Abstract
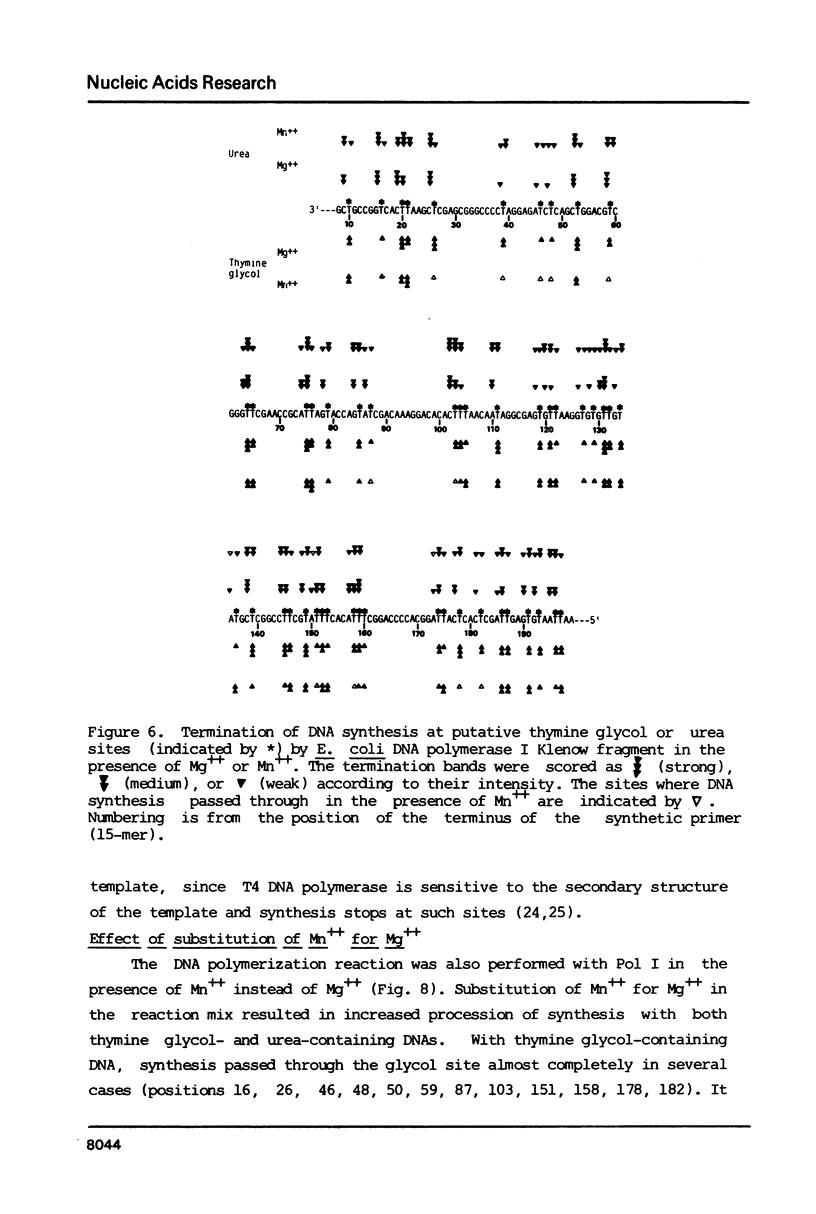
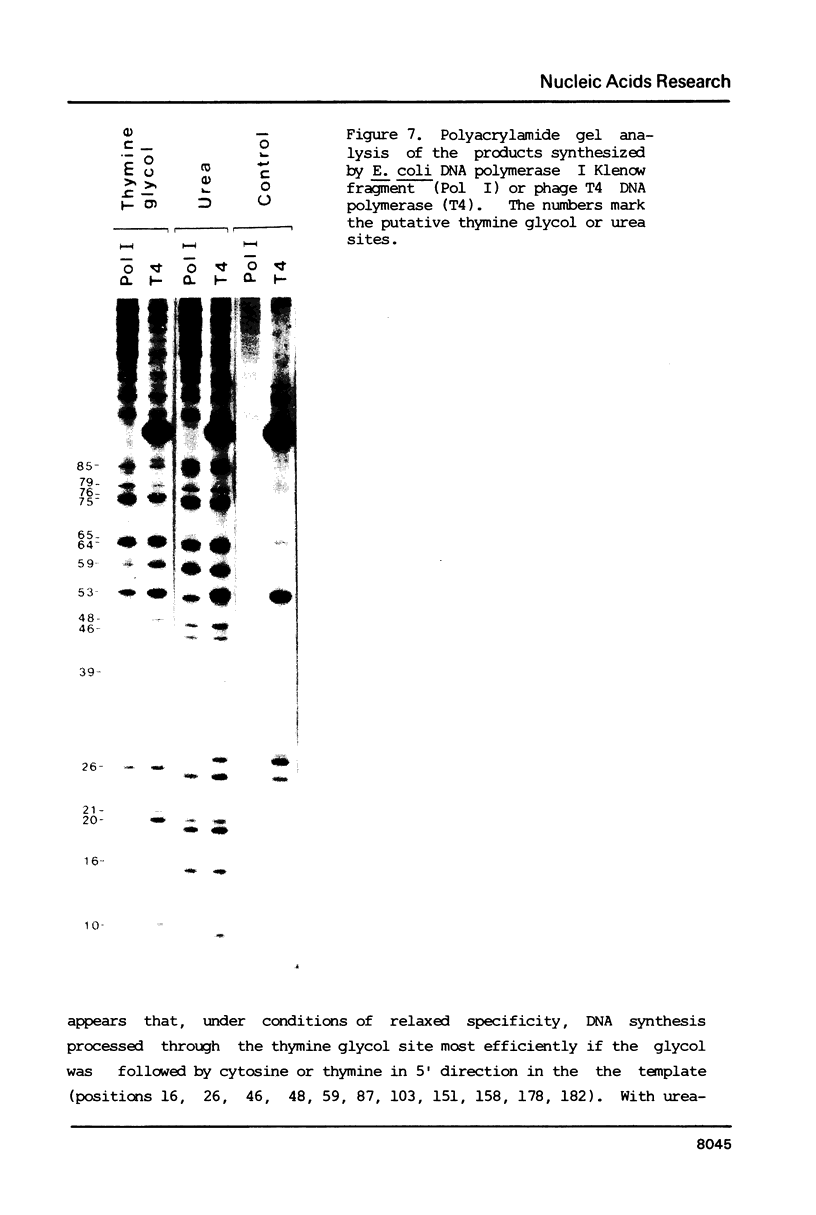
Thymine glycols were produced in M13 DNA in a concentration dependent manner by treating the DNA with osmium tetroxide (OsO4). For the formation of urea-containing M13 DNA, OsO4-oxidized DNA was hydrolyzed in alkali (pH 12) to convert the thymine glycols to urea residues. With both thymine glycol- and urea-containing M13 DNA, DNA synthesis catalyzed by Escherichia coli DNA polymerase I Klenow fragment was decreased in proportion to the number of damages present in the template DNA. Sequencing gel analysis of the products synthesized by E. coli DNA polymerase I and T4 DNA polymerase showed that DNA synthesis terminated opposite the putative thymine glycol site and at one nucleotide before the putative urea site. Substitution of manganese for magnesium in the reaction mix resulted in increased processivity of DNA synthesis so that a base was incorporated opposite urea. With thymine glycol-containing DNA, processivity in the presence of manganese was strongly dependent on the presence of a pyrimidine 5' to the thymine glycol in the template.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beer M., Stern S., Carmalt D., Mohlhenrich K. H. Determination of base sequence in nucleic acids with the electron microscope. V. The thymine-specific reactions of osmium tetroxide with deoxyribonucleic acid and its components. Biochemistry. 1966 Jul;5(7):2283–2288. doi: 10.1021/bi00871a017. [DOI] [PubMed] [Google Scholar]
- Cathcart R., Schwiers E., Saul R. L., Ames B. N. Thymine glycol and thymidine glycol in human and rat urine: a possible assay for oxidative DNA damage. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5633–5637. doi: 10.1073/pnas.81.18.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Englund P. T. The effect of template secondary structure on vaccinia DNA polymerase. J Biol Chem. 1979 Aug 25;254(16):7820–7826. [PubMed] [Google Scholar]
- Demple B., Halbrook J., Linn S. Escherichia coli xth mutants are hypersensitive to hydrogen peroxide. J Bacteriol. 1983 Feb;153(2):1079–1082. doi: 10.1128/jb.153.2.1079-1082.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINK R. M., MCGAUGHEY C., CLINE R. E., FINK K. Metabolism of intermediate pyrimidine reduction products in vitro. J Biol Chem. 1956 Jan;218(1):1–7. [PubMed] [Google Scholar]
- Frenkel K., Goldstein M. S., Teebor G. W. Identification of the cis-thymine glycol moiety in chemically oxidized and gamma-irradiated deoxyribonucleic acid by high-pressure liquid chromatography analysis. Biochemistry. 1981 Dec 22;20(26):7566–7571. doi: 10.1021/bi00529a035. [DOI] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Hariharan P. V., Achey P. M., Cerutti P. A. Biological effect of thymine ring saturation in coliphage phiX174-DNA. Radiat Res. 1977 Feb;69(2):375–378. [PubMed] [Google Scholar]
- Hariharan P. V., Cerutti P. A. Excision of damaged thymine residues from gamma-irradiated poly(dA-dT) by crude extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3532–3536. doi: 10.1073/pnas.71.9.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan P. V. Determination of thymine ring saturation products of the 5,6-dihydroxydihydrothymine type by the alkali degradation assay. Radiat Res. 1980 Mar;81(3):496–498. [PubMed] [Google Scholar]
- Hillebrand G. G., Beattie K. L. Influence of template primary and secondary structure on the rate and fidelity of DNA synthesis. J Biol Chem. 1985 Mar 10;260(5):3116–3125. [PubMed] [Google Scholar]
- Huang C. C., Hearst J. E., Alberts B. M. Two types of replication proteins increase the rate at which T4 DNA polymerase traverses the helical regions in a single-stranded DNA template. J Biol Chem. 1981 Apr 25;256(8):4087–4094. [PubMed] [Google Scholar]
- Iida S., Hayatsu H. The permanganate oxidation of thymidine and thymidylic acid. Biochim Biophys Acta. 1971 Jan 1;228(1):1–8. doi: 10.1016/0005-2787(71)90541-7. [DOI] [PubMed] [Google Scholar]
- Iida S., Hayatsu H. The permanganate oxidation of thymine. Biochim Biophys Acta. 1970 Jul 16;213(1):1–13. doi: 10.1016/0005-2787(70)90002-x. [DOI] [PubMed] [Google Scholar]
- Katcher H. L., Wallace S. S. Characterization of the Escherichia coli X-ray endonuclease, endonuclease III. Biochemistry. 1983 Aug 16;22(17):4071–4081. doi: 10.1021/bi00286a013. [DOI] [PubMed] [Google Scholar]
- Moore P. D., Bose K. K., Rabkin S. D., Strauss B. S. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1981 Jan;78(1):110–114. doi: 10.1073/pnas.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P., Strauss B. S. Sites of inhibition of in vitro DNA synthesis in carcinogen- and UV-treated phi X174 DNA. Nature. 1979 Apr 12;278(5705):664–666. doi: 10.1038/278664a0. [DOI] [PubMed] [Google Scholar]
- Rabkin S. D., Strauss B. S. A role for DNA polymerase in the specificity of nucleotide incorporation opposite N-acetyl-2-aminofluorene adducts. J Mol Biol. 1984 Sep 25;178(3):569–594. doi: 10.1016/0022-2836(84)90239-0. [DOI] [PubMed] [Google Scholar]
- Rajagopalan R., Melamede R. J., Laspia M. F., Erlanger B. F., Wallace S. S. Properties of antibodies to thymine glycol, a product of the radiolysis of DNA. Radiat Res. 1984 Mar;97(3):499–510. [PubMed] [Google Scholar]
- Refolo L. M., Conley M. P., Sambamurti K., Jacobsen J. S., Humayun M. Z. Sequence context effects in DNA replication blocks induced by aflatoxin B1. Proc Natl Acad Sci U S A. 1985 May;82(10):3096–3100. doi: 10.1073/pnas.82.10.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roti J. L., Cerutti P. A. Letter: Gamma-ray induced thymine damage in mammalian cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1974 Apr;25(4):413–417. doi: 10.1080/09553007414550491. [DOI] [PubMed] [Google Scholar]
- Sagher D., Strauss B. Abasic sites from cytosine as termination signals for DNA synthesis. Nucleic Acids Res. 1985 Jun 25;13(12):4285–4298. doi: 10.1093/nar/13.12.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagher D., Strauss B. Insertion of nucleotides opposite apurinic/apyrimidinic sites in deoxyribonucleic acid during in vitro synthesis: uniqueness of adenine nucleotides. Biochemistry. 1983 Sep 13;22(19):4518–4526. doi: 10.1021/bi00288a026. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaper R. M., Loeb L. A. Depurination causes mutations in SOS-induced cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1773–1777. doi: 10.1073/pnas.78.3.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss B., Rabkin S., Sagher D., Moore P. The role of DNA polymerase in base substitution mutagenesis on non-instructional templates. Biochimie. 1982 Aug-Sep;64(8-9):829–838. doi: 10.1016/s0300-9084(82)80138-7. [DOI] [PubMed] [Google Scholar]
- Teoule R., Bert C., Bonicel A. Thymine fragment damage retained in the DNA polynucleotide chain after gamma irradiation in aerated solutions. II. Radiat Res. 1977 Nov;72(2):190–200. [PubMed] [Google Scholar]
- VAN HALTEREN M. B. Artefacts in the chromatography of mixtures of amino- acids containing cysteine. Nature. 1951 Dec 22;168(4288):1090–1091. doi: 10.1038/1681090b0. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Koiwai O., Suzuki R., Tada M. Arrest of DNA elongation by DNA polymerases at guanine adducts on 4-hydroxyaminoquinoline 1-oxide-modified DNA template. Cancer Res. 1984 May;44(5):1867–1870. [PubMed] [Google Scholar]






