treatment such as pioglitazone, metformin and aerobic exercise. The authors divided 27 T2DM individuals into 3 groups (10 pioglitazone, 9 using metformin for 6 months, and 7 having a 3-month exercise program). After the treatment period, fetuin-A levels decreased significantly in the group using pioglitazone. Consequently, the authors stated that pioglitazone may partially regulate insulin resistance by decreasing fetuin-A levels.
Yilmaz et al. [11] divided patients into 2 groups in terms of proteinuria, <500 mg/day and ≥500 mg/day, and measured visfatin, fetuin-A, adiponectin and hsCRP levels. The authors observed significantly lower fetuin-A levels in the diabetic group and a negative correlation with proteinuria. Yilmaz et al. [11] expressed that a urinary loss of fetuin-A, which is a vasoprotective protein, may contribute to increased cardiovascular disease risk in these patients.
In the present study, fetuin-A levels in the T2DM group were lower compared to the control group which was compatible with Yilmaz et al. [11] and not compatible with Ix et al. [10], Takata et al. [15], and Mori et al. [16].
The authors of the present study measured urine albumin levels in the T2DM group. Microalbuminuria levels of the T2DM patient group were 55.07±81.15 mg/day (mean±standard deviation) and the mean was over the reference range. This result is an indicator of diabetic nephropathy developing in the T2DM patient group. Although serum fetuin-A levels were lower overall in the T2DM group compared to control group, stating that fetuin-A levels were not different according to the presence of proteinuria was difficult because of the small number of subjects and this was a limitation of the present study.
HbA1c, which is positively correlated with glucose levels and increased in T2DM, has a role in development of microvascular complications. Correlation between HbA1c and visfatin or fetuin-A in both study groups was not observed. A possible reason is that the result may be due to the effect of drug treatments masking a correlation or visfatin and fetuin-A may have roles in different mechanisms in T2DM pathogenesis.
After dividing groups in terms of HbA1c and microalbuminuria, between groups of HbA1c ≥7% and HbA1c <7%, statistically significant differences in terms of duration of DM, BMI, urea, creatinine, TC, HDL-C, LDL-C, microalbuminuria, visfatin, and fetuin-A levels could not be found.
Similarly, there was no significant difference between the T2DM group having microalbuminuria (≥30 mg/day) and the normoalbuminuria group (<30 mg/day) in terms of duration of DM, BMI, glucose, urea, creatinine, TC, HDL-C, LDL-C, HbA1c, visfatin, and fetuin-A levels.
Consequently, low serum levels of fetuin-A in the T2DM patient group may suggest fetuin-A is a factor having a role in T2DM pathogenesis, although neither visfatin nor fetuin-A were correlated with HbA1c and albuminuria levels in patients with T2DM.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Guerre-Millo M. Adipose tissue hormones. J Endocrinol Invest. 2002;25:855–861. doi: 10.1007/BF03344048. [DOI] [PubMed] [Google Scholar]
- 2.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ognjanovic S, Bao S, Yamamoto SY, Garibay-Tupas J, Samal B, Bryant-Greenwood GD. Genomic organization of the gene coding for human pre-B-cell colony enhancing factor and expression in human fetal membranes. J Mol Endocrinol. 2001;26:107–117. doi: 10.1677/jme.0.0260107. [DOI] [PubMed] [Google Scholar]
- 4.Berndt J, Kloting N, Kralisch S, Kovacs P, Fasshauer M, Schon MR, Stumvoll M, Bluher M. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54:2911–2916. doi: 10.2337/diabetes.54.10.2911. [DOI] [PubMed] [Google Scholar]
- 5.Haider DG, Schaller G, Kapiotis S, Maier C, Luger A, Wolzt M. The release of the adipocytokine visfatin is regulated by glucose and insulin. Diabetologia. 2006;49:1909–1914. doi: 10.1007/s00125-006-0303-7. [DOI] [PubMed] [Google Scholar]
- 6.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 7.Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, Shin SJ, Lee YJ. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:295–299. doi: 10.1210/jc.2005-1475. [DOI] [PubMed] [Google Scholar]
- 8.Hammarstedt A, Pihlajamaki J, Rotter Sopasakis V, Gogg S, Jansson PA, Laakso M, Smith U. Visfatin is an adipokine, but it is not regulated by thiazolidinediones. J Clin Endocrinol Metab. 2006;91:1181–1184. doi: 10.1210/jc.2005-1395. [DOI] [PubMed] [Google Scholar]
- 9.Mori K, Emoto M, Araki T, Yokoyama H, Lee E, Teramura M, Koyama H, Shoji T, Inaba M, Nishizawa Y. Effects of pioglitazone on serum fetuin-A levels in patients with type 2 diabetes mellitus. Metabolism. 2008;57:1248–1252. doi: 10.1016/j.metabol.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Ix JH, Wassel CL, Kanaya AM, Vittinghoff E, Johnson KC, Koster A, Cauley JA, Harris TB, Cummings SR, Shlipak MG. Health ABC Study. Fetuin-A and incident diabetes mellitus in older persons. JAMA. 2008;300:182–188. doi: 10.1001/jama.300.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yilmaz MI, Saglam M, Qureshi AR, Carrero JJ, Caglar K, Eyileten T, Sonmez A, Cakir E, Oguz Y, Vural A, Yenicesu M, Stenvinkel P, Lindholm B, Axelsson J. Endothelial dysfunction in type-2 diabetics with early diabetic nephropathy is associated with low circulating adiponectin. Nephrol Dial Transplant. 2008;23:1621–1627. doi: 10.1093/ndt/gfm828. [DOI] [PubMed] [Google Scholar]
- 12.Dogru T, Sonmez A, Tasci I, Bozoglu E, Yilmaz MI, Genc H, Erdem G, Gok M, Bingol N, Kilic S, Ozgurtas T, Bingol S. Plasma visfatin levels in patients with newly diagnosed and untreated type 2 diabetes mellitus and impaired glucose tolerance. Diabetes Res Clin Pract. 2007;76:24–29. doi: 10.1016/j.diabres.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Sandeep S, Velmurugan K, Deepa R, Mohan V. Serum visfatin in relation to visceral fat, obesity, and type 2 diabetes mellitus in Asian Indians. Metabolism. 2007;56:565–570. doi: 10.1016/j.metabol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Krzyzanowska K, Krugluger W, Mittermayer F, Rahman R, Haider D, Shnawa N, Schernthaner G. Increased visfatin concentrations in women with gestational diabetes mellitus. Clin Sci (Lond) 2006;110:605–609. doi: 10.1042/CS20050363. [DOI] [PubMed] [Google Scholar]
- 15.Takata H, Ikeda Y, Suehiro T, Ishibashi A, Inoue M, Kumon Y, Terada Y. High glucose induces transactivation of the alpha2-HS glycoprotein gene through the ERK1/2 signaling pathway. J Atheroscler Thromb. 2009;16:448–456. doi: 10.5551/jat.no950. [DOI] [PubMed] [Google Scholar]
- 16.Mori K, Emoto M, Yokoyama H, Araki T, Teramura M, Koyama H, Shoji T, Inaba M, Nishizawa Y. Association of serum fetuin-A with insulin resistance in type 2 diabetic and nondiabetic subjects. Diabetes Care. 2006;29:468. doi: 10.2337/diacare.29.02.06.dc05-1484. [DOI] [PubMed] [Google Scholar]