Abstract
BACKGROUND AND PURPOSE
Glucagon-like peptide-1 (GLP-1) receptors are widely expressed in neural tissues and diminish neuronal degeneration or induce neuronal differentiation. The aim of this study was to investigate the effect of the GLP-1 pathway on peripheral nerves in streptozotocin-induced diabetic rats.
EXPERIMENTAL APPROACH
Diabetic and nondiabetic rats were treated with the GLP-1 receptor agonist, synthetic exendin-4 (i.p., 1 nmol·kg−1·day−1) or placebo for 24 weeks, and current perception threshold values, cAMP levels and nerve fibre size in the sciatic nerve were measured. We also investigated GLP-1 receptor expression, quantitative changes in PGP9.5-positive intraepidermal nerve fibres and cleaved caspase 3–stained Schwann cells by immunohistochemistry.
KEY RESULTS
GLP-1 receptor expression was detected in the sciatic nerve and skin. After exendin-4 treatment, the increase seen in current perception threshold values at 2000 and 250 Hz in diabetic rats was reduced. Also, the decrease in myelinated fibre size or axon/fibre area ratio in the sciatic nerve and the loss of intraepidermal nerve fibre in the skin of diabetic rats were ameliorated. These responses were closely associated with the attenuation of Schwann cell apoptosis and improvement in the cAMP level in exendin-4-treated diabetic rats, compared with placebo-treated animals.
CONCLUSION AND IMPLICATIONS
Synthetic exendin-4 may prevent peripheral nerve degeneration induced by diabetes in an animal model, supporting the hypothesis that GLP-1 may be useful in peripheral neuropathy. The neuroprotection is probably attributable to GLP-1 receptor activation, antiapoptotic effects and restoration of cAMP content.
Keywords: apoptosis, cAMP, diabetic neuropathy, exendin-4, GLP-1 pathway
Introduction
Glucagon-like peptide-1 (GLP-1) is secreted from enteroendocrine L cells in response to digestion of food and exhibits insulinotropic and beta-cell-proliferating effects (Drucker, 2003). However, its potential as a therapy in diabetic subjects is limited by its short biological half-life because of degradation mediated by dipeptidyl peptidase IV (DPP-IV). Exendin-4, which displays ligand-binding affinity to the GLP-1 receptor (nomenclature follows Alexander et al., 2009) and acts as an agonist, is more useful for treating diabetes because of its resistance to degradation by DPP-IV (Thorens et al., 1993; Parkes et al., 2001). Some studies have suggested that exendin-4 or exenatide (synthetic exendin-4) prevents beta-cell failure, stimulates insulin release, reduces food intake and body weight and improves glycaemic control and metabolic anomalies in humans and rodents with diabetes mellitus (Egan et al., 2003; Verspohl, 2009). By modulating the GLP-1 receptor, treatments with 1 nmol·kg−1 exendin-4 and 0.05 µg·kg−1 exendin-4 analog (E4a) are reported to ameliorate diabetic nephropathy and retinopathy, two microvascular complications that are commonly found in diabetes along with diabetic neuropathy (Park et al., 2007; Zhang et al., 2009). The close relationship of diabetic microvascular complications and neuropathy suggested to us the possibility that exendin-4 could also provide neuroprotective effects (Girach and Vignati, 2006).
GLP-1 receptors are expressed widely in the central and peripheral nervous systems, and evidence is accumulating that they are active (Goke et al., 1995; Nakagawa et al., 2004). GLP-1 and exendin-4 mediate differentiation in PC12 cells and protect neurons from damage in vitro, through the activation of the GLP-1 receptor and a cascade involving cAMP (Perry et al., 2002a,b;). GLP-1 and a receptor agonist offer protective effects in neuronal cells in addition to in insulin-secreting cells, by coupling to trophic and antiapoptotic signalling pathways (Perry et al., 2002a; 2003; Li et al., 2003). Some studies indicate that intracerebroventricular (i.c.v.) injection of GLP-1 and infusion of exendin-4 attenuate the neuronal apoptosis and nerve injury induced by kainate (During et al., 2003) and pyridoxine (Perry et al., 2007) in vivo. GLP-1 or exendin-4 also retarded neuron degeneration and stimulated cell proliferation by attenuating the toxicity of amyloid-β and oxidative challenge, findings that reinforce the potential value of incretins in treating Parkinson's disease and Alzheimer's disease (Perry et al., 2003; Li et al., 2009a,b; 2010;). We recently suggested that a DPP-IV inhibitor might prevent peripheral nerve degeneration in an induced diabetes model by enhancing the endogenous levels of GLP-1 (Jin et al., 2009). Therefore, GLP-1-receptor activation mediated by GLP-1 or exendin-4 has been implicated in several processes including the mediation of cyclic AMP, the action of neurotrophic factors and the antiapoptosis pathway in a variety of nerve insults. The objective of the present study was to clarify whether synthetic exendin-4 had a therapeutic effect against a model of diabetic peripheral neuropathy and to investigate the possible mechanism(s), in streptozotocin-induced diabetic rats.
Methods
Animals and study design
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of the Chonbuk National University Medical School. Male Sprague-Dawley rats weighing 240 to 260 g each were housed in a 12 h light/dark room, at a constant temperature of 24°C, with food and water available ab libitum. Diabetes was induced by a single intraperitoneal injection of streptozotocin (Sigma, St. Louis, MO, USA; 60 mg·kg−1 body weight) dissolved in 0.1 mol·L−1 citrate buffer (pH 4.5). Age-matched control rats were injected with an equal volume of vehicle (sodium citrate buffer). Diabetes was verified 48 h later by evaluating tail vein blood glucose levels by Precision Xtra Plus (Abbot Laboratories, MediSence Products, Bedford, MA, USA). Rats with a blood glucose level higher than 3 mg·mL−1 after overnight fasting were studied. Two weeks after the verification of diabetes (designated as week 0), either normal or diabetic rats were randomly divided into two groups (six to eight per group). Synthetic exendin-4 (exenatide; Amylin Pharmaceuticals, San Diego, CA, USA) was dissolved in normal saline and administered intraperitoneally at 1 nmol·kg−1. Normal and diabetic rats were treated with the compound or vehicle once a day for 24 weeks.
Body weight, blood glucose, oral glucose tolerance test (OGTT), insulin level and HbA1c measurements
Body weight and tail blood glucose were measured after overnight fasting every 4 weeks. In week 24, following overnight fasting, the rats received 2 g·kg−1 body weight of dextrose by gastric gavage, and blood glucose was assayed at hour 0, 0.5, 1, 2, 3 during an OGTT. At hour 0, 1 and 3, blood was sampled from the tail vein and serum insulin levels were measured using an ELISA (enzyme-linked immunosorbent assay) kit (Linco Research, St. Charles, MO, USA). HbA1c was measured by a NycoCard READER II by boronate affinity assay in weeks 12 and 24.
Current perception threshold measurement
The current perception threshold was examined as reported previously (Liu et al., 2010), to quantify nerve dysfunction in weeks 0, 8, 16 and 24. Sine-waves were delivered by Neurometer CPT/C (Neurotron Inc., Baltimore, MD, USA), with stimuli intensities for 2000, 250 and 5 HZ increased 40, 20 and 10 µA per second respectively. The current perception threshold was defined as the minimum intensity value that caused a lift in the hind paw, or appearance of vocalization or agitation.
Radioimmunoassay of cAMP concentration in sciatic nerve
All the rats were killed 4 h after administration of exendin-4 or placebo in week 24, and the left sciatic nerves were immediately dissected, frozen in liquid nitrogen and kept at −80°C until assay. For measurement of cAMP concentration, 25 mg tissue was minced in 0.5 mL ice-cold trichloroacetic acid (6%) and homogenized at 4°C in a Polytron homogenizer (Brinkman, Westbury, NY, USA). The homogenate was centrifuged at 1000×g for 10 min at 4°C, and the supernatant extracted with water-saturated ether three times and dried using a Speedvac concentrator (Savant, Farmingdale, NY, USA). The pellet was treated with 0.5 mL NaOH (1 N), ultrasonicated and used for protein determination (Bradford method). The cAMP levels were measured by equilibrated radioimmunoassay as described previously (Park et al., 2002). Briefly, standards or samples were taken up in a final volume of 100 µL of 50 mM sodium acetate buffer (pH 4.8) containing theophylline (8 mM), and 100 µL of diluted cAMP antiserum (Calbiochem-Novabiochem, San Diego, CA, USA) and iodinated 2′-0-monosuccinyl-adenosine 3′,5′-cyclic monophosphate tyrosyl methyl ester ([125I]-ScAMP-TME, 10 000 cpm per 100 µL) were added. [125I]-ScAMP-TME was prepared as described previously (Cui et al., 2000), by the Department of Physiology, Chonbuk National University. Samples were then incubated for 24 h at 4°C. For the acetylation reaction, 5 µL of a mixture of acetic anhydride and triethylamine (1:2 dilution) were added to the assay tube before antiserum and tracer were also added. Bound form was separated from the free form by charcoal suspension, and the supernatant was counted in a gamma counter (Packard Instrument; Meriden, CT, USA). The cAMP concentration was expressed as fmol·mg−1 extracted protein.
Histopathology
Skin biopsies of the hind left index, left middle, right index and right middle toe were performed in weeks 0, 8, 16 and 24 respectively. After death, segments of right sciatic nerve and skin samples of the hind left dorsum were obtained. Each sciatic nerve sample (separated into three segments for staining with toluidine blue, anti-cleaved caspase 3 and anti-GLP-1 receptor) and skin tissue [separated into two samples for staining with anti-protein gene product 9.5 (PGP9.5) and anti-GLP-1 receptor] was subjected to morphometric or immunohistochemical analysis. One segment of sciatic nerve tissue was post-fixed overnight in 4% paraformaldehyde prior to embedding in JB-4. A 1.5 µm transverse section was cut on a Leica RM2165 microtome (Leica Microsystems, Inc., Bannockburn, IL, USA) and stained with toluidine blue.
For fluorescence immunohistochemistry using antibody against the GLP-1 receptor, samples were frozen in liquid nitrogen and cut into 10 µm sections with a Microm HM525 cryostat (Microm, Walldorf, Germany) before fixing with 4% paraformaldehyde at room temperature for 20 min and staining as described below. For staining using antibodies against PGP9.5 and cleaved caspase 3, skin and sciatic nerve samples were fixed with PLP (2% paraformaldehyde, 0.075 M lysine, 0.05 M phosphate buffer pH 7.4, 0.01 M sodium meta-periodate) solution for 12 h. After thorough rinsing in PBS, and successive transfer to 20% and 30% glycerol in 0.1 M phosphate buffer for 12 h each at 4°C, all tissues were cryoprotected with Tissue-tec O.C.T. compound (Miles, Elkhart, IN, USA). Skin sections of 40 µm and sciatic nerve sections of 10 µm were cut with a sliding microtome. Sciatic nerve segments used for immunohistochemistry were sectioned longitudinally, and skin sections were perpendicular to the epidermal layer. Following washes, the preparations were blocked in serum-free protein block (Dako, Carpinteria, CA, USA) supplemented with 1% donkey serum for 1 h on a shaker table at room temperature. Then sections were incubated with rabbit anti-PGP 9.5 (1:800; Abd Serotec, Oxford, UK), anti-cleaved caspase 3 (1:150; Chemicon International, Inc., Temecula, CA, USA) or anti-GLP-1 receptor (1:150; Abcam, Inc., Cambridge, MA, USA) at 4°C overnight in Antibody Diluents (Dako) supplemented with 0.5% donkey serum and 0.25% Triton-X. The non-immune serum was used as a control. After complete washing, immunoreactivity was visualized with donkey anti-rabbit Alexa Fluor (488) (1:500; Invitrogen, Lidingo, Sweden) in a dark room. The anti-cleaved caspase 3 labelled section was subsequently counterstained with DAPI. After washing with PBS as above, sections were mounted with fluorescent mounting medium (Dako). Images were collected and analyzed using a Carl Zeiss Axioskop2 plus microscope (Carl Zeiss, Goettingen, Germany) and Axiovision 5.1 program.
Quantifications of myelinated fibre, apoptotic Schwann cell and innervation
Photomicrographs of myelinated fibre, cleaved caspase 3–stained Schwann cells and intraepidermal nerve fibre were captured using a digital camera (Axiocam HRC, Carl Zeiss) with a final magnifications of 400× for myelinated fibres and Schwann cells, and 100× for nerve fibre. In sciatic nerve, myelinated fibre or axonal area represented by the outer or inner border of myelin sheath was measured with analySIS image software (Soft Imaging Systems GmbH, Munster, Germany), and the axon/fibre area ratio was determined. Fibre number per square millimetre (fibre density) and the fraction of endoneurial area occupied by myelinated fibres (fibre occupancy) were calculated. All cleaved caspase 3–stained cells with an elongated nucleus located within the basal lamina in the sciatic nerve are reported to be Schwann cells (Saito et al., 2009). These were double stained by S-100 in our preliminary experiments. In determining apoptotic Schwann cells, when the nuclear shape or location was not clear, the cell was not counted. In the endoneurial space, Schwann cells exhibiting cleaved caspase 3 immunoreactivity in the nucleus were calculated as percent of the total number of DAPI-stained cells using Image J software (ImageJ, U. S. National Institutes of Health, Bethesda, MD, USA). In the skin, intraepidermal PGP9.5-positive nerve fibres per unit length of epidermis (mm) was used to determine the cutaneous innervation (Hirai et al., 2000). From each tissue, 12 random sections and two photomicrographs per section were counted and measured by two independent investigators without knowledge of the treatments.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM), and one-way analysis of variance (anova) with Duncan's post hoc test was used for comparisons between experimental groups. Data were considered statistically significant if P < 0.05. Statistical analysis was performed using the SPSS 12.0 software (SPSS Inc., Chicago, IL, USA).
Results
Effects on food intake, body weight, blood glucose, OGTT, HbA1c and serum insulin levels
The average food intake in the exendin-4-treated diabetic group over the 24 weeks was reduced by 16.7% compared to the vehicle-treated group (P < 0.05, Table 1). No significant difference was observed between the treated and non-treated normal groups. In weeks 12 and 24, exendin-4 treatment tended to decrease the body weight, fasting blood glucose and HbA1c levels in both normal and diabetic rats, although the differences were not significant. Glucose level was lower in the exendin-4 treated diabetic rats than in non-treated diabetic rats during the OGTT at the 1 h time point, but not at other times (P < 0.05, Figure 1). No differences were seen between normal groups throughout the OGTT, and administration of exendin-4 did not change the serum insulin level.
Table 1.
Effects of exendin-4 on food intake, body weight, blood glucose, HbA1c, and insulin level in nondiabetic and diabetic rats (n = 6–8)
| Food intake (g·day−1) | Body weight (g) | Blood glucose (mg·mL−1) | HbA1c level (%) | Insulin level ng·mL−1 | |||
|---|---|---|---|---|---|---|---|
| Groups | 24 weeks | Week 0 | Week 24 | Week 0 | Week 24 | Week 24 | Week 24 |
| NOR | 22.8 ± 1.8 | 347 ± 3 | 627 ± 16 | 0.91 ± 0.05 | 0.96 ± 0.03 | 3.53 ± 0.07 | 4.18 ± 0.28 |
| NOR + EXE-4 | 22.2 ± 1.0 | 348 ± 2 | 617 ± 7 | 0.89 ± 0.04 | 0.87 ± 0.04 | 3.27 ± 0.15 | 4.66 ± 0.12 |
| DM | 46.7 ± 1.5## | 252 ± 8## | 269 ± 12## | 3.87 ± 0.13## | 4.15 ± 0.27## | 8.08 ± 0.24## | 0.26 ± 0.02## |
| DM + EXE-4 | 40.0 ± 1.3* | 253 ± 8 | 254 ± 7 | 3.95 ± 0.08 | 3.82 ± 0.13 | 7.84 ± 0.22 | 0.24 ± 0.05 |
Average food intake over 24 weeks, body weight, fasting blood glucose, HbA1c and insulin level (at hour 3 during oral glucose tolerance test) in week 0 or 24 for each group are expressed as mean ± SEM.
P < 0.01 DM versus NOR
P < 0.05 DM + EXE-4 versus DM.
NOR = vehicle-treated nondiabetic group; DM = -vehicle-treated diabetic group; EXE-4 = treated with 1 nmol·kg−1·day−1 exendin-4.
Figure 1.

Changes in oral glucose tolerance test (OGTT) in nondiabetic and diabetic rats with or without exendin-4. Data are expressed as mean ± SEM for six to eight animals. NOR = vehicle-treated nondiabetic group; DM = vehicle-treated diabetic group; EXE-4 = treated with 1 nmol·kg−1·day−1 exendin-4. ##P < 0.01 vs. nondiabetic rats; *P < 0.05 versus vehicle treated diabetic rats.
Effect on current perception threshold
In our study, the current perception threshold value in week 0 was considered to be the normal value. In the vehicle-treated diabetic group, thresholds at 2000, 250 and 5 Hz showed a trend towards an increase, from week 16, and a significant change at 2000 Hz was seen in week 24, compared with week 0 (P < 0.05). Intraperitoneal administration of 1 nmol·kg−1 exendin-4 significantly decreased the thresholds at 2000 Hz in weeks 16 and 24 (P < 0.05 and P < 0.05), and at 250 Hz in week 24 (P < 0.05) (Figure 2), although differences in the threshold at 5 Hz were not significant throughout the 24 weeks (data not shown). No differences were observed between the exendin-4 treated and non-treated nondiabetic groups over the 24 weeks (data not shown). Data from normal glucose groups were not compared with diabetic groups because of the marked difference in body weight (Hoybergs et al., 2008; Liu et al., 2010).
Figure 2.

Effects of exendin-4 treatment on current perception thresholds at frequencies of 2000 and 250 HZ in diabetic rats over 24 weeks. Data are expressed as mean ± SEM. NOR = vehicle-treated nondiabetic group; DM = vehicle-treated diabetic group; EXE-4 = treated with 1 nmol·kg−1·day−1 exendin-4. *P < 0.05 vs. vehicle-treated diabetic rats; $P < 0.05 week 24 versus week 0 in vehicle-treated diabetic group.
Alterations in sciatic nerve structure
The mean myelinated fibre area and axon/fibre area ratio in the sciatic nerve were reduced by 26% and 16% in non-treated diabetic rats compared to age-matched nondiabetic rats (P < 0.01 and P < 0.05, respectively, Table 2 and Figure 3). Exendin-4 treatment significantly increased the wrinkled fibre area and the axon/fibre size ratio of diabetic rats (P < 0.05 and P < 0.05 respectively). The fibre density and fibre occupancy of diabetic groups did not differ markedly from those of nondiabetic groups. No values in the exendin-4 treated nondiabetic group were different from the placebo group.
Table 2.
Morphometric data of myelinated fibress in nondiabetic and diabetic rats that were treated with or without exendin-4
| Groups | Mean fibre area (µm2) | Axon/fibre ratio (%) | Fibre density number·(mm2)−1 | Fibre occupancy (%) |
|---|---|---|---|---|
| NOR | 69.4 ± 2.9 | 42.8 ± 1.9 | 7814 ± 203 | 60.5 ± 2.4 |
| NOR + EXE-4 | 69.9 ± 2.7 | 42.0 ± 1.1 | 8031 ± 165 | 59.2 ± 2.2 |
| DM | 51.6 ± 2.6## | 35.9 ± 0.9# | 8668 ± 146 | 58.4 ± 1.9 |
| DM + EXE-4 | 62.1 ± 2.7* | 40.3 ± 1.1* | 8260 ± 264 | 58.7 ± 2.1 |
Data are expressed as mean ± SEM NOR = vehicle-treated nondiabetic group; DM = vehicle-treated diabetic group; EXE-4 = treated with 1 nmol·kg−1·day−1 exendin-4.
P < 0.05
P < 0.01 DM versus NOR
P < 0.05 DM + EXE-4 versus DM.
Figure 3.

Morphology of sciatic nerve cross-sections stained with toluidine blue in nondiabetic (A and B) and diabetic rats (C and D) treated with (B and D) or without exendin-4 (A and C). Exendin-4 treatment prevented the reduction in calibre of myelinated fibres in diabetic rats. Bar = 20 µm.
Change of skin innervation
Immunoreactive profiles were clearly visualized in sections incubated with anti-rat PGP 9.5, anti-GLP-1 receptor or anti-caspase 3, and no clear immunoreactivity was seen in sections stained with non-immune serum. The nerve fibre densities of the hind toe were not different among the four groups in week 0 or in week 8. In weeks 16 and 24, the number of epidermal nerve fibres per mm of hind toe in the non-treated diabetic group decreased significantly compared with the nondiabetic group. However, administration of exendin-4 significantly prevented nerve fibre deficit in both week 16 (8.00 ± 0.27 vs. 6.19 ± 0.13·mm−1, P < 0.05) and week 24 (7.44 ± 0.30 vs. 5.58 ± 0.33·mm−1, P < 0.01). The nerve fibre density of the hind dorsum in the exendin-4 treated diabetic group (4.84 ± 0.37·mm−1) was significantly increased (P < 0.05) in comparison with the non-treated diabetic group (3.13 ± 0.35·mm−1) in week 24, which was in agreement with findings in the toe (Figure 4). In the exendin-4 treated and non-treated nondiabetic groups, the differences in nerve fibre densities were not significant over the 24 weeks.
Figure 4.

(A) Representative microphotographs of PGP9.5-immunoreactive intraepidermal nerve fibres in the dorsum and toes of nondiabetic and diabetic rats with or without exendin-4. Skin 40-µm sections were collected in week 24 from six to eight rats per group. (B and C) Bar and line graphs of epidermal innervation in the dorsum (B) and toe (C) in weeks 0, 8, 16 and/or 24. NOR = vehicle-treated nondiabetic group; DM = vehicle-treated diabetic group; EXE-4 = treated with 1 nmol·kg−1·day−1 exendin-4. #P < 0.05 DM versus NOR; *P < 0.05 and **P < 0.01 DM + EXE-4 versus DM. Bar = 100 µm.
GLP-1 receptor expression in the skin and sciatic nerve
We focused on changes in intraepidermal nerve fibre and sciatic nerve in our study, so GLP-1 receptor expression in these tissues was investigated. As shown in Figure 5, no obvious signal was visible in the skin or sciatic nerve with the negative control non-immune serum. In all four experimental groups, the GLP-1 receptor was detectable in skin, especially in the epidermis and around the hair follicles. The receptor also was apparent in the sciatic nerve and perhaps appeared to be predominantly associated with the nerve fibre. These findings suggested a possible neuroprotective effect of the GLP-1 receptor on the sciatic nerve and intraepidermal nerve fibres.
Figure 5.

(A and C) Expression of GLP-1 receptors in sciatic nerve and skin in week 24. GLP-1 receptor was expressed predominantly in epidermis (arrowhead) and around skin hair follicles (arrow) of the skin. (B and D) No immunoreactivity was observed in the non-immune serum controls for sciatic nerve and skin. Sections of 10 µm were stained with rabbit anti-GLP-1 receptor or normal rabbit serum. Bar = 100 µm.
Assessment of Schwann cell apoptosis in sciatic nerve
Schwann cells have a remarkable potential for axonal regeneration and neurotrophic support in the peripheral nerve, so the number of cleaved caspase 3–stained Schwann cells was studied (Bunge, 1994). Positive Schwann cells, expressed as a percent of the total number of DAPI-stained cells, were significantly increased in diabetic rats compared with the nondiabetic rats (P < 0.05). However, the increase was decreased by 31% after exendin-4 treatment (P < 0.05, Figure 6).
Figure 6.
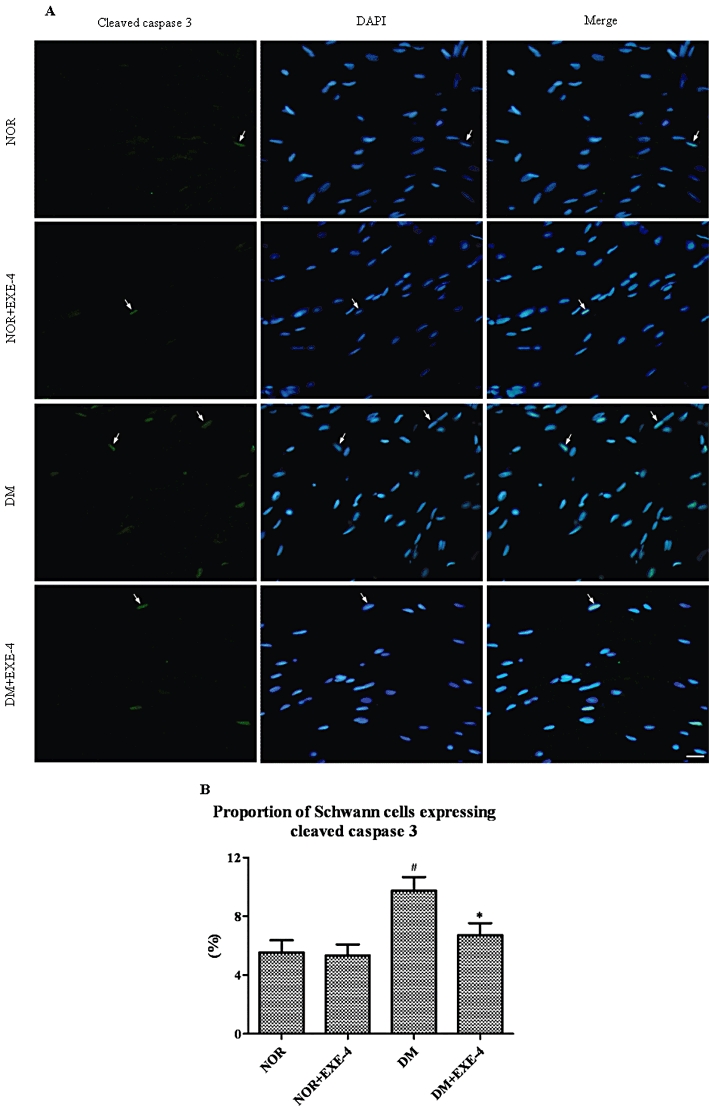
(A) Double-labelling fluorescence of sciatic nerves in nondiabetic and diabetic rats with or without exendin-4. Arrows indicate the nuclei of apoptotic Schwann cells that were dual-stained by anti-cleaved caspase 3 (green) and DAPI (blue-white). (B) Bar graph shows cleaved caspase 3-stained Schwann cells as a percentage of total number of DAPI-stained cells. NOR = vehicle treated nondiabetic group; DM = vehicle-treated diabetic group; EXE-4 = treated with 1 nmol·kg−1·day−1 exendin-4. #P < 0.05 DM versus NOR; *P < 0.05 DM + EXE-4 versus DM. Bar = 20 µm.
Effect on cAMP content in sciatic nerve
The cAMP levels in the sciatic nerves of vehicle-treated diabetic rats was reduced by 27% at 4 h after administration of exendin-4 compared with age-matched nondiabetic rats (P < 0.05, Figure 7). Intraperitoneal administration of 1 nmol·kg−1 exendin-4 completely restored the cAMP levels of diabetic rats.
Figure 7.
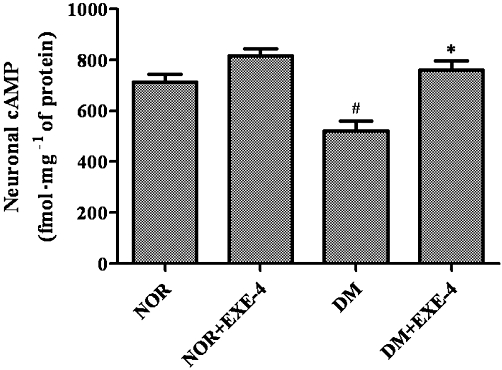
Effects of exendin-4 treatment on cAMP levels in sciatic nerves of nondiabetic and diabetic rats. Data are expressed as mean ± SEM. NOR = vehicle-treated nondiabetic group; DM = vehicle-treated diabetic group; EXE-4 = treated with 1 nmol·kg−1·day−1 exendin-4. #P < 0.05 DM versus NOR; *P < 0.05 DM + EXE-4 versus DM.
Discussion and conclusions
In our study, the insulin content, although at a low level, did not significantly change along with glucose uptake during OGTT in diabetic rat in week 24 (0.35 ± 0.06 ng·mL−1 at hour 1 vs. 0.26 ± 0.02 ng·mL−1 at hour 3, P = 0.12), which was different from nondiabetic rat (7.28 ± 0.56 ng·mL−1 at hour 1 vs. 4.18 ± 0.28 ng·mL−1 at hour 3, P = 0.008). These findings suggest that streptozotocin-induced diabetes resembles type 1 or late-stage type 2 diabetes in our study. In this state, there is a therapeutic limitation in glucose control by monotherapy with exendin-4 or another incretin-based agent and increased insulin secretion is unlikely. These results are parallel to previous studies on streptozotocin-induced diabetic rats or long-standing type 1 diabetes (even by multiple daily injection of lower-dose synthetic exendin-4) (Villanueva-Penacarrillo et al., 2001; Jin et al., 2009; Rother et al., 2009). Incretins possibly offer lesser effects on expanding beta-cell mass, modulating pathogenetic autoimmune and suppressing postprandial hyperglycaemia (only at 1 h time point during OGTT) in the models with almost whole pancreas destruction than that with autoimmune insulin-dependent diabetes mellitus (IDDM) (Bosi, 2010; Raman et al., 2010). Therefore, in our study, the neuroprotection provided by exendin-4 is likely not to be attributable to the glucose-lowering effect on peripheral nerves. Significant differences following exendin-4 treatment were not observed in the decrease of body weights in any of the rats and food intake in nondiabetic rodents could be due to the fact that the duration of exendin-4 administration differed from previous study (Mack et al., 2006).
The morphological characteristics of diabetic neuropathy are primarily a loss of nerve fibres, so assessing cutaneous innervation is considered to be a reliable means of diagnosing and staging diabetic neuropathy (Hirai et al., 2000; Beiswenger et al., 2008). Therefore, we quantified the density of intraepidermal nerve fibres as detected by a neuronal marker, PGP9.5. In our 24 week study, the attenuation of cutaneous nerve fibre damage in diabetic rats suggests that synthetic exendin-4 exhibits beneficial neuroprotective properties that partly prevent development of diabetic neuropathy. Another characteristic change of neurodegeneration is the atrophy of myelinated fibres associated with a reduction in axonal size in the peripheral nerves (Jakobsen, 1979; Yagihashi, 1997). Diabetes-induced atrophy of myelinated fibres, especially in axons, may result from abnormalities of neurofilaments, including impaired supply, decreased axonal flow and reduced synthesis (Medori et al., 1985; Yagihashi et al., 1990). In our study, evidence of fibre atrophy in the sciatic nerve of non-treated diabetic rats was reflected in a decreased mean fibre area. A reduction in axon/ fibre size ratio, to some extent, suggests axonal atrophy, although this requires further analysis of the relationship between axonal size and myelin spiral length by electron microscopy, a sensitive method of detecting axonal atrophy (O'Neill and Gilliatt, 1987). Exendin-4 treatment attenuated the morphometric abnormalities of myelinated nerve fibres, probably through the protection of axonal neurofilaments, for example by preventing neurofilament loss. Current perception threshold is a sensitive and convenient indicator of early diabetic neuropathy and has been used in clinical and animal studies to assess nerve dysfunction in diabetes (Ionescu-Tirgoviste et al., 1987; Jin et al., 2009). Our data (Figure 2) implied that therapy with exendin-4 could alleviate hypoesthesia, as characterized by enhanced current perception threshold, which might be a sign of sensory loss.
GLP-1 or exendin-4 increased viability in GLP-1 receptor over-expressing SH-SY5Y cells, whereas it did not confer protection in cells from GLP-1 receptor knockout (–/–) mice (Li et al., 2009b; 2010;). These facts suggest that exendin-4 provides neuroprotective properties by GLP-1 receptor activation. Previous studies demonstrated that the GLP-1 receptor was found in peripheral neurons of male rats and in the epidermis or hair follicles of newborn mice (Nakagawa et al., 2004; List et al., 2006; Vahl et al., 2007). Our results confirmed that this receptor was also present in the sciatic nerve and skin of adult rats (Figure 5). This suggests that the activation of GLP-1 receptors has a crucial part in preventing sciatic nerve fibre wrinkling and protecting intraepidermal nerve fibres against loss and hypofunction. Moreover, hyperglycaemia leads to down-regulation of GLP-1 receptor expression in diabetic rodent models (Xu et al., 2007). However, this difference was not observed in our study by immunohistochemistry (data not shown). The isolation and quantification of GLP-1 receptors in sciatic nerve and skin await further study in the future. It is possible that exendin-4 offers neuroprotection by up-regulating GLP-1 receptor expression in diabetic rats, just as it functions in ameliorating other diabetic complications with same-dose peptide therapy (Park et al., 2007).
An antiapoptotic effect mediated by the GLP-1 receptor has been suggested in neural, beta, renal and retinal cells following hyperglycaemia or other insults (Li et al., 2003; 2009b; Park et al., 2007; Zhang et al., 2009). Therefore, one of the most important mechanisms underlying the neuroprotection may be coupling to antiapoptotic signalling pathways. We found many more cleaved caspase 3-stained Schwann cells in diabetic rats than in nondiabetic animals, which could contribute to impairment of axonal function and efficiency of fibre regeneration. Interestingly, the apoptotic cells were significantly decreased after treatment with 1 nmol·kg−1·day−1 synthetic exendin-4. This is likely to indicate that GLP-1 receptor signalling directly modifies the susceptibility to apoptotic injury, normalizes the calibre of myelinated nerve fibres or axons and reduces the loss of intraepidermal nerve fibres, although the mechanism by which this occurs is not understood. Similar to other neurotrophins, the neurotrophic functions of GLP-1 and exendin-4 are identified through modulation of neuronal cell survival (Perry et al., 2002a; Perry and Greig, 2003). Our data also showed that exendin-4 protected Schwann cells, which are able to promote the regeneration of axons or myelinated nerve fibres, and reduce neurofilament loss from apoptosis. Both Schwann cells and neurofilaments provide a versatile source of trophic factors for peripheral nerves (Bunge, 1994). These findings suggest that the neurotrophic signalling pathway may represent a potential target for antiapoptosis and neuroprotection mediated by a GLP-1 receptor agonist.
To further observe the actions of synthetic exendin-4 in our experiment, the pharmacokinetics was investigated using a fluorescent immunoassay kit (Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA) in a parallel group of diabetic rats in week 4. Exendin-4 reached peak plasma concentration (474 ± 59 pg·mL−1) at approximately 1 h after incretin treatment. Plasma exendin-4 had a estimated half-life (t1/2) of 1–3 h, and a certain concentration of the peptide (94 ± 37 pg·mL−1) was still maintained at 6 h; however, exendin-4 level decreased to the undetectable level at 24 h post dose in all of the treated rodents (data not shown), which was similar to the previous data from the patients (Fineman et al., 2003; Kothare et al., 2008). It is possible that the circulating exendin-4 exerts neuroprotection at least during 6 h after administration, since the peptide concentration at this time point is higher than 50 pg·mL−1, a level previously shown to possess biological activity (Taylor et al., 2005). Interestingly, we observed that the decreased cAMP level in the sciatic nerve of diabetic rats was ameliorated, 4 h after administration of exendin-4. This supports the hypothesis that the levels of cAMP, stimulated by exendin-4 (within some time after administration) binding to GLP-1 receptor mediates signal transduction involved in protection against development of diabetic neuropathy. The secondary messenger is likely to be important in regulating processes, such as antiapoptosis, that work against oxidative stress and for neurotrophic support (Yu and Jin, 2009). In addition, decreased cAMP content, which positively correlates with impaired Na+–K+–ATPase activity and contributes to the development of diabetic neuropathy, has been established in the sciatic nerve of diabetic rats. The attenuation of the lowered cAMP level following treatment with cilostazol and other agents restores functional impairment and morphological changes by modulating Na+–K+–ATPase activity (Shindo et al., 1993; Suh et al., 1999). Therefore, exendin-4 might be neuroprotective by raising cAMP levels and thus affecting metabolic disturbances mediated by a reversible defect in Na+–K+–ATPase in the peripheral nerve.
In conclusion, synthetic exendin-4 appeared to ameliorate diabetic peripheral neuropathy in skin and sciatic nerve, including functional and morphological abnormalities, probably through activating the GLP-1 receptor. The GLP-1 receptor may act through signalling pathways involved in apoptosis and cAMP to enhance neuroprotection. These findings have important implications for the GLP-1 pathway in the treatment of diabetic peripheral neuropathy.
Acknowledgments
We thank Dr Chen Zhao for the technical support and Ji Hyun Kim for the valuable assistance with histology. This work was supported in part by Chonbuk National University Hospital Research Fund 2010 and the Medical Science Education Program for the 21st Century of Chonbuk National University Medical School.
Glossary
Abbreviations
- DPP-IV
dipeptidyl peptidase-IV
- GLP-1
Glucagon-like peptide-1
- OGTT
oral glucose tolerance test
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th Edition. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiswenger KK, Calcutt NA, Mizisin AP. Epidermal nerve fiber quantification in the assessment of diabetic neuropathy. Acta Histochem. 2008;110:351–362. doi: 10.1016/j.acthis.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosi E. Time for testing incretin therapies in early type 1 diabetes? J Clin Endocrinol Metab. 2010;95:2607–2609. doi: 10.1210/jc.2009-2741. [DOI] [PubMed] [Google Scholar]
- Bunge RP. The role of the Schwann cell in trophic support and regeneration. J Neurol. 1994;242(Suppl 1):S19–S21. doi: 10.1007/BF00939235. [DOI] [PubMed] [Google Scholar]
- Cui X, Lee SJ, Kim SZ, Kim SH, Cho KW. Effects of pituitary adenylate cyclase activating polypeptide27 on cyclic AMP efflux and atrial dynamics in perfused beating atria. Eur J Pharmacol. 2000;402:129–137. doi: 10.1016/s0014-2999(00)00514-8. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Glucagon-like peptide-1 and the islet beta-cell: augmentation of cell proliferation and inhibition of apoptosis. Endocrinology. 2003;144:5145–5148. doi: 10.1210/en.2003-1147. [DOI] [PubMed] [Google Scholar]
- During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- Egan JM, Bulotta A, Hui H, Perfetti R. GLP-1 receptor agonists are growth and differentiation factors for pancreatic islet beta cells. Diabetes Metab Res Rev. 2003;19:115–123. doi: 10.1002/dmrr.357. [DOI] [PubMed] [Google Scholar]
- Fineman MS, Bicsak TA, Shen LZ, Taylor K, Gaines E, Varns A, et al. Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care. 2003;26:2370–2377. doi: 10.2337/diacare.26.8.2370. [DOI] [PubMed] [Google Scholar]
- Girach A, Vignati L. Diabetic microvascular complications – can the presence of one predict the development of another? J Diabetes Complications. 2006;20:228–237. doi: 10.1016/j.jdiacomp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7:2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Hirai A, Yasuda H, Joko M, Maeda T, Kikkawa R. Evaluation of diabetic neuropathy through the quantitation of cutaneous nerves. J Neurol Sci. 2000;172:55–62. doi: 10.1016/s0022-510x(99)00290-7. [DOI] [PubMed] [Google Scholar]
- Hoybergs YM, Biermans RL, Meert TF. The impact of bodyweight and body condition on behavioral testing for painful diabetic neuropathy in the streptozotocin rat model. Neurosci Lett. 2008;436:13–18. doi: 10.1016/j.neulet.2008.02.051. [DOI] [PubMed] [Google Scholar]
- Ionescu-Tirgoviste C, Pruna S, Bajenaru O, Cheta D, Mincu I. The perception threshold to an electric stimulus deeply applied in the lower limbs in normal and diabetic subjects. Diabetes Res Clin Pract. 1987;3:249–256. doi: 10.1016/s0168-8227(87)80048-7. [DOI] [PubMed] [Google Scholar]
- Jakobsen J. Early and preventable changes of peripheral nerve structure and function in insulin-deficient diabetic rats. J Neurol Neurosurg Psychiatry. 1979;42:509–518. doi: 10.1136/jnnp.42.6.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin HY, Liu WJ, Park JH, Baek HS, Park TS. Effect of dipeptidyl peptidase-IV (DPP-IV) inhibitor (Vildagliptin) on peripheral nerves in streptozotocin-induced diabetic rats. Arch Med Res. 2009;40:536–544. doi: 10.1016/j.arcmed.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Kothare PA, Linnebjerg H, Isaka Y, Uenaka K, Yamamura A, Yeo KP, et al. Pharmacokinetics, pharmacodynamics, tolerability, and safety of exenatide in Japanese patients with type 2 diabetes mellitus. J Clin Pharmacol. 2008;48:1389–1399. doi: 10.1177/0091270008323750. [DOI] [PubMed] [Google Scholar]
- Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem. 2003;278:471–478. doi: 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- Li Y, Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, et al. GLP-1 Receptor Stimulation Reduces Amyloid-beta Peptide Accumulation and Cytotoxicity in Cellular and Animal Models of Alzheimer's Disease. J Alzheimers Dis. 2009a;19:1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci USA. 2009b;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tweedie D, Mattson MP, Holloway HW, Greig NH. Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J Neurochem. 2010;113:1621–1631. doi: 10.1111/j.1471-4159.2010.06731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List JF, He H, Habener JF. Glucagon-like peptide-1 receptor and proglucagon expression in mouse skin. Regul Pept. 2006;134:149–157. doi: 10.1016/j.regpep.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Liu WJ, Jin HY, Park JH, Baek HS, Park TS. Effect of rimonabant, the cannabinoid CB1 receptor antagonist, on peripheral nerve in streptozotocin-induced diabetic rat. Eur J Pharmacol. 2010;637:70–76. doi: 10.1016/j.ejphar.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Mack CM, Moore CX, Jodka CM, Bhavsar S, Wilson JK, Hoyt JA, et al. Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes (Lond) 2006;30:1332–1340. doi: 10.1038/sj.ijo.0803284. [DOI] [PubMed] [Google Scholar]
- Medori R, Autilio-Gambetti L, Monaco S, Gambetti P. Experimental diabetic neuropathy: impairment of slow transport with changes in axon cross-sectional area. Proc Natl Acad Sci USA. 1985;82:7716–7720. doi: 10.1073/pnas.82.22.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, et al. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci. 2004;110:36–43. doi: 10.1016/j.autneu.2003.11.001. [DOI] [PubMed] [Google Scholar]
- O'Neill JH, Gilliatt RW. Adaptation of the myelin sheath during axonal atrophy. Acta Neuropathol. 1987;74:62–66. doi: 10.1007/BF00688339. [DOI] [PubMed] [Google Scholar]
- Park CW, Kim HW, Ko SH, Lim JH, Ryu GR, Chung HW, et al. Long-term treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J Am Soc Nephrol. 2007;18:1227–1238. doi: 10.1681/ASN.2006070778. [DOI] [PubMed] [Google Scholar]
- Park JK, Cui Y, Kim MK, Kim YG, Kim SH, Kim SZ, et al. Effects of extracorporeal shock wave lithotripsy on plasma levels of nitric oxide and cyclic nucleotides in human subjects. J Urol. 2002;168:38–42. [PubMed] [Google Scholar]
- Parkes D, Jodka C, Smith P, Nayak S, Rinehart L, Gingerich R, et al. Pharmacokinetic actions of exendin-4 in the rat: comparison with glucagon-like peptide-1. Drug Dev Res. 2001;53:260–267. [Google Scholar]
- Perry T, Greig NH. The glucagon-like peptides: a double-edged therapeutic sword? Trends Pharmacol Sci. 2003;24:377–383. doi: 10.1016/S0165-6147(03)00160-3. [DOI] [PubMed] [Google Scholar]
- Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther. 2002a;302:881–888. doi: 10.1124/jpet.102.037481. [DOI] [PubMed] [Google Scholar]
- Perry T, Lahiri DK, Chen D, Zhou J, Shaw KT, Egan JM, et al. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther. 2002b;300:958–966. doi: 10.1124/jpet.300.3.958. [DOI] [PubMed] [Google Scholar]
- Perry T, Lahiri DK, Sambamurti K, Chen D, Mattson MP, Egan JM, et al. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res. 2003;72:603–612. doi: 10.1002/jnr.10611. [DOI] [PubMed] [Google Scholar]
- Perry T, Holloway HW, Weerasuriya A, Mouton PR, Duffy K, Mattison JA, et al. Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Exp Neurol. 2007;203:293–301. doi: 10.1016/j.expneurol.2006.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman VS, Mason KJ, Rodriguez LM, Hassan K, Yu X, Bomgaars L, et al. The role of adjunctive exenatide therapy in pediatric type 1 diabetes. Diabetes Care. 2010;33:1294–1296. doi: 10.2337/dc09-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rother KI, Spain LM, Wesley RA, Digon BJ, 3rd, Baron A, Chen K, et al. Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes. Diabetes Care. 2009;32:2251–2257. doi: 10.2337/dc09-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H, Kanje M, Dahlin LB. Delayed nerve repair increases number of caspase 3 stained Schwann cells. Neurosci Lett. 2009;456:30–33. doi: 10.1016/j.neulet.2009.03.075. [DOI] [PubMed] [Google Scholar]
- Shindo H, Tawata M, Onaya T. Cyclic adenosine 3′,5′-monophosphate enhances sodium, potassium-adenosine triphosphatase activity in the sciatic nerve of streptozotocin-induced diabetic rats. Endocrinology. 1993;132:510–516. doi: 10.1210/endo.132.2.7678791. [DOI] [PubMed] [Google Scholar]
- Suh KS, Oh SJ, Woo JT, Kim SW, Yang IM, Kim JW, et al. Effect of cilostazol on the neuropathies of streptozotocin-induced diabetic rats. Korean J Intern Med. 1999;14:34–40. doi: 10.3904/kjim.1999.14.2.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K, Kim D, Nielsen LL, Aisporna M, Baron AD, Fineman MS. Day-long subcutaneous infusion of exenatide lowers glycemia in patients with type 2 diabetes. Horm Metab Res. 2005;37:627–632. doi: 10.1055/s-2005-870529. [DOI] [PubMed] [Google Scholar]
- Thorens B, Porret A, Buhler L, Deng SP, Morel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9–39) an antagonist of the receptor. Diabetes. 1993;42:1678–1682. doi: 10.2337/diab.42.11.1678. [DOI] [PubMed] [Google Scholar]
- Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148:4965–4973. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]
- Verspohl EJ. Novel therapeutics for type 2 diabetes: incretin hormone mimetics (glucagon-like peptide-1 receptor agonists) and dipeptidyl peptidase-4 inhibitors. Pharmacol Ther. 2009;124:113–138. doi: 10.1016/j.pharmthera.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Villanueva-Penacarrillo ML, Puente J, Redondo A, Clemente F, Valverde I. Effect of GLP-1 treatment on GLUT2 and GLUT4 expression in type 1 and type 2 rat diabetic models. Endocrine. 2001;15:241–248. doi: 10.1385/ENDO:15:2:241. [DOI] [PubMed] [Google Scholar]
- Xu G, Kaneto H, Laybutt DR, Duvivier-Kali VF, Trivedi N, Suzuma K, et al. Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes. 2007;56:1551–1558. doi: 10.2337/db06-1033. [DOI] [PubMed] [Google Scholar]
- Yagihashi S. Nerve structural defects in diabetic neuropathy: do animals exhibit similar changes? Neurosci Res Commun. 1997;21:25–32. [Google Scholar]
- Yagihashi S, Kamijo M, Watanabe K. Reduced myelinated fiber size correlates with loss of axonal neurofilaments in peripheral nerve of chronically streptozotocin diabetic rats. Am J Pathol. 1990;136:1365–1373. [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Jin T. New insights into the role of cAMP in the production and function of the incretin hormone glucagon-like peptide-1 (GLP-1) Cell Signal. 2009;22:1–8. doi: 10.1016/j.cellsig.2009.09.032. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Q, Zhang J, Lei X, Xu GT, Ye W. Protection of exendin-4 analogue in early experimental diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2009;247:699–706. doi: 10.1007/s00417-008-1004-3. [DOI] [PubMed] [Google Scholar]


