Abstract
Background
Peripheral arterial disease (PAD) is a common manifestation of systemic atherosclerosis and is associated with significant morbidity and mortality. Diabetes is known to increase the risk of PAD two- to four-fold. The prevalence of PAD in Korean diabetic patients has not been established. In this study, we investigated the prevalence of PAD in Korean patients with type 2 diabetes attending a large university hospital and analyzed the factors associated with PAD.
Methods
A total of 2,002 patients with type 2 diabetes who underwent ankle-brachial index (ABI) measurement in an outpatient clinic were enrolled. PAD was defined as an ABI ≤0.9. Clinical characteristics of 64 patients with PAD were compared with those of 192 age- and sex-matched control patients without PAD.
Results
Of the 2,002 type 2 diabetic patients, 64 (3.2%) were diagnosed as having PAD. PAD was associated with higher prevalences of retinopathy, nephropathy, neuropathy, cerebrovascular and coronary artery disease. Patients with PAD had higher systolic blood pressure and serum triglyceride level and reported higher pack-years of smoking. Multivariate analysis showed that the presence of micro- and macrovascular complications and high systolic blood pressure are factors independently associated with PAD.
Conclusion
The prevalence of PAD in diabetic patients was 3.2%, suggesting that the prevalence in Korean diabetic patients is lower than that of patients in Western countries.
Keywords: Diabetes mellitus, type 2; Peripheral arterial disease; Prevalence; Risk factors
INTRODUCTION
Peripheral arterial disease (PAD), atherosclerotic occlusion of the arteries to the legs, is an important manifestation of systemic atherosclerosis [1]. Both symptomatic and asymptomatic PAD are associated with a significantly increased risk for cardiovascular mortality [2]. It has been reported that patients with PAD, even in the absence of a history of myocardial infarction or ischemic stroke, have approximately the same relative risk of death from cardiovascular causes as do patients with a history of coronary or cerebrovascular disease [3].
One of the objective measures of PAD is the ankle-brachial index (ABI), defined as the ratio between the systolic arterial pressure at the ankle level and that in the left or right brachial artery [4]. PAD is defined as an ABI ≤0.9 [5]. This measurement is valuable for early detection of PAD and is also an indicator of generalized atherosclerosis [6].
It has been reported that diabetes is associated with a two-to four-fold increase in the incidence of PAD compared with that in non-diabetic subjects [7]. Among the U.S. adult population ≥40 years of age, the prevalence of PAD is 9.5% in diabetic subjects, two-fold higher than the 4.5% prevalence in non-diabetic subjects [8]. Accordingly, the American Diabetes Association (ADA) consensus statement recommends that ABI should be performed as a screening measure in all diabetic individuals >50 years of age [9].
While many PAD studies have been conducted in Western countries, little is known about the prevalence of PAD in Asian populations. PAD-SEARCH (peripheral arterial disease - screening and evaluation of diabetic patients in Asian regions characterized by high risk factors), the first international study to investigate the prevalence of PAD in Asian type 2 diabetic patients, reported that 11.7% of Asian patients with type 2 diabetes have PAD [10]. However, this study may have not reflected the general prevalence of PAD in Asian type 2 diabetic patients because it included only type 2 diabetic patients over 50 years of age having one or more risk factors such as smoking, hypertension or dyslipidemia. The aim of the present study was to investigate the prevalence of PAD in Korean type 2 diabetic patients attending a university hospital and to characterize the factors associated with PAD.
METHODS
Study subjects
The study subjects consisted of 2,002 consecutive type 2 diabetic patients who underwent ABI measurement at the Diabetes Center of Asan Medical Center between February and July 2009. Type 2 diabetes was defined as onset age ≥25 years and fasting C-peptide level >1.0 ng/mL. Among the 2,061 patients in whom ABI was measured during the study period, 59 patients with the following conditions were excluded from the study: 1) onset age younger than 25 years (n=14), 2) type 1 diabetes (n=30), and 3) an ABI >1.4 (n=15). The study protocol was approved by the Institutional Review Board of Asan Medical Center.
Study design
We analyzed the prevalence of PAD in all type 2 diabetic patients who underwent ABI during the study period. To characterize the factors associated with PAD, we performed a case-control study. For each patient with PAD, we selected age- and sex-matched control patients without PAD and retrospectively examined the medical records. We obtained information about age, sex, body mass index (BMI), duration of diabetes, systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting plasma glucose (FBS), 2-hour postprandial glucose (2hPG), C-peptide, hemoglobin A1c (HbA1c), total cholesterol, high density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, smoking history, and various complications of diabetes. All laboratory results were based on the tests performed on the day of the ABI exam. Diabetic retinopathy included both non-proliferative and proliferative retinopathy diagnosed by the ophthalmologist. Diabetic nephropathy included microalbuminuria (defined as an albumin-to-creatinine ratio between 30 and 300 µg/mg in a random urine spot collection), overt proteinuria (defined as an albumin-to-creatinine ratio >300 µg/mg), and azotemia. Diabetic neuropathy was defined as having typical symptoms and/or evidence of reduced vibratory sensation or impairment on nerve conduction. Diagnosis of cerebrovascular (CVA) and coronary artery disease (CAD) were dependent on a history of ischemic events and/or demonstration of vascular stenosis by radiologic or other measures (i.e., brain magnetic resonance imaging [MRI], coronary angiography, or coronary computerized tomography [CT]). All results of the diagnostic tests were based on the final examinations on the day of the ABI exam.
Measurement of the ABI
The ABI was calculated for each leg by dividing the SBP obtained at the ankle level in the respective leg by the SBP of the brachial artery. Measurements were performed with the participants in the supine position. The examination was performed by trained technicians using VP-1000® (Colin Co., Komaki, Japan).
Assessment of PAD
The lesser ABI of the two legs was used to determine the presence of PAD because disease often occurs unilaterally. We used an ABI ≤0.9 as a criterion for the diagnosis of PAD according to the guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) [5]. This cut-off point has a sensitivity of 95% and a specificity of 99% for detecting a significant stenotic limb lesion (at least 50% of arterial lumen) [11]. An ABI ≤0.4 was regarded as severe PAD [1,5].
Statistical analysis
Data were analyzed using the software program SPSS version 15.0 (SPSS Inc., Chicago, IL, USA) and SAS software package version 9.1 (SAS Inc., Cary, NC, USA). Continuous variables were expressed as the mean±standard deviation, and categorical variables as a percentage. Student's t-test and the chi-square test were used to compare continuous and categorical differences, respectively. Age-adjusted prevalence estimates were calculated using the direct method, which were age adjusted to the 2005 Korean population [12]. Multivariate conditional logistic regression analysis was performed using PAD as the dependent variable and smoking, duration of diabetes, SBP, serum total cholesterol, and presence of microvascular or macrovascular complication as the independent variables. The control group was selected using the stratified random sampling method to be matched for age and sex with the case group. A P value less than 0.05 was considered statistically significant.
RESULTS
Prevalence of PAD
The overall prevalence of PAD in the patients was 3.2% (n=64). The proportion of severe PAD among patients with PAD was 4.7% (n=3). The age-adjusted prevalence rate in the population [12] was 1.8%.
Comparative analysis of the prevalence of PAD according to age and sex
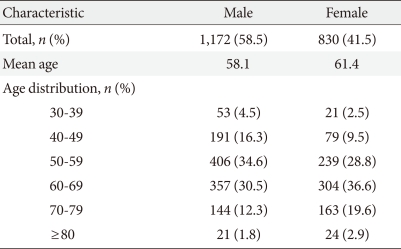
Baseline characteristics of the study population are presented in Table 1. Men comprised 58.5% of the subjects, and the mean age of the subjects was 59.5 years. The prevalence of PAD was 4.3% and 1.7% in the male and female subjects, respectively.
Table 1.
Characteristics of the patients (n=2,002)
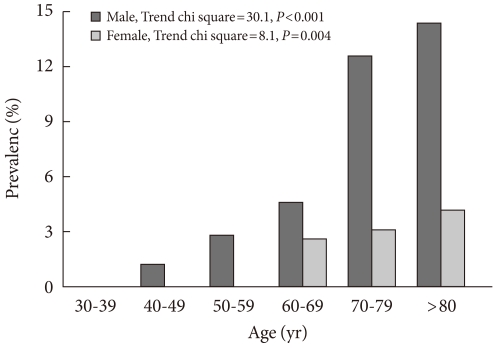
Fig. 1 shows the prevalence rates of PAD according to age and sex. The trend for the increase according to age was statistically significant in both sexes (P<0.001). PAD was more prevalent in male subjects than in female subjects, regardless of age.
Fig. 1.
The prevalence rates of peripheral arterial disease according to age and sex.
Characteristics of the PAD group
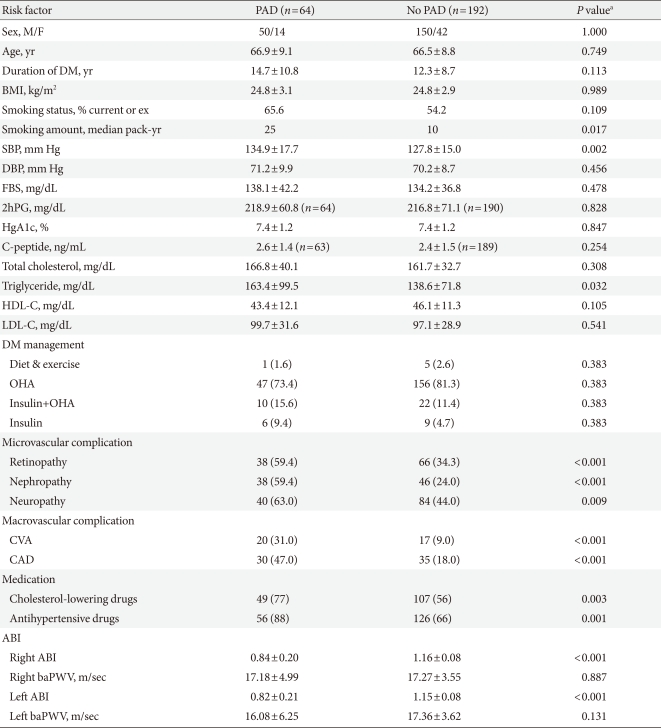
The mean age of the PAD group was 66.9±9.1 years, and 78.0% (n=50) were male (Table 2). When the clinical characteristics of these patients were compared with those of the age- and sex-matched control group, SBP and serum triglyceride levels were significantly higher in the PAD group than in the control group. A history of smoking was found in 65.6% and 54.2% of the PAD and control groups, respectively. The amount of smoking was significantly higher in the PAD group than in the control group. No significant differences were found in duration of diabetes, BMI, FBS, 2hPG, HbA1c, C-peptide, total cholesterol, LDL cholesterol or HDL cholesterol. The PAD group had higher rates of all microvascular and macrovascular complications than did the control group. More patients in the PAD group were being treated with medications for hypercholesterolemia or hypertension.
Table 2.
Clinical characteristics of the study groups
Values are presented as the mean±standard deviation or number (%).
PAD, peripheral arterial disease; DM, diabetes mellitus; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBS, fasting blood sugar; 2hPG, plasma glucose two hours after a meal; HbA1c, hemoglobin A1c; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; OHA, oral hypoglycemic agent; CVA, cerebrovascular accident; CAD, coronary artery disease; ABI, ankle-brachial index; baPWV, brachial-ankle pulse wave velocity.
aP<0.05 was considered significant.
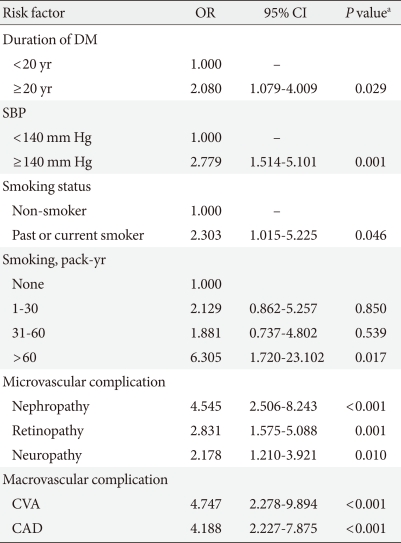
Table 3 shows the odds ratio (OR) of PAD according to clinical status analyzed by univariate conditional logistic regression analysis. Those with a diabetic duration ≥20 years had a significantly higher risk of PAD (OR, 2.08; 95% confidence interval [CI], 1.08 to 4.01; P≤0.029). The OR for SBP ≥140 mm Hg as a risk factor for PAD was 2.78 (95% CI, 1.51 to 5.10; P≤0.001). The OR for smoking status as a risk factor for PAD was 2.30 (95% CI, 1.02 to 5.23; P≤0.046).
Table 3.
Odds ratios of PAD according to clinical status: results from univariate conditional logistic regression analysis
PAD, peripheral arterial disease; OR, odds ratio; CI, confidence interval; DM, diabetes mellitus; SBP, systolic blood pressure; CVA, cerebrovascular accident; CAD, coronary artery disease.
aP<0.05 was considered significant.
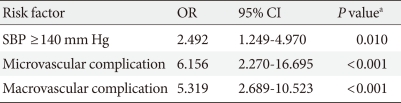
In order to determine the factors independently associated with PAD, we performed multivariate conditional logistic regression analysis (Table 4). Presence of one or more components of microvascular and macrovascular complications was categorized as microvascular and macrovascular complication, respectively. Higher SBP and the presence of microvascular and/or macrovascular complications were independently associated with PAD.
Table 4.
Adjusted odds ratios of PAD for various independent indicators: results from multivariate conditional logistic regression analysis
Included in the logistic regression model were smoking, duration of diabetes, SBP, serum total cholesterol, and presence of microvascular or macrovascular complication.
PAD, peripheral arterial disease; OR, odds ratio; CI, confidence interval; SBP, systolic blood pressure.
aP<0.05 was considered significant.
DISCUSSION
The prevalence of PAD in the general population has been reported to be varied [6,13-15]. It is generally believed that diabetes increases the risk of PAD two- to four-fold [7]. Most of the previous data on the prevalence of PAD came from Europeans or Americans. In a German population aged ≥65 years, subjects with diabetes had a higher prevalence of PAD in comparison with that of non-diabetics (26.3% vs. 15.3%) [16]. In a U.S. population aged 50 to 70 years, the prevalence of PAD in diabetic patients was 22.0% [17]. PAD was found in 21.1% of newly diagnosed type 2 diabetic Italian patients aged >30 years [18].
In our study, the prevalence of PAD in Korean type 2 diabetic patients aged ≥25 years was considerably lower (3.2%) than that in Western countries. The prevalence in patients ≥50 years of age was 3.7% (men 5.2%, women 1.9%). In agreement with our study, a previous nationwide survey performed by the Committee of the Korean Diabetes Association on the Epidemiology of Diabetes Mellitus also reported that 3.0% of Korean diabetic patients attending 13 tertiary hospitals in Korea had PAD [19]. Similarly, the prevalence of PAD in an urban South Indian population was 3.2% in non-diabetic subjects and 6.3% in diabetic patients (≥20 years of age) [20]. Conversely, in the PAD-SEARCH study, an international study that investigated the prevalence of PAD in Asian type 2 diabetic patients (>50 years of age, having one or more risk factors for atherosclerosis), 17.7% of the patients had PAD [10]. The prevalence of PAD in Chinese diabetic patients (≥35 years of age) was even higher (32.2%) [21].
The cause of these differences between studies performed in Asian populations is not known. In addition, the reason the results of our study demonstrated a lower prevalence of PAD in Korean type 2 diabetic patients than that in the Western population is not known. However, this may not be due to simple selection bias because the study subjects were randomly selected from patients attending a large university hospital. In this hospital, milder diabetic patients without apparent diabetic complications are usually referred back to private clinics. The hospital is located in the southern part of Seoul, and more than 70% of the patients come from urban areas. If the lower rate of PAD in Korean diabetic patients is real, this may be related to a lower prevalence of obesity, an important risk factor for atherosclerosis, in this country [22]. Other previous studies [23,24] have shown associations between obesity and manifestations of PAD among diabetic subjects. However, incidences of CAD and ischemic CVA are increasing in Asian populations due to the Westernization of life style [25-27]. It thus remains to be seen whether the prevalence of PAD in Korean diabetic patients will increase in the future.
The prevalence of PAD in women is usually lower than that in men, and it has been suggested that onset of PAD in women usually starts 10 to 20 years later than that in men [28]. Accordingly, the prevalence of PAD in women in our study was lower than that in men, and, in fact, no women <60 years of age had PAD.
As with previous studies [24,29-31], significant differences in SBP, triglyceride, and pack-years of smoking were found between subjects with and without PAD in our study. This finding suggests that these factors may be particularly important in the development of PAD in diabetic populations. We did not find a significant difference in total, LDL- or HDL-cholesterol level between patients with and without PAD. These results are inconsistent with the Framingham Study [32] and other studies [24,29,33]. It is possible that some of these differences in risk factors may be caused by cholesterol-lowering drugs. More patients in the PAD group in our study were taking medications for hypercholesterolemia, which may have masked the real differences in cholesterol.
It is well known that very strong associations exist between PAD and other atherosclerotic disorders [1-3]. However, independent association of PAD with microvascular complications was not expected. According to the ticking clock hypothesis [34], the risk for CAD starts to increase long before the onset of clinical diabetes, while microvascular complications develop only after the onset of diabetes. However, several previous studies also have shown that peripheral neuropathy [7], diabetic retinopathy [35,36] and renal insufficiency [37,38] are associated with PAD. This can be partly explained by the steno hypothesis [39], which posits that microvascular complications (i.e., albuminuria) reflect widespread vascular damages in the entire body.
The present study has some limitations. First, our results were affected by a selection bias. The sample was not population-based and consisted of individuals who visited a single large university hospital. In addition, our study did not include all diabetic patients who visited in our hospital during the study period. Second, because this study was cross-sectional, we were unable to determine whether risk factors were causally related to the ABI values. Third, we excluded the subjects with an ABI >1.4. Calcified, poorly compressible vessels in the elderly diabetic patients can artificially elevate values. The Strong Heart Study (SHS) recommended that ABI >1.4 should not be considered normal [40]. However, the overall prevalence of PAD would not have changed much (3.2% → 3.9%) even if these subjects were classified as having PAD.
In summary, this study showed that the prevalence of PAD might be quite low in Korean patients with type 2 diabetes in comparison with that in Western countries. It remains to be seen whether Westernization will increase the prevalence of PAD in Korean diabetic patients in the future.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–1621. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 2.Murabito JM, Evans JC, Nieto K, Larson MG, Levy D, Wilson PW. Prevalence and clinical correlates of peripheral arterial disease in the Framingham Offspring Study. Am Heart J. 2002;143:961–965. doi: 10.1067/mhj.2002.122871. [DOI] [PubMed] [Google Scholar]
- 3.Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelmark M, Polak JF, Powe NR, Siscovick D The Cardiovascular Health Study Group. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19:538–545. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- 4.Begelman SM, Jaff MR. Noninvasive diagnostic strategies for peripheral arterial disease. Cleve Clin J Med. 2006;73(Suppl 4):S22–S29. doi: 10.3949/ccjm.73.suppl_4.s22. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 6.Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, Wolfson SK. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee D. Peripheral and cerebrovascular atherosclerotic disease in diabetes mellitus. Best Pract Res Clin Endocrinol Metab. 2009;23:335–345. doi: 10.1016/j.beem.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, Burt V, Curtin L, Engelgau M, Geiss L 1999-2000 national health and nutrition examination survey. Prevalence of lowerextremity disease in the US adult population >=40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care. 2004;27:1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 9.Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47:921–929. doi: 10.1016/j.jacc.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 10.Rhee SY, Guan H, Liu ZM, Cheng SW, Waspadji S, Palmes P, Tai TY, Suwanwalaikorn S, Kim YS PAD-SEARCH Study Group. Multi-country study on the prevalence and clinical features of peripheral arterial disease in Asian type 2 diabetes patients at high risk of atherosclerosis. Diabetes Res Clin Pract. 2007;76:82–92. doi: 10.1016/j.diabres.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 11.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. Trans Atlantic Inter-Society Consensus (TASC) J Vasc Surg. 2000;31(1 Pt 2):S1–S296. [PubMed] [Google Scholar]
- 12.Korea National Statistical Office: Korean statistical information service. [cited 2011 Aug 30]. Available from: http://www.kosis.kr.
- 13.Zheng ZJ, Sharrett AR, Chambless LE, Rosamond WD, Nieto FJ, Sheps DS, Dobs A, Evans GW, Heiss G. Associations of ankle-brachial index with clinical coronary heart disease, stroke and preclinical carotid and popliteal atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 1997;131:115–125. doi: 10.1016/s0021-9150(97)06089-9. [DOI] [PubMed] [Google Scholar]
- 14.Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20:384–392. doi: 10.1093/ije/20.2.384. [DOI] [PubMed] [Google Scholar]
- 15.Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral arterial disease in the elderly: The Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18:185–192. doi: 10.1161/01.atv.18.2.185. [DOI] [PubMed] [Google Scholar]
- 16.Lange S, Diehm C, Darius H, Haberl R, Allenberg JR, Pittrow D, Schuster A, von Stritzky B, Tepohl G, Trampisch HJ. High prevalence of peripheral arterial disease and low treatment rates in elderly primary care patients with diabetes. Exp Clin Endocrinol Diabetes. 2004;112:566–573. doi: 10.1055/s-2004-830408. [DOI] [PubMed] [Google Scholar]
- 17.Beach KW, Bedford GR, Bergelin RO, Martin DC, Vandenberghe N, Zaccardi M, Strandness DE., Jr Progression of lower-extremity arterial occlusive disease in type II diabetes mellitus. Diabetes Care. 1988;11:464–472. doi: 10.2337/diacare.11.6.464. [DOI] [PubMed] [Google Scholar]
- 18.Faglia E, Caravaggi C, Marchetti R, Mingardi R, Morabito A, Piaggesi A, Uccioli L, Ceriello A SCAR (SCreening for ARteriopathy) Study Group. Screening for peripheral arterial disease by means of the ankle-brachial index in newly diagnosed type 2 diabetic patients. Diabet Med. 2005;22:1310–1314. doi: 10.1111/j.1464-5491.2005.01612.x. [DOI] [PubMed] [Google Scholar]
- 19.Lim S, Kim DJ, Jeong IK, Son HS, Chung CH, Koh G, Lee DH, Won KC, Park JH, Park TS, Ahn J, Kim J, Park KG, Ko SH, Ahn YB, Lee I. A nationwide survey about the current status of glycemic control and complications in diabetic patients in 2006: The Committee of the Korean Diabetes Association on the Epidemiology of Diabetes Mellitus. Korean Diabetes J. 2009;33:48–57. [Google Scholar]
- 20.Premalatha G, Shanthirani S, Deepa R, Markovitz J, Mohan V. Prevalence and risk factors of peripheral vascular disease in a selected South Indian population: the Chennai Urban Population Study. Diabetes Care. 2000;23:1295–1300. doi: 10.2337/diacare.23.9.1295. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Luo Y, Xu Y, Yang J, Zheng L, Hasimu B, Yu J, Hu D. Risk factors of peripheral arterial disease and relationship between low ankle - brachial index and mortality from all-cause and cardiovascular disease in Chinese patients with type 2 diabetes. Circ J. 2007;71:377–381. doi: 10.1253/circj.71.377. [DOI] [PubMed] [Google Scholar]
- 22.Kim DM, Ahn CW, Nam SY. Prevalence of obesity in Korea. Obes Rev. 2005;6:117–121. doi: 10.1111/j.1467-789X.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 23.Kreines K, Johnson E, Albrink M, Knatterud GL, Levin ME, Lewitan A, Newberry W, Rose FA. The course of peripheral vascular disease in non-insulin-dependent diabetes. Diabetes Care. 1985;8:235–243. doi: 10.2337/diacare.8.3.235. [DOI] [PubMed] [Google Scholar]
- 24.Kannel WB, D'Agostino RB, Wilson PW, Belanger AJ, Gagnon DR. Diabetes, fibrinogen, and risk of cardiovascular disease: the Framingham experience. Am Heart J. 1990;120:672–676. doi: 10.1016/0002-8703(90)90026-t. [DOI] [PubMed] [Google Scholar]
- 25.Kuller LH. Ethnic differences in atherosclerosis, cardiovascular disease and lipid metabolism. Curr Opin Lipidol. 2004;15:109–113. doi: 10.1097/00041433-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Egusa G, Watanabe H, Ohshita K, Fujikawa R, Yamane K, Okubo M, Kohno N. Influence of the extent of westernization of lifestyle on the progression of preclinical atherosclerosis in Japanese subjects. J Atheroscler Thromb. 2002;9:299–304. doi: 10.5551/jat.9.299. [DOI] [PubMed] [Google Scholar]
- 27.Woo KS, Chook P, Raitakari OT, McQuillan B, Feng JZ, Celermajer DS. Westernization of Chinese adults and increased subclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19:2487–2493. doi: 10.1161/01.atv.19.10.2487. [DOI] [PubMed] [Google Scholar]
- 28.Diehm C, Schuster A, Allenberg JR, Darius H, Haberl R, Lange S, Pittrow D, von Stritzky B, Tepohl G, Trampisch HJ. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis. 2004;172:95–105. doi: 10.1016/s0021-9150(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 29.Uusitupa MI, Niskanen LK, Siitonen O, Voutilainen E, Pyorala K. 5-year incidence of atherosclerotic vascular disease in relation to general risk factors, insulin level, and abnormalities in lipoprotein composition in non-insulin-dependent diabetic and nondiabetic subjects. Circulation. 1990;82:27–36. doi: 10.1161/01.cir.82.1.27. [DOI] [PubMed] [Google Scholar]
- 30.Paisey RB, Arredondo G, Villalobos A, Lozano O, Guevara L, Kelly S. Association of differing dietary, metabolic, and clinical risk factors with macrovascular complications of diabetes: a prevalence study of 503 Mexican type II diabetic subjects. I. Diabetes Care. 1984;7:421–427. doi: 10.2337/diacare.7.5.421. [DOI] [PubMed] [Google Scholar]
- 31.MacGregor AS, Price JF, Hau CM, Lee AJ, Carson MN, Fowkes FG. Role of systolic blood pressure and plasma triglycerides in diabetic peripheral arterial disease. The Edinburgh Artery Study. Diabetes Care. 1999;22:453–458. doi: 10.2337/diacare.22.3.453. [DOI] [PubMed] [Google Scholar]
- 32.Kannel WB, Skinner JJ, Jr, Schwartz MJ, Shurtleff D. Intermittent claudication: incidence in the Framingham Study. Circulation. 1970;41:875–883. doi: 10.1161/01.cir.41.5.875. [DOI] [PubMed] [Google Scholar]
- 33.Maser RE, Wolfson SK, Jr, Ellis D, Stein EA, Drash AL, Becker DJ, Dorman JS, Orchard TJ. Cardiovascular disease and arterial calcification in insulin-dependent diabetes mellitus: interrelations and risk factor profiles. Pittsburgh Epidemiology of Diabetes Complications Study-V. Arterioscler Thromb. 1991;11:958–965. doi: 10.1161/01.atv.11.4.958. [DOI] [PubMed] [Google Scholar]
- 34.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263:2893–2898. doi: 10.1001/jama.263.21.2893. [DOI] [PubMed] [Google Scholar]
- 35.Bosevski M, Meskovska S, Tosev S, Peovska I, Asikov I, Georgievska-Ismail LJ. Risk factors for development of critical limb ischemia: a survey of diabetic vs. nondiabetic population. Prilozi. 2006;27:89–96. [PubMed] [Google Scholar]
- 36.Mostaza JM, Suarez C, Manzano L, Cairols M, Lopez-Fernandez F, Aguilar I, Diz Lois F, Sampedro JL, Sanchez-Huelva H, Sanchez-Zamorano MA Merito Study Group. Sub-clinical vascular disease in type 2 diabetic subjects: relationship with chronic complications of diabetes and the presence of cardiovascular disease risk factors. Eur J Intern Med. 2008;19:255–260. doi: 10.1016/j.ejim.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 37.De Graauw J, Chonchol M, Poppert H, Etgen T, Sander D. Relationship between kidney function and risk of asymptomatic peripheral arterial disease in elderly subjects. Nephrol Dial Transplant. 2011;26:927–932. doi: 10.1093/ndt/gfq455. [DOI] [PubMed] [Google Scholar]
- 38.Wasmuth S, Baumgartner I, Do DD, Willenberg T, Saguner A, Zwahlen M, Diehm N. Renal insufficiency is independently associated with a distal distribution pattern of symptomatic lower-limb atherosclerosis. Eur J Vasc Endovasc Surg. 2010;39:591–596. doi: 10.1016/j.ejvs.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 39.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32:219–226. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- 40.Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, Howard BV. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]