Abstract
Background:
Nitric oxide (NO) is one of the many chemical mediators involved in inflammatory processes. In addition to periapical inflammation, NO can have a role in periapical healing. The purpose of this study was to evaluate the effect of aminoguanidine (AG) as a selective inhibitor of inducible nitric oxide synthase (iNOS) on the degree of healing response of periapical lesions of the canine teeth of cats.
Methods:
In this interventional experimental study, the root canals of 48 cat canine teeth were infected with cat dental plaque and sealed. After induction of periapical lesions, root canal therapy (RCT) was performed. On the day of RCT phase, the cats were administered either AG (experimental group) or normal saline (control group), which was continued on a daily basis until the day of sacrifice. Four canine teeth in one cat served as negative and positive controls. The animals were sacrificed 6 weeks after RCT. The healing response of the periapical zones was analyzed histologically. The mean scores of healing for the two groups were compared using Mann–Whitney U test.
Results:
The mean scores of healing for the AG group (2.45±0.508) were significantly higher than those of the control group (2±0.510) (P<0.05).
Conclusion:
The use of an iNOS selective inhibitor such as AG can accelerate the healing process in periapical lesions.
Keywords: Aminoguanidine, apical periodontitis, healing, nitric oxide, nitric oxide synthase
INTRODUCTION
Apical periodontitis is viewed as a dynamic encounter between microbial factors and host defenses, which results in local inflammation and resorption of periapical hard tissue.[1] Pulpal infections first elicit an immune response in the dental pulp and stimulate a secondary immune response to the bacteria in the periapical region following total pulp necrosis. In this situation, complex immunologic mechanisms are activated that protect the pulp and periapical region but also cause host tissue destruction and mediate periapical bone resorption.
Healing of periapical lesions following root canal therapy (RCT) is an essential process to recovery of normal anatomic structures. Healing is a highly coordinated process that includes a series of overlapping phases: inflammation, cell proliferation, matrix deposition and tissue remodeling.[2] Inflammation plays an important role in healing process because the first step of healing in every tissue is an inflammatory reaction.[3] After the primary inflammatory phase subsides, the components of tissue start to proliferate and the response inches toward healing phase.[4]
As with inflammatory reactions, many chemical mediators with overlapping functions are involved in healing process. Nitric oxide (NO) is one of these chemical mediators, which, depending on the site of production and the concentration produced, can demonstrate several different biologic effects. NO is a highly reactive free radical (soluble gas) that may act as a pro-inflammatory or anti-inflammatory mediator in human tissue. This molecule is synthesized by three distinct isoforms of nitric oxide synthase (NOS).[5] Physiologic NO is synthesized by neuronal and endothelial isoforms of NOS in small amounts for a short period of time. However, in inflammatory events and wound healing, NO is synthesized in high concentrations by inducible isoform (iNOS) which can have detrimental effects on various cellular processes.[6–7] NO generated by iNOS has a distinct role in cellular process, including the induction of apoptosis,[8] inhibition of mitochondrial respiration[9] and regulation of oxidative phosphorylation, in addition to its cytotoxic effects on target cells.[10] The role of NO in wound healing is complex and multifactorial. NO released by infiltrating leukocytes certainly plays a role in the inflammatory phase of healing. NO is also known to promote angiogenesis, but the link between angiogenesis and wound healing remains poorly defined.[11]
The nucleophilic hydrazine compound, aminoguanidine (AG), possesses multiple biological effects including prevention of advanced glycation end products (AGEP) formation,[12] inhibition of diamino oxidase[13] and selective inhibition of iNOS.[14] Misko et al, showed that it selectively inhibited iNOS without inducing side effects such as increased blood pressure.[15] It was over 50-fold more effective at inhibiting the enzymatic activity of iNOS than endothelial or neuronal isoforms of NOS.[16]
The aim of this study was to evaluate the effect of AG as a selective inhibitor of iNOS on the degree of healing in periapical lesions of the canine teeth of cats.
MATERIALS AND METHODS
This study was approved by the Ethics Committee of the Isfahan University of Medical Sciences. For the purpose of this study, the cat model was selected because of its availability, ease of handling and anesthetizing, and useful pulpal and periapical anatomy.[17]
Animals
Forty-eight canine teeth of 1-year-old male cats (n=12) were used in this prospective, interventional experimental study, with intact crowns and healthy periapical and periodontal tissue in clinical examination and pretreatment radiographs. Necessary vaccinations were performed for all the experimental animals.
Inducing periapical lesions
General anesthesia was induced with 0.02 mg/kg intramuscular 2% acepromazine (Alfasan, Woerden, Holland) and 10 mg/kg intramuscular ketamine hydrochloride (Alfasan, Woerden, Holland). Buccal infiltration of 2% lidocaine with 1:100,000 epinephrine (Xylocaine, Dentsply Pharmaceutical, York, PA, USA) was injected at the apex. The maxillary and mandibular canines were isolated with rubber dam and pulp exposure was performed by removal of the two-third coronal portions of the crown. Direct access to the canal was made using gates glidden drills (Mani, Utsunomiya, Japan). The working length was determined by inserting a #15 file (Mani, Utsunomiya, Japan) and taking a radiograph. The pulp was removed by a barbed broach (Zipperer, Munich, Germany). Then, a paper point (Mani, Utsunomiya, Japan) soaked with cat tooth plaque was inserted into the canal and access openings were sealed with IRM cement (Dentsply Pharmaceutical, York, PA, USA). Four canine teeth in one cat served as negative and positive controls.
Treatment intervention
There were six animals (24 canine teeth) in each of the control and experimental groups. After the presence of periapical lesions was established using radiographs (after 8 weeks), RCT was initiated using passive step-back technique (apical to file #35 and coronal to file #60). Two milliliters of 5.25% sodium hypochlorite (Sigma-Aldrich Inc, St Louis, MO, USA) was used as irrigant after each instrument. The canals were dried using paper points and were obturated using lateral condensation technique with gutta-percha (Mani, Utsunomiya, Japan) and AH26 sealer (Dentsply, De Trey, Konstanz, Germany). The required amount (25 mg/kg) of aminoguanidine hydrochloride (Sigma-Aldrich Inc, St Louis, MO, USA) for each cat was dissolved in 2 ml of sterile normal saline. On the day of RCT phase of the treatment, the cats were given an intramuscular injection of either AG (experimental group) or normal saline (control group), and injections were continued on a daily basis until the day of sacrifice.
Histologic evaluation
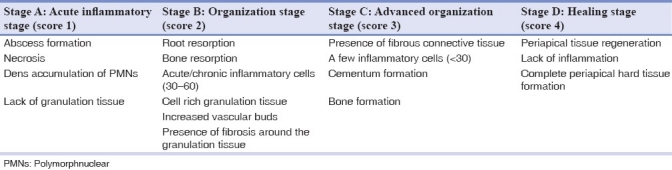
The animals were sacrificed 6 weeks after RCT phase. Vital perfusion was applied so that periapical tissue became fixed before degeneration. The specimens were fixed in 10% neutral buffered formalin and demineralized in 7% nitric acid. The specimens were dehydrated, embedded in paraffin and sectioned along the canine in a buccolingual plane for hematoxylin and eosin staining. Five 5-μm sections (separated by 50 μm), which included the apex of canine and periapical bone, were prepared for each specimen. The histology of the periapical zones was analyzed under a light microscope (Carl Zeiss 452904-9901, Oberkachen, Germany) by an expert pathologist using a single-blind protocol. Stages of healing from A to D, as defined in Table 1, were used to score the specimens.[18]
Table 1.
Stages of healing
Statistical analysis
The mean scores of periapical healing in two experimental and control groups were compared using the Mann–Whitney U test. A probability value of P<0.05 was considered a significant difference between the groups.
RESULTS
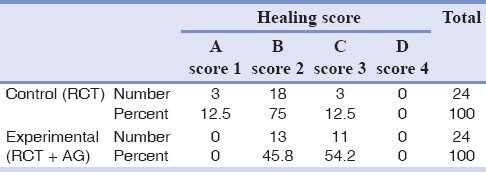
The mean scores of healing for the AG and control groups were 2.45±0.510 and 2±0.508, respectively. The distribution of healing grades within AG and control groups is presented in Table 2. Positive and negative control teeth received a histological score of 1.00 and 4.00, respectively. The difference between the AG and control groups was statistically significant (P=0.005). Thus, AG as a selective iNOS inhibitor significantly improved the degree of periapical healing in cats.
Table 2.
Distribution of healing scores within aminoguanidine (AG) and control groups
DISCUSSION
Basic science researchers have studied the role of NO in inflammatory diseases such as rheumatoid arthritis,[10] diabetes[14] and bone remodeling.[19] Additionally, researchers in dentistry have evaluated the function of NO in inflammatory processes of oral mucosa,[20] periodontal tissues,[21–22] pulp[23] and periapical areas.[24] Although reactive intermediates of oxygen and nitrogen, such as NO, are frequently found at inflammatory and healing sites, their function in pulp and periapical tissues is not yet completely clear. Considering that NO function can affect the different phases of healing in a apical periodontitis setting, a better understanding of the role of NO in the pathophysiology of healing process in periapical lesions can be helpful in future pharmacological interventions. Thus, to obtain a better understanding of the complex healing process in the periapical area, the present animal model was designed to investigate the role of NO in periapical lesion repair.
A number of studies have shown the pro-inflammatory nature of NO in pulp and surrounding tissues. Kawanishi et al, suggested that NO can be responsible for the infiltration of immunocompetent cells in the development of pulpitis.[23] Investigations by da Silva et al, Law et al, and Fan et al, all showed greater concentrations of the NOS enzyme in inflamed zones in comparison to non-inflamed areas of the pulp.[25–27] Di Paola et al, reported that when AG was used to reduce the NO concentration in rats with induced marginal periodontitis, the density of inflammatory cells and alveolar bone resorption decreased.[28] Hama et al, explained the mechanisms regarding the role of NO in the progression of periapical inflammation.[29] Our research group, in the first of series of investigations on NO, demonstrated that AG administration significantly reduced the inflammatory intensity in periapical lesions of cats,[30] which was consistent with the findings of the present study. The results of the current investigation showed that AG as a selective inhibitor of iNOS can promote the healing of periapical region. Since the first phase of tissue healing is an inflammatory process, the acceleration in periapical healing in this study may be attributed to subsidence of local inflammatory conditions due to inhibition of iNOS in the periapical area.
In addition to the inflammatory phase, the effect of AG and NO on the components of the other phases of healing must be considered. Fibroblasts are the major cell type involved in the healing process. It has been suggested that the improved healing of periapical lesions can be due to the antioxidant effect of AG on fibroblasts. Wang et al, showed that AG-treated fibroblasts from human lung tissue (2BS cells) revealed phenotypic characteristic of young cells, even by the end of incubation period,[31] whereas when these cells were transferred to normal medium (with no AG), a senescent phenotype appeared after 1 week. They suggested that AG could be helpful for the growth and proliferation of fibroblasts. It appears that AG maintains the dividing state of cells or prevents the entry of cells from M or S phase into the G0 phase of the cell cycle. The results of this study support the findings of our research.
One of the essential aspects of wound healing is the formation of collagen by fibroblasts in which the first step is the hydroxylation of proline by prolyl hydroxylase enzyme.[32] Conflicting results have been reported on the effect of NO on collagen metabolism. In Shukla et al′s study, circular wounds were made on the dorsal aspects of rats. Administration of L_NG_Nitroarginine methyl ester (L-NANE), an inhibitor of NOS, intraperitoneally at 3, 10 and 30 mg/kg doses significantly increased hydroxyproline (HP) content at all doses.[33] Histological observation of L-NAME treated wound supports biochemical findings showing less congestion and edema, considerably decreased inflammatory cells and tissue necrosis and increased fibroblastic proliferation. The presence of thick collagen fibers was a major difference, which was not present in the control group. However, when L-arginine (biologic substrate for NO) was applied topically, the HP content of wound decreased by 21% which may have resulted from increased NO concentration. Since NO is well known to inhibit iron containing enzymes, it could decrease collagen formation by inhibiting prolyl hydroxylase which is an oxygenase type of enzyme requiring iron for its activity.[34] The results of the present investigation are similar to the observations in Shukla et al′s study.
On the other hand, Schaffer et al, showed that when S-methyl isothiouronium (MITU), a competitive inhibitor of NOS, was applied to wounds during the healing process, the HP content of the tissue decreased significantly.[35] Even though the reasons for such discrepancies are not clear, the divergent results obtained from these experimental studies can be attributed to differences between animals and models used, the type and dose of NOS inhibitors used and especially the time points at which NOS inhibitors were administered.
Angiogenesis, another component of wound healing, can be affected by iNOS inhibitors. NO can stimulate the proliferation of endothelial cells, protect them from apoptosis and mediate vascular endothelium growth factor (VEGF) production.[36] It seems NO is essential for the activity of pro-angiogenic cytokines. The pharmacological blockage of NO prevents both VEGF-induced endothelial cell proliferation and mitogen-activated protein (MAP) kinase.[37] Also, it has been shown that NO is involved in the conversion of VEGF from an inert to an angiogenic state.[38] However, the link between angiogenesis and wound healing remains poorly defined. Increased blood circulation during the inflammatory phase of healing causes the influx of more inflammatory mediators which in turn delays the healing process. Conversely, formation of new vessels is considered essential in the proliferative phase of healing.[39]
AG is regarded as a selective iNOS inhibitor, but in the presence of calcium, calmodulin and other cofactors, it can also inhibit endothelial and neuronal isoforms of NOS which are responsible for beneficial effects such as bone loss prevention. It seems that many harmful effects observed as a consequence of NOS inhibition could be connected to improper dose prescription. Leitao et al, showed that AG at 5 and 10 mg/kg doses could significantly reduce bone loss in experimental models of rat with induced periodontitis lesions, while 100 mg/kg dose could not inhibit alveolar bone loss or local inflammatory changes.[22] This finding could be attributed to inhibition of physiologic NOS when AG is used in high concentrations. In order to achieve the desired effects, a continuous, low-dose administration of NOS inhibitors may be more beneficial than bolus high-dose administrations.[40]
In conclusion, the present study provides in vivo evidence implicating a role for NO in the healing process in the periapical area. The results of this study support the use of iNOS selective inhibitors such as AG to promote healing in periapical lesions [Figure 1]. Considering the facts that NO has a wide range of biological functions, AG can have other systemic effects, and there have been limited studies in dentistry on the processes mediated by AG and NO, further research is required to comprehensively evaluate the role of AG in healing process. Future research can focus on different concentrations of AG and its mechanism of action. In addition, the effects of iNOS selective promoters and other iNOS selective inhibitors on the healing process should be evaluated.
Figure 1.
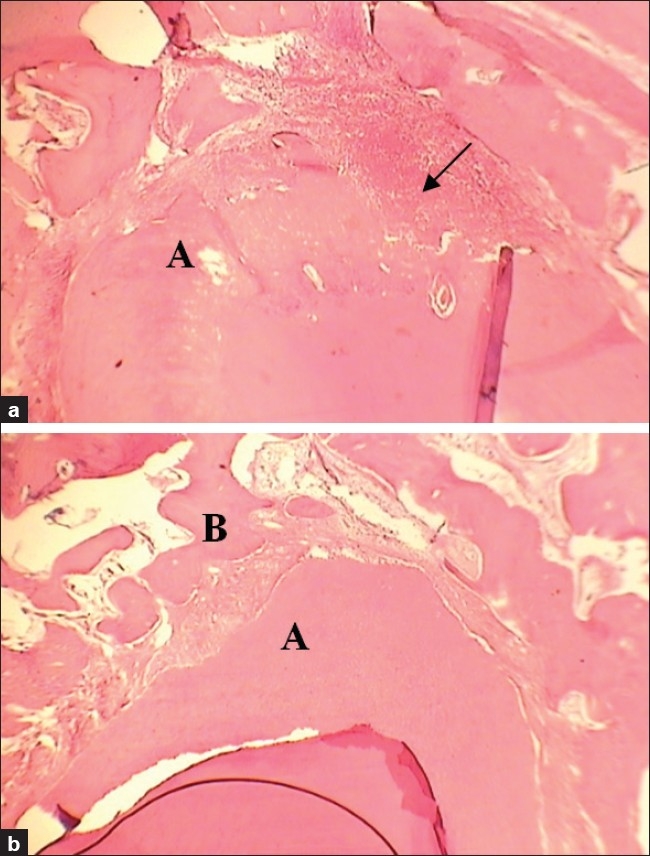
(a) Histological view of the control group showing severe infiltration of inflammatory cells and apical resorption (arrow) corresponding to healing score 1.00 (H and E: ×100). (b) Histological view of the AG group showing minimal inflammatory cells, bone formation, and fibrous connective tissue formation corresponding to healing score 3.00 (H and E: ×100). A: Apex B: Bone
Footnotes
Source of Support: Isfahan University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Sundqvist G. Ecology of the root canal flora. J Endod. 1992;18:427–30. doi: 10.1016/S0099-2399(06)80842-3. [DOI] [PubMed] [Google Scholar]
- 2.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 3.Catto ME. Healing (repair) and hypertrophy. In: Anderson, editor. In: Muir Text Book of Pathology. London: Edward Arnold Ltd; 1980. pp. 77–87. [Google Scholar]
- 4.Seltzer S. Endodontology biologic considerations in endodontic procedures. 2nd ed. Philadelphia: Lea and Febiger; 1988. pp. 389–438. [Google Scholar]
- 5.Chartrain NA, Geller DA, Koty PP, Sitrin NF, Nussler AK, Hoffman EP, et al. Molecular cloning, structure, and chromosomal localization of the human inducible nitric oxide synthase gene. J Biol Chem. 1994;269:6765–72. [PubMed] [Google Scholar]
- 6.Snyder SH, Bredt DS. Biological roles of nitric oxide. (74-7).Sci Am. 1992;266:68–71. doi: 10.1038/scientificamerican0592-68. [DOI] [PubMed] [Google Scholar]
- 7.Yasuhara R, Suzawa T, Miyamoto Y, Wang X, Takami M, Yamada A, et al. Nitric oxide in pulp cell growth, differentiation, and mineralization. J Dent Res. 2007;86:163–8. doi: 10.1177/154405910708600211. [DOI] [PubMed] [Google Scholar]
- 8.Tunbridge AJ, Stevanin TM, Lee M, Marriott HM, Moir JW, Read RC, et al. Inhibition of macrophage apoptosis by Neisseria meningitides requires nitric oxide detoxification mechanisms. Infect Immun. 2006;74:729–33. doi: 10.1128/IAI.74.1.729-733.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: Effect on HIF1 alpha. Science. 2003;302:1975–8. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 10.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, et al. Mitochondrial biogenesis in mammals: The role of endogenous nitric oxide. Science. 2003;299:896–9. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 11.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036–44. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlassara H. Recent progress on the biologic and clinical significance of advanced glycosylation end products. J Lab Clin Med. 1994;124:19–30. [PubMed] [Google Scholar]
- 13.Sessa A, Perin A. Diamine oxidase in relation to diamine and polyamine metabolism. Agents Actions. 1994;43:69–77. doi: 10.1007/BF02005768. [DOI] [PubMed] [Google Scholar]
- 14.Corbett JA, Tilton RG, Chang K, Hasan KS, Ido Y, Wang JL, et al. Aminoguanidine: A novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992;41:552–6. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- 15.Misko TP, Moore WM, Kasten TP, Nickols GA, Corbett JA, Tilton RG, et al. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993;233:119–25. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- 16.Corbett JA, McDaniel ML. The use of Aminoguanidine: A selective iNOS inhibitor, to evaluate the role of nitric oxide in the development of autoimmune diabetes. Methods. 1996;10:21–30. doi: 10.1006/meth.1996.0074. [DOI] [PubMed] [Google Scholar]
- 17.Torabinejad M, Bakland LK. An animal model for the study of immunopathogenesis of periapical lesions. J Endod. 1978;4:273–7. doi: 10.1016/S0099-2399(78)80143-5. [DOI] [PubMed] [Google Scholar]
- 18.Leonardo MR, da Silva LA, Leonardo Rde T, Utrilla LS, Assed S. Histological evaluation of therapy using a calcium hydroxide dressing for teeth with incompletely formed apices and periapical lesions. J Endod. 1993;19:348–52. doi: 10.1016/s0099-2399(06)81361-0. [DOI] [PubMed] [Google Scholar]
- 19.Collin-Osdoby P, Rothe L, Bekker S, Anderson F, Osdoby P. Decreased nitric oxide levels stimulate osteoclastogenesis and bone resorption both in vitro and in vivo on the chick chorioallantoic membrane in association with neoangiogenesis. J Bone Miner Res. 2000;15:474–88. doi: 10.1359/jbmr.2000.15.3.474. [DOI] [PubMed] [Google Scholar]
- 20.Hirose M, Ishihara K, Saito A, Nakagawa T, Yamada S, Okuda K. Expression of cytokines and inducible nitric oxide synthase in inflamed gingival tissue. J Periodontol. 2001;72:590–7. doi: 10.1902/jop.2001.72.5.590. [DOI] [PubMed] [Google Scholar]
- 21.Gaspirc B, Masera A, Skaleric U. Immunolocalization of inducible nitric oxide synthase in localized juvenile periodontitis patients. Connect Tissue Res. 2002;43:413–8. doi: 10.1080/03008200290000628. [DOI] [PubMed] [Google Scholar]
- 22.Leitao RF, Ribeiro RA, Chaves HV, Rocha FA, Lima V, Brito GA. Nitric oxide synthase inhibition prevents alveolar bone resorption in experimental periodontitis in rats. J Periodontol. 2005;76:956–63. doi: 10.1902/jop.2005.76.6.956. [DOI] [PubMed] [Google Scholar]
- 23.Kawanishi HN, Kawashima N, Suzuki N, Suda H, Takagi M. Effects of an inducible nitric oxide synthase inhibitor on experimentally induced rat pulpitis. Eur J Oral Sci. 2004;112:332–7. doi: 10.1111/j.1600-0722.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 24.Lin SK, Kok SH, Lin LD, Wang CC, Kuo MY, Lin CT, et al. Nitric Oxide promotes the progression of periapical lesion via inducing macrophage and osteoblast apoptosis. Oral Microbiol Immunol. 2007;22:24–9. doi: 10.1111/j.1399-302X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- 25.da Silva LP, Issa JP, Del Bel EA. Action of nitric oxide on healthy and inflamed hman dental pulp tissue. Micron. 2008;39:797–801. doi: 10.1016/j.micron.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Law AS, Baumgardner KR, Meller ST, Gebhart GF. Localization and changes in NADPH-diaphorase reactivity and nitric oxide synthase immunoreactivity on rat pulp following tooth preparation. J Dent Res. 1999;78:1585–95. doi: 10.1177/00220345990780100301. [DOI] [PubMed] [Google Scholar]
- 27.Fan W, Huang F, Li C, Qu H, Gao Z, Leng S, et al. Involvement of NOS/NO in the development of chronic dental inflammatory pain in rats. Brain Res Rev. 2009;59:324–32. doi: 10.1016/j.brainresrev.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Di Paola R, Marzocco S, Mazzon E, Dattola F, Rotondo F, Britti D, et al. Effect of aminoguanidine in ligature-induced periodontitis in rats. J Dent Res. 2004;83:343–8. doi: 10.1177/154405910408300414. [DOI] [PubMed] [Google Scholar]
- 29.Hama S, Takeichi O, Saito I, Ito K. Involvement of inducible nitric oxide synthase and receptor for advanced glycation end products in periapical granulomas. J Endod. 2007;33:137–41. doi: 10.1016/j.joen.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Farhad AR, Razavi S, Jahadi S, Saatchi M. Use of aminoguanidine: A selective inducible nitric oxide synthase inhibitor, to evaluate the role of nitric oxide in periapical inflammation. J Oral Sci. 2011;53:225–30. doi: 10.2334/josnusd.53.225. [DOI] [PubMed] [Google Scholar]
- 31.Wang PC, Zhang J, Zhang ZY, Tong TJ. Aminoguanidine delays the replicative senescence of human diploid fibroblasts. Chin Med J (Engl) 2007;120:2028–35. [PubMed] [Google Scholar]
- 32.Fujimoto D, Tarniya N. Incorporation of 18O from air into hydroxyproline by chick embryo. Biochem J. 1962;84:333–5. doi: 10.1042/bj0840333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shukla A, Rasik AM, Shankar R. Nitric oxide inhibits wounds collagen synthesis. Mol Cell Biochem. 1999;200:27–33. doi: 10.1023/a:1006977513146. [DOI] [PubMed] [Google Scholar]
- 34.Lancaster JR, Jr, Hibbs JB., Jr EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc Natl Acad Sci USA. 1990;87:1223–7. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaffer MR, Tantry U, Gross SS, Wasserburg HL, Barbul A. Nitric oxide regulates wound healing. J Surg Res. 1996;63:237–40. doi: 10.1006/jsre.1996.0254. [DOI] [PubMed] [Google Scholar]
- 36.Frank S, Kämpfer H, Wetzler C, Pfeilschifter J. Nitric oxide drives skin repair: Novel functions of an established mediator. Kidney Int. 2002;61:882–8. doi: 10.1046/j.1523-1755.2002.00237.x. [DOI] [PubMed] [Google Scholar]
- 37.Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, et al. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest. 1997;99:2625–34. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong M, Elson G, Legarda D, Leibovich SJ. Production of vascular endothelial growth factor by murine macrophages: Regulation by hypoxia, lactate, and the inducible nitric oxide synthase pathway. Am J Pathol. 1998;153:587–98. doi: 10.1016/S0002-9440(10)65601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwentker A, Billiar TR. Inducible nitric oxide synthase: From cloning to therapeutic applications. World J Surg. 2002;26:772–8. doi: 10.1007/s00268-002-4051-7. [DOI] [PubMed] [Google Scholar]
- 40.Kirkebøen KA, Strand OA. The role of nitric oxide in sepsis: An overview. Acta Anaesthesiol Scand. 1999;43:275–88. doi: 10.1034/j.1399-6576.1999.430307.x. [DOI] [PubMed] [Google Scholar]


