Abstract
Background:
The aim of this study was to evaluate the effect of adding acidulated phosphate fluoride (APF) to phosphoric acid etchant on shear bond strength (SBS) and adhesive remnant index (ARI) of orthodontic brackets bonded to etched teeth.
Methods:
In this in vitro experimental study, 40 human premolars were etched with 37% phosphoric acid solution (Dentsply) blended with 0, 25%, 50%, and 75% fractions of 1.23% APF (Dentsply). The brackets (Mini Dyna Lock, 3M) were bonded (Transbond XT, 3M) and were subjected to 96 hours of 37°C incubation and thermocycling procedures (2000 cycles, 5–55°C, dwell time = 30 s). Then, they were debonded at 1-mm crosshead speed to measure the SBS. The ARI was estimated at 10× magnification. The data were analyzed using the tests one-way analysis of variance (ANOVA), Tukey, chi-square, one-sample t-test, and Spearman correlation.
Results:
The SBS of the groups control, 25%, 50%, and 75% APF were 11.90±2.72, 8.01±3.13, 5.40±2.51, and 3.27±2.01 MPa, respectively. Mean ARI scores of these groups were 2.4 (control), 4.3, 4.7, and 4.8, respectively. According to the Tukey′s test, only the mean SBS of the second group (25%) was not different from the control group (P=0.091).
Conclusion:
Adding about 20–25% of 1.23% APF to the phosphoric acid etchant might considerably reduce the amount of residual adhesive, without compromising the SBS.
Keywords: Acidulated phosphate fluoride, dental materials, orthodontics, phosphoric acid, topical fluorides
INTRODUCTION
Enamel etching is an essential step in bracket bonding protocols. By selectively demineralizing hydroxyapatite crystals of enamel rods, it exposes micropores on the enamel, leaving a much broader area to allow the adhesive material to interlock. Thus, the bracket might resist the masticatory forces. However, the etchant might trim down 5–10 μm of the enamel surface, which can result in a permanent demineralization of enamel surface.[1] Also, it might predispose the tooth to formation of caries or decalcifications beneath or adjacent to the bracket.[2,3] Another damage to enamel during orthodontic treatments occurs during bonding and debonding procedures. About 40–50 μm of the enamel surface might be scratched while performing these procedures.[4–6] Albeit the enamel might remineralize its fluoride-rich layer,[5] such an extent is not always reversible by remineralization procedures.[7] In addition, the enamel surface might undergo some other types of damage (e.g. fractures) during debonding or adhesive removal steps, which might correlate with the efficacy of the etchant.[1,8]
Fluoride is a highly beneficial, yet easily available, dental material. It might reduce the solubility of enamel crystals by changing their formulation. Therefore, it might be used to protect the enamel from many types of damages such as caries or decalcifications.[3,9] The acidic dental materials such as etchants used in bracket bonding in orthodontic treatments might increase the caries rate.[1] Therefore, fluoride might be utilized to reduce this iatrogenic damage, when applied prior to, while, or after etching, by directly affecting the enamel structure or interfering with microorganisms′ metabolism, reducing the level of acidic metabolites.[1] Its addition to the etchant solution is suggested as a method to directly and indirectly reduce the adverse effects of etching.[1] However, a fluoride-incorporated etchant might lack in efficacy, negatively affecting the shear bond strength (SBS) of bonded brackets and leading to bracket failure during the treatment, although controversy exists over this.[1,10–17] In this regard, Kim et al,[1] and Takahashi et al,[11] concluded that optimum levels of incorporated fluoride might be quite beneficial without reducing the bond strength, while excessive fluoride concentrations deteriorate the bond strength. Thornton et al,[12] also stated that the incorporation of fluoride to phosphoric acid did not impede the etching effect on enamel. Their results were in line with those of Kawabata et al,[15] who showed an insignificant difference between the SBS values of brackets, and a greater amount of remnant adhesive on enamel which could reduce fracture possibility. Zhang et al,[16] concluded that 2% NaF added to 35% phosphoric acid had both the best bond strength and the best preventive effect against enamel caries. Nevertheless, Garcia-Godoy et al,[13] reported a significant difference between the SBS values of brackets bonded to etched surfaces using fluoridated and non-fluoridated etchants. Meng et al,[3] and Kimura et al,[9] failed to find any statistically significant differences between the extents of remaining adhesive, which implied that the fluoridation of etchant solution would not reduce enamel damage.
A slight reduction in the SBS might reduce the remnant adhesives, probably lowering the damages caused by debonding procedures.[1] Therefore, orthodontists might use this effect to reduce enamel damages caused by debonding procedures as well as to partly protect the teeth from decalcification. Fluoride is available as different forms and controversy exists over its results.[1] Although the literature comprises the mentioned studies on this subject,[1,10–17] to our knowledge only one study has assessed the effect of fluoride as acidulated phosphate fluoride (APF) form.[1] This study evaluated the effect of adding different amounts of 1.23% APF to 37% phosphoric acid etchant on its efficacy in producing appropriate in vitro shear bond strengths (6–8 MPa),[18] which might imply a successful in vivo experience.[5] Furthermore, the adhesive remnant index (ARI) which can relate to the extent of enamel damage was assessed.[8,19]
MATERIALS AND METHODS
In this experimental in vitro study, 40 intact, recently extracted human first premolars were acquired from 10 orthodontic patients. The exclusion criteria were the presence of hypocalcification, hypoplasia, enamel fractures, and any history of bleaching. After disinfection with 0.1% thymol and collecting the sample in less than 6 months, the sample was randomly grouped in a way that each group included a tooth from each patient, and the number of maxillary and mandibular premolars was equal in each group.[4,5]
Bonding with non-fluoridated and fluoridated etchants
A 37±0.5% phosphoric acid etchant (Dentsply, York, PA, USA), and a 1.23±0.1% APF composed of sodium fluoride and hydrofluoric acid (Dentsply) were blended in various proportions, 2 weeks prior to the experiment. The groups (n=10) comprised the following: group 1: only phosphoric acid solution (PA); group 2: 75% PA and 25% APF; group 3: 50% PA and 50% APF; and group 4: 25% PA and 75% APF.
After pumice prophylaxis and rinsing, the teeth in each group were etched with one of the etchant types for 30 s and were rinsed (30 s) and dried (15 s). The brackets (Mini Dyna Lock standard size 0.018, 3M Unitek, Monrovia, CA, USA) were bonded with a thin layer of adhesive primer (Transbond XT, 3M Unitek) and the bonding composites were respectively smeared on the etched enamel and the brackets′ bases. Then, with the use of a bracket-positioning gauge, they were bonded to the middle of buccal surface, 4 mm away from the cusp. In order to standardize the adhesive thickness, the bracket was pressed against the tooth using a force gauge (1303 16 oz, ETM, Monrovia, CA, USA) with 300-g compressive force.[4,5] After removing the excess materials, a calibrated halogen light curing unit (Arialux Blue Point, Apadanatak, Tehran, Iran) was used to light cure the brackets for 40 s (10 s per mesial, distal, occlusal, and apical sides).
Aging
The specimens were stored in an incubator at 37°C for 96 hours. This was followed by thermocycling (2000 cycles, 5–55°C, dwell time = 30 s).
SBS
The specimens were mounted in self-cure acrylic resin cylinders (diameter = 3 cm, height = 20 mm). As a standardizing template, an archwire (0.018×0.025 mm) was bent in a stepped shape with one middle upper and two lower steps (all on the horizontal plane) adjoining with 25-mm vertical segments [Figure 1]. The middle upper step was completely fitted in the bracket slot, and using the two lower steps, the buccal surface and the bracket slot were positioned vertically and horizontally, respectively [Figure 1].[4,5,20] To measure the SBS, the specimens were debonded with a calibrated test machine including a wedge-shaped blade (Universal Testing Machine 1195, Instron, Canton, MA, USA). The edge (but not the slope) of the blade exerted the occlusogingival shear force to the occlusal sides of the bracket wings at 1 mm/min crosshead speed. To calculate the SBS, the breakage force magnitude was divided by the size of the bonding area (10 mm2).[5]
Figure 1.
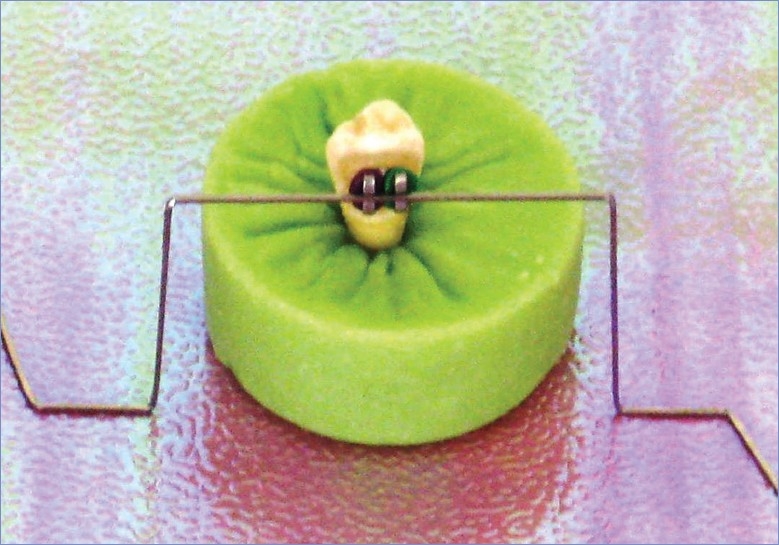
Mounting the specimen with the bracket slot standing horizontally. The bracket is color coded with o-rings
ARI
After debonding the specimens, the ARI was evaluated using a stereomicroscope (E200, Nikon, Japan) under 10× magnification. This index included five grades: (1) all the adhesive remained on the enamel; (2) more than 90% of adhesive remained; (3) between 10% and 90% of the adhesive remained; (4) less than 10% remained; and (5) indicated the absence of residual adhesive on enamel.[1,4,14]
Statistical analysis
Data were analyzed using the tests one-way analysis of variance (ANOVA), Tukey, chi-square, one-sample t-test, and Spearman correlation coefficient. The SBS values <6 MPa were considered bond failure.[1,18] The level of significance was predetermined at 0.05.
RESULTS
Three out of 13 participants were excluded due to having some teeth within the exclusion criteria. Participants′ mean age was 16.1±5.8 years (min=12, max=25 years); of them, 40% were males. Until collecting the sample, none of the teeth were stored in distilled water for more than 6 months.
A considerable proportion of the sample underwent bond failure (SBS<6) [Table 1]. According to the chi-square test, this was significant (P<0.001). All the control specimens had SBS values >6 MPa. The numbers of the specimens with SBS values >6 MPa were 7, 4, and 1 in groups 2, 3 and 4, respectively. The one-way ANOVA with ≥0.8 power showed a significant difference (P<0.001) between the groups. The Tukey post hoc test failed to detect a significant difference in the SBS values between the groups 1 (control) and 2, or groups 3 and 4 [Table 2]; however, groups 3 and 4 were significantly different from the control group (P<0.001).
Table 1.
Descriptive statistics of SBS

Table 2.
Results of the Tukey test

The Spearman correlation coefficient showed a significant negative correlation between the APF concentration and the SBS (Rho=–0.786, P<0.01).
The one-sample t-test showed that only the group 2 (25% APF) had SBS values similar to the values 6 and 8 MPa. The mean SBS of the group 1 (control) was significantly greater than 6 and 8 MPa and the values of groups 3 and 4 were significantly lower [Table 1, Figure 2].
Figure 2.
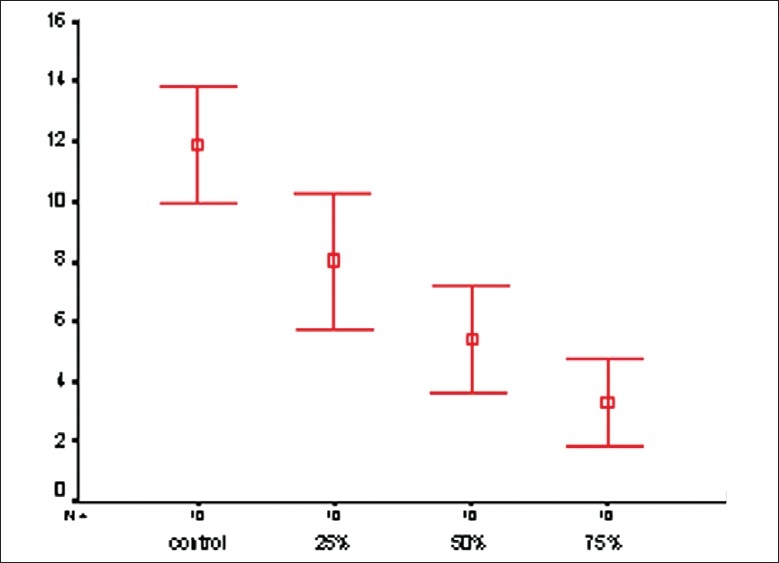
The mean SBS (and 95% CI) of the groups (MPA)
The ARI was significantly different (P<0.001) between the four groups, on analyzing with chi-square test [Table 3]. Nonparametric correlation coefficient test revealed a significant negative correlation between the APF concentration and the amount of remnant adhesive (Rho=–0.709, P<0.01).
Table 3.
Distribution of ARI scores across the sample

DISCUSSION
In the present study, the proportion of additional fluoride correlated significantly and negatively with the bond strength. According to the coefficients of variation, the most and the least reliable mean bond strengths belonged to the control group and group 4, respectively. Topical application of fluoride on the enamel surface (which includes its incorporation to the etchant solution)[1,9] might compromise the SBS because of (1) reducing the proportion of the active etchant agent, (2) the emergence of fluorapatite and calcium fluoride crystals which might increase its resistance to acid, (3) waning the enamel′s surface energy, (4) increasing the maturation rate of enamel, and (5) the alkaline nature of fluoride ions which could reduce the etchant′s pH, compromising its efficacy and reducing the etching depth.[1,2,9]
The findings of this study concerning the SBS were in harmony with certain other studies[1,11,12,15–17] and in contrast to some others.[3,9,13] The probable reason for such controversies might be various etching durations which in the study of Meng et al,[3] might be insufficient (15 s) to etch either control or experimental specimens, while in the study of Garcia-Godoy et al,[13] it was extended (60 s) which might disrupt enamel tags in the control group, diminishing the SBS more in the control group than in the experimental one. All the supporting studies reported that optimum levels of fluoride which differed on a case by case basis might be useful, while concentrations greater or lesser might be ineffective.[1,11,12,15–17] The failure of the other studies in finding such an optimum level[3,9,13] might be caused by methodological differences, such as the concentrations, etching times, fluoride solution types, and the brands used.
Adding APF to the phosphoric acid etchant reduced the amount of remnant adhesive. This was consistent with Kim et al′s[1] study. In both studies, the resin was mostly debonded from the enamel surface which might be clinically favorable since it might reduce the enamel damage following the debonding stage.[4] The incorporation of fluoride might neutralize the etchant by chemical mechanisms or simply reducing its concentration or both. Some studies[9,17] failed to observe such a significant difference in the ARI scores. These differences might be attributed to the use of different materials and other methodological differences such as the presence of thermal cycling.
The 25% APF group showed appropriate results. This fraction is considerably smaller than 50–66% APF which was proposed by Kim et al.[1] In their study, despite the presence of thermocycling, all the mean SBS values were much higher than the values in this study, allowing them to select a group with a higher proportion of fluoride. This difference might relate to material brands.[5] The suggested optimum proportion of APF in this study was more conservative. Moreover, it should be taken into consideration that the score 4 of ARI means less than 10% remaining adhesive which is near to 0% residual adhesive represented by the score 5.[4] Therefore, according to this study′s findings, the ARI did not differ considerably between groups 2, 3, and 4 [Table 3], and adding higher amounts of APF seems to be ineffective and unnecessary for further reducing the ARI.
In such studies, there should be control groups in which the etchant gel is thinned only by distilled water in order to determine whether the effect of fluoride is attributed to chemical mechanisms or attenuation of the etchant or both. However, this was absent in many other studies as well.[1,13–15] Some investigators have considered SBS range 6–10 MPa as an in vitro requirement for a successful bond,[1] while others have stated 6-8 MPa.[4,5,14,18] This study focused on the consistent lower limit (6 MPa) while comparing the SBS with both 8 and 10 MPa values. The group 2 showed a favorable result compared to 6 and 8 MPa. Its result was only slightly lower than 10 MPa (P=0.075) which was acceptable. Considering the trend of the SBS across the groups [Figure 3], it seems that the proportion of APF might be slightly reduced (e.g. about 20%) to produce a bond strength significantly greater than 6 MPa, while still providing appropriate ARI scores.
Figure 3.
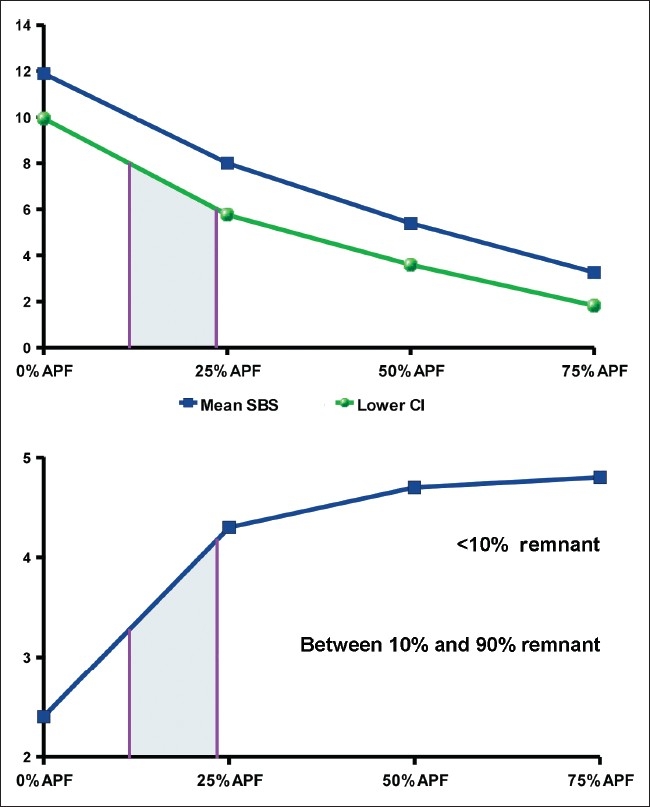
By increasing the APF:etchant ratio, the SBS and the amount of remnant adhesive decreased. The vertical band corresponds to a range of APF ratios that can result in approximately at least 6–8 MPa SBS values
This study was limited by certain factors. We could not certainly conclude whether this apparent effect of fluoride on the SBS and the ARI might be replicated, in part, only by reducing the concentration of etchant using only distilled water. There should be corresponding control groups in which the etchant gel is attenuated only by water in percentages equal to the experimental groups. However, this was absent in the other studies as well.[1,10–15] Further clinical studies are required to evaluate these findings as there are limitations over generalizability of ex vivo to clinical results.[1,5,9]
CONCLUSION
About 20–25% of 1.23% APF might be added to 37% phosphoric acid etchant to considerably reduce the residual adhesive, while maintaining appropriate SBS levels.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kim M, Lim B, Chang W, Lee Y, Rhee S, Yang H. Phosphoric acid incorporated with acidulated phosphate fluoride gel etchant effects on bracket bonding. Angle Orthod. 2005;75:678–84. doi: 10.1043/0003-3219(2005)75[678:PAIWAP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Cacciafesta V, Sfondrini M, Calvi D, Scribante A. Effect of fluoride application on shear bond strength of brackets bonded with a resin-modified glass-ionomer. Am J Orthod Dentofac Orthop. 2005;127:580–3. doi: 10.1016/j.ajodo.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Meng C, Wang W, Yeh I. Fluoridated etching on orthodontic bonding. Am J Orthod Dentofac Orthop. 1997;112:259–62. doi: 10.1016/S0889-5406(97)70253-1. [DOI] [PubMed] [Google Scholar]
- 4.Khosravanifard B, Nemati-Anaraki S, Nili S, Rakhshan V. Assessing the effects of three resin removal methods and bracket sandblasting on shear bond strength of metallic orthodontic brackets and enamel surface. Orthod Waves. 2011;70:27–38. [Google Scholar]
- 5.Khosravanifard B, Rakhshan V, Saadatmand A. Effects of blood and saliva contamination on shear bond strength of metal orthodontic brackets and evaluating certain methods for reversing the effect of contamination. Orthod Waves. 2010;69:156–63. [Google Scholar]
- 6.Schuler F, Van Waes H. SEM-evaluation of enamel surfaces after removal of fixed orthodontic appliances. Am J Dent. 2003;16:390. [PubMed] [Google Scholar]
- 7.Willmot D. White lesions after orthodontic treatment: Does low fluoride make a difference? J Orthod. 2004;31:235. doi: 10.1179/146531204225022443. [DOI] [PubMed] [Google Scholar]
- 8.Brosh T, Kaufman A, Balabanovsky A, Vardimon A. In vivo debonding strength and enamel damage in two orthodontic debonding methods. J Biomech. 2005;38:1107–13. doi: 10.1016/j.jbiomech.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Kimura T, Dunn W, Taloumis L. Effect of fluoride varnish on the in vitro bond strength of orthodontic brackets using a self-etching primer system. Am J Orthod Dentofac Orthop. 2004;125:351–6. doi: 10.1016/j.ajodo.2003.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Grajower R, Glick A, Gedalia I, Kochavi D. Tensile strength of the bond between resin to enamel etched with phosphoric acid containing fluoride. J Oral Rehabil. 1979;6:267–72. doi: 10.1111/j.1365-2842.1979.tb01504.x. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi Y, Arakawa Y, Matsukubo T, Takeuchi M. The effect of sodium fluoride in acid etching solution on sealant bond and fluoride uptake. J Dent Res. 1980;59:625. doi: 10.1177/00220345800590031201. [DOI] [PubMed] [Google Scholar]
- 12.Thornton J, Retief D, Bradley E, Jr, Denys F. The effect of fluoride in phosphoric acid on enamel fluoride uptake and the tensile bond strength of an orthodontic bonding resin. Am J Orthod Dentofac Orthop. 1986;90:91. doi: 10.1016/0889-5406(86)90039-9. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Godoy F, Hubbard G, Storey A. Effect of a fluoridated etching gel on enamel morphology and shear bond strength of orthodontic brackets. Am J Orthod Dentofac Orthop. 1991;100:163–70. doi: 10.1016/S0889-5406(05)81523-9. [DOI] [PubMed] [Google Scholar]
- 14.Keçik D, Çehreli S, Sar Ç, Ünver B. Effect of acidulated phosphate fluoride and casein phosphopeptide–amorphous calcium phosphate application on shear bond strength of orthodontic brackets. Angle Orthod. 2008;78:129–33. doi: 10.2319/122506-529.1. [DOI] [PubMed] [Google Scholar]
- 15.Kawabata R, Hayakawa T, Kasai K. Modification of 4-META/MMA-TBB resin for safe debonding of orthodontic brackets--influence of the addition of degradable additives or fluoride compound. Dent Mater J. 2006;25:524–32. doi: 10.4012/dmj.25.524. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Feng C, Ma J, Hong Y. Effect of fluorided etching fluid on the shear bond strength and the deposition on the surface of enamel. Shanghai Kou Qiang Yi Xue. 2004;13:393. [PubMed] [Google Scholar]
- 17.Ma J, Zhang Y, Hong Y. Effects on the shear bond strength of orthodontic brackets with different concentration of fluorided etching fluid. Hua Xi Kou Qiang Yi Xue Za Zhi. 2005;23:397. [PubMed] [Google Scholar]
- 18.Reynolds I. A review of direct orthodontic bonding. Br J Orthod. 1975;2:171–8. [Google Scholar]
- 19.Habibi M, Nik T, Hooshmand T. Comparison of debonding characteristics of metal and ceramic orthodontic brackets to enamel: An in-vitro study. Am J Orthod Dentofac Orthop. 2007;132:675–9. doi: 10.1016/j.ajodo.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 20.Khosravanifard B, Nemati-Anaraki S, Faraghat S, Sajjadi SH, Rakhshan H, Rakhshan V. Efficacy of 4 surface treatments in increasing the shear bond strength of orthodontic brackets bonded to saliva-contaminated direct composites. Orthod Waves. 2011;70:65–70. [Google Scholar]


