Abstract
BACKGROUND AND PURPOSE
Hyperbilirubinaemia and cholestasis are two major forms of liver abnormality. The Chinese herb Yin Chin has been used for thousands of years to treat liver dysfunctions. In mice, this herb and its principal ingredient scoparone were found to accelerate the clearance of bilirubin accompanied by the induction of uridine diphosphate-5′-glucuronosyltransferase-1A1 (UGT1A1), a bilirubin processing enzyme. The aim of this study was to determine whether scoparone induces the expression of human UGT1A1. In addition, the expression of the bile salt export pump (BSEP), a transporter of bile acids, was determined.
EXPERIMENTAL APPROACH
Primary human hepatocytes and hepatoma line Huh7 were treated with scoparone, chenodeoxycholic acid (CDCA) or both. The expression of UGT1A1 and BSEP mRNA was determined. The activation of the human BSEP promoter reporter by scoparone was determined in Huh7 cells by transient transfection and in mice by bioluminescent imaging. The metabolism of scoparone was investigated by recombinant CYP enzymes and pooled human liver microsomes.
KEY RESULTS
Scoparone did not enhance the expression of either human BSEP or, surprisingly, UGT1A1. However, scoparone significantly potentiated the expression of BSEP induced by CDCA. Consistent with this, scoparone potentiated the stimulant effect of CDCA on the human BSEP promoter. This potentiation was enhanced by co-transfection of cytochrome P4501A2 but abolished by the PKC inhibitor GF109203X.
CONCLUSIONS AND IMPLICATIONS
Scoparone and Yin Chin normalize liver function primarily by enhancing the secretion of bile acids, and this effect probably varies depending on the metabolic rate of scoparone.
Keywords: scoparone, bile salt export pump (BSEP), potentiation of BSEP expression, CYP1A2, CYP2B6, GF109203X, CAR, FXR, phosphorylation
Introduction
The liver is the largest internal organ and has diverse functions, notably nutrient processing, protein synthesis, lipid catabolism and detoxification (Santoro et al., 2007; Bock and Bock-Hennig, 2010; Villarroya et al., 2010). Hepatic homeostasis is therefore critical in maintaining the overall physiology of the entire body. In many cases, hepatic dysfunction leads to altered functions of other organs (Romero-Gómez et al., 2001; Ferenci et al., 2002; Tandon, 2003). For example, as many as 50% patients with liver cirrhosis experience episodes of encephalopathy (Romero-Gómez et al., 2001), a syndrome associated with the global impairment of the brain. The prevalence of hepatic dysfunction is high, and it affects more than 10% of Americans (Liver Foundation, 2009). Genetic defect (Pietrangelo, 2009), viral infection (Ferri et al., 2010), alcohol consumption (Cederbaum et al., 2009) and certain therapeutic agents (Calderon et al., 2010) are all found to trigger liver dysfunction and cause liver diseases. Liver illness is a major cause of mortality as well (IPA, 2007). Worldwide, liver cancer and chronic liver diseases are the eighth leading cause of death (IPA, 2007).
Liver diseases are usually manifested with elevated bilirubin, bile acids and hepatic enzymes in the blood (Dolz et al., 1989; Vítek and Ostrow, 2009; Paumgartner, 2010). Bilirubin is a breakdown product of haeme from haemoglobin (Vítek and Ostrow, 2009). Normally, bilirubin is transported from the spleen and conjugated in the liver with glucuronic acid by uridine diphosphate-5′-glucuronosyltransferase-1A1 (UGT1A1) (Ieiri et al., 2004; Vítek and Ostrow, 2009). The conjugated bilirubin is secreted into the bile by transporters such as multidrug resistance-related protein 2 (MRP2) (Ieiri et al., 2004; Hirouchi et al., 2005). Bile acids, on the other hand, are cholesterol derivatives and formed in the liver (Ho et al., 2010; Stieger, 2010). Like glucuronidated bilirubin, bile acids are eliminated through the bile. However, bile acids are secreted by the bile salt export pump (BSEP) (Stieger, 2010). Both genetic and non-genetic factors reportedly impair the functions of BSEP, resulting in cholestasis (increased bile acids) (Paumgartner, 2010; Stieger, 2010). Patients with the progressive familial intrahepatic cholestasis type II have multiple mutations in the BSEP gene (Kagawa et al., 2008). Cyclosporine A, a widely used immunosuppressant, induces cholestasis in both humans and animals (Kis et al., 2009).
Nevertheless, elevated hepatic bile acids and bilirubin have direct toxic effects on the liver (Rodrigues et al., 2002; Trauner et al., 2008; Perez and Briz, 2009). Therefore, for liver and many non-liver diseases, one of the therapeutic objectives is to lower bilirubin and/or bile acids. While therapeutic strategies are usually taken directly towards the underlying causes of hyperbilirubinaemia (Paul et al., 2004; Cuperus et al., 2009) and cholestasis (Pérez Fernández et al., 2004; Paumgartner, 2010), several herbs are used simply to normalize liver functions in general (Huang et al., 2004; Lee et al., 2007; Wang et al., 2008; Chen et al., 2009). For thousands of years, the Chinese herb Yin Chin has been used to treat liver illness. Yin Chin-containing decoctions and its principal ingredient scoparone (SC) (Huang et al., 2004; Tan et al., 2008; Murat Bilgin et al., 2011) have been studied to elucidate its mechanisms of action. In mice, the decoction and SC are shown to accelerate the clearance of bilirubin, and the acceleration is accompanied by an induction of the expression of UGT1A1 and MRP2 (Huang et al., 2004). These effects, however, are absent in mice deficient in the constitutive androstane receptor (CAR) (Huang et al., 2004).
The present study was performed to extend the observations made in mice and designed to test the hypothesis that SC induces the hepatic expression of genes involved in the elimination of bilirubin and bile acids in humans. Primary hepatocytes were treated with this compound, and the expression of UGT1A1 and BSEP was determined. In contrary to the hypothesis, SC did not induce the expression of UGT1A1 or BSEP, even at 100 µM. However, SC 10 µM significantly potentiated the increase in BSEP induced by chenodeoxycholic acid (CDCA). Consistent with this effect, SC potentiated the ability of CDCA to stimulate the human BSEP promoter, and this potentiation was much enhanced in cells transfected with cytochrome P4501A2 (CYP1A2). The human BSEP promoter was robustly activated in SC-treated mice and mouse bsep was significantly enhanced in these mice as well.
Methods
Chemicals and supplies
Hanks balanced salt solution, SC, William's medium E (WME) and GF109203X were purchased from Sigma (St. Louis, MO). Eagle's minimum Essential medium (EMEM), high fidelity Platinum Taq DNA polymerase, insulin–transferrin–selenium (ITS) G supplement were purchased from Invitrogen (Carlsbad, CA). Dual-Luciferase Reporter Assay System was from Promega (Madison, WI). Supersomes™ expressing cytochrome P450s (CYP) and control lysates were obtained from Gentest (Woburn, MA). Fetal bovine serum was from HyClone laboratories (Logan, UT). The antibodies against glyceradehyde-3-phosphate dehydrogenase (GAPDH) or lamin B1 were from Abcam (Cambridge, UK). The goat anti-rabbit IgG conjugated with horseradish peroxidase was from Pierce (Rockford, IL). Human primary hepatocytes were obtained from the Liver Tissues Procurement and Distribution System (University of Minnesota) or CellzDirect (Pittsboro, NC). Unless otherwise specified, all other reagents were purchased from Fisher Scientific (Fair Lawn, NJ).
Reverse transcription-quantitative PCR (RT-qPCR)
Primary hepatocytes and hepatoma cells were cultured and treated as described previously (Yang et al., 2007). A total of five donors were included with three males (21 and 48 years old) and two females (35 and 72 years old). Among the donors, three were Caucasian and two African American. None of them was smoker. Upon arrival, media were replaced with rich WME containing ITS supplement and penicillin (100 U·mL−1)/streptomycin (10 µg·mL−1) (Ma et al., 2005). Total RNA (1 µg) was subjected to reverse transcription in a total volume of 25 µL (Ma et al., 2005; Yang et al., 2007). The resultant cDNAs were diluted eight times, and quantitative PCR was conducted with TaqMan Gene Expression Assay (Applied Biosystems, Foster City, CA). The TaqMan assay identification numbers were as follows: BSEP, Hs00184824_m1; CYP2B6, Hs03044636_m1; UGT1A1, Hs02511055_s1; GAPDH, 4352934E; RNA polymerase II, Hs00172187_m1; mouse bsep, Mm00445156_m1; mouse GAPDH, Mm99999915_g1. The PCR amplification was conducted in a total volume of 20 µL containing universal PCR master mixture (10 µL), gene-specific TaqMan assay mixture (1 µL) and cDNA template (6 µL). The mRNA levels were normalized according to the level of GAPDH, and the normalization of selected samples was confirmed based on the level of RNA polymerase II. Amplification and quantification were done with the Applied Biosystems 7500 Real-Time PCR System.
Co-transfection assays
Various BSEP promoter reporters and the farnesoid X receptor (FXR) construct were described elsewhere (Deng et al., 2006; 2007;). The FXR phosphorylation mutants were kindly provided by Dr Bart Staels of Institut National de la Santé et de la Recherche Médicale (Gineste et al., 2008). Huh7 cells were plated in 48-well plates in DMEM supplemented with 10% fetal bovine serum at a density of 6 × 104 cells per well. Transfection was conducted by FuGene HD (Roche, Indianapolis, IN). Transfection mixtures contained 50 ng of a reporter plasmid and 5 ng of TK-Renilla luciferase plasmid. In some cases, a CYP expression construct was used in the co-transfection, and the corresponding vector was used to equalize the total amount of plasmid DNA. CYP1A2 expression construct was purchased from Origene (Baltimore, MD), and the CYP3A4 expression construct was described elsewhere (Zhu et al., 2000). Cells were transfected for 12 h and the medium was replaced with fresh medium supplemented with 1% fetal bovine serum. The treatment lasted for 24 h, and the cells were washed once with PBS and collected by scraping. The collected cells were subjected to two cycles of freeze/thaw. The reporter enzyme activities were assayed with a Dual-Luciferase Reporter Assay System. This system contained two substrates, which were used to determine the activities of two luciferases sequentially. The firefly luciferase activity, which represented the reporter activity, was initiated by mixing an aliquot of lysates (10 µL) with Luciferase Assay Reagent II. Then the firefly luminescence was quenched, and the Renilla luminescence was simultaneously activated by adding Stop & Glo Reagent to the sample tubes. The firefly luminescence signal was normalized based on the Renilla luminescence signal.
In vivo activation of the human BSEP promoter and induction of mouse bsep
Mice (∼22 g, n = 3 per group) were injected with the human BSEP promoter reporter (10 µg·mL−1) via the tail vein in a volume equivalent to 10% of the body weight in 5 s. Next day, mice were given an i.p. injection of SC, 10 mg·kg−1 in suspension or the same volume of saline. The treatment was repeated twice 12 h apart. Four hours after the last injection, mice were injected i.p. with d-luciferin (∼200 µL of 15 mg·mL−1 depending on the weight). After the mice had been anaesthetized with isoflurane, bioluminescent images were obtained by Xenogen IVIS 100 for 60 s at 15 min post d-luciferin injection. The mice were later killed, and the livers were collected. Total RNA was isolated and analysed for levels of mouse bsep by RT-qPCR. The mRNA level of mouse GAPDH was used to normalize the mRNA level of mouse bsep. All mice were allowed free access to Purina Rodent Chow 5001 and water. All animal care and experimental procedures were in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Rhode Island.
Metabolism of SC by recombinant CYPs and pooled liver microsomes
Metabolism of SC was conducted with pooled human liver microsomes and various recombinant CYPs including CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C18 and 3A4. The incubation was performed in a total volume of 100 µL containing potassium phosphate buffer (50 mM, pH 7.4), EDTA (1 mM), MgCl2 (3 mM), NADP+(1 mM), glucose 6-phosphate (5 mM), glucose 6-phosphate dehydrogenase (1 U·mL−1), SC (20 µM) and a CYP (1 pmol), or pooled human liver microsomes (20 µg). SC was dissolved in acetonitrile, and the final concentration of the solvent was 0.1%. After a 3 min pre-incubation, reactions were started by addition of the NADPH-regenerating system and incubated at the same temperature for 60 min. The reactions were terminated by addition of 200 µL ice-cold acetonitrile, and the precipitated protein was removed by centrifugation (12 000 ×g for 15 min at 4°C). The metabolism was monitored by HPLC (Elite Chrom) with a Chromolith SpeedROD column RP-18e (Merck, Germany). The supernatants (10–30 µL) of the reaction mixtures were injected and separated by a discontinuous gradient: 2% acetonitrile for 1 min, 15% acetonitrile for 15 min and 35% for 8 min at a flow rate of 2 mL·min−1. The metabolic profiles were monitored by a diode array detector at 323 nm. The chemical identity of purified metabolite (M1) was determined in 60:40 acetonitrile by LC-MS (QSTAR Elite quadrupole time-of-flight). The parent compound and the metabolite M1 have the m/z ratio of 207 and 193, respectively.
Western analysis
Nuclear and cytoplasmic extracts were prepared with a nuclear extract kit (Active Motif, Carlsbad, CA). Samples were resolved by 7.5% SDS-PAGE in a mini-gel apparatus and transferred electrophoretically to nitrocellulose membranes. After non-specific binding sites were blocked with 5 % non-fat milk, the blots were incubated with an antibody against CYP2B6, CAR, GAPDH or lamin B. The antibodies against CYP2B6 and CAR were prepared with synthetic peptides and purified as described previously (Zhu et al., 2000). The sequences of CYP2B6 and CAR were as follows: CYP2B6, NH2-CIDTYLLHMEKEK-COOH; CAR, NH2-SPDKPGVTQRDEIDQC-COOH. The specificity was established with the corresponding recombinant proteins. The primary antibodies were localized with goat anti-rabbit IgG conjugated with horseradish peroxidase. Horseradish peroxidase activity was detected with a chemiluminescent kit (SuperSignal West Pico). To determine the level of the house-keeping gene proteins, the blots were first incubated at 50°C for 40 min in the striping buffer (2% SDS, 100 mM 2-mercaptoethanol and 62.5 mM Tris–HCl, pH 7.0), blocked with 5 % non-fat milk and then incubated with an antibody against a house-keeping gene protein. The chemiluminescent signal was captured by KODAK Image Station 2000 (Eastman Kodak Company, Rochester, NY, USA) and the relative intensities were quantified by KODAK 1D Image Analysis Software.
Other analyses
Protein concentrations were determined with a BCA assay (Pierce) based on albumin standard. Data are presented as mean ± SD of at least three separate experiments, except where results of blots are shown in which case a representative experiment is depicted in the figures. Statistical significance between two means was made according to one-way anova followed by a Duncan's multiple comparison test (P < 0.05). Letters or asterisks were used to indicate data points for the comparisons.
Results
SC induces the expression of CYP2B6 but not UGT1A1 or BSEP
Human primary hepatocytes were treated with SC at 10–100 µM, which spanned the concentrations used in the mouse study by Huang et al. (2004). Five individual donors were included. The treatment lasted for 24 h, and the expression was monitored by RT-qPCR, and selective samples were analysed by Western blotting. In addition to UGT1A1 and BSEP, the level of CYP2B6 mRNA was also determined. Inclusion of CYP2B6 was based on the finding that the nuclear receptor CAR was found to support the action of SC in knockout mice (Huang et al., 2004), and the CYP2B6 gene is a CAR target gene (Wang et al., 2004).
The results on the induction of mRNA are summarized in Figure 1A. With only one exception, SC caused no statistically significant increase in any of the genes at any concentrations assayed. CYP2B6 was significantly increased at 100 µM, the highest concentration used in this study (Figure 1A). SC at 50 µM increased CYP2B6 mRNA by 81%, but this increase did not reach statistical significance (P = 0.101) (Figure 1A). We next tested whether this concentration causes a measurable increase in CYP2B6 protein. Consistent with the evident increase in CYP2B6 mRNA, the level of CYP2B6 protein was markedly increased (Figure 1B). To determine whether SC at this concentration causes nuclear translocation of CAR, nuclear and cytoplasmic fractions were prepared and analysed for the presence of this receptor in the subcellular fractions. As shown in Figure 1B, SC markedly increased the nuclear accumulation of CAR (nCAR) accompanied by decreased cytoplasmic CAR (cCAR) (Figure 1B).
Figure 1.
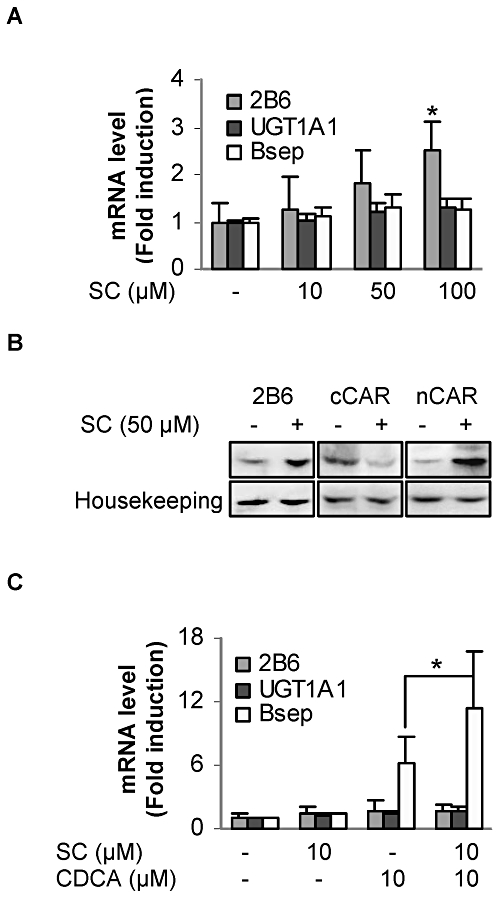
Induction of CYP2B6, UGT1A1 and BSEP and nuclear translocation of CAR. (A) Induction of CYP2B6, UGT1A1 and BSEP in human primary hepatocytes treated with SC at 10–100 µM or the vehicle DMSO for 24 h. Total RNAs were isolated and subjected to RT-qPCR analysis for the mRNA levels of CYP2B6, UGT1A1 and BSEP. *Significant difference from DMSO-treated hepatocytes. (B) Western blot analysis of CYP2B6 and CAR. Pooled cell lysates (10 µg), nuclear proteins (20 µg) or cytoplasmic proteins (20 µg) were subjected to SDS-PAGE and transferred electrophoretically to Trans-Blot nitrocellulose membranes. The immunoblots were blocked in 5% non-fat dry milk and then incubated with the antibody against CYP2B6 or CAR. The blots were detected with chemiluminescent substrate. To re-probe for the house keeping genes, the blots were striped, blocked again and incubated with GAPDH or lamin B1 antibody. (C) Potentiation of the induction of BSEP in human primary hepatocytes treated with SC (10 µM), CDCA (10 µM) or both for 24 h. Total RNAs were isolated and subjected to RT-qPCR analysis for the mRNA levels of CYP2B6, UGT1A1 and BSEP. *Significant difference between CDCA and SC/CDCA co-treatment (n = 5).
The non-significant increase in UGT1A1 and BSEP was unexpected. We next tested whether SC alters the expression of BSEP in response to CDCA, a bile acid known to induce BSEP (Deng et al., 2006). Likewise, primary hepatocytes were treated with SC, CDCA or both. Both CDCA and SC were assayed at 10 µM, a concentration that is widely used under laboratory conditions (Huang et al., 2004; Kaimal et al., 2009). The concentration of total bile acids in the blood in cholestasis ranges from 10 to 30 µM (Brites et al., 1998), and it is estimated that SC reaches 10–20 µM in the liver after oral administration; this is based on a pharmacokinetic study in rats (Yu et al., 2007). As expected, CDCA efficaciously increased BSEP but had little effect on CYP2B6 or UGT1A1 (Figure 1C). Likewise, SC (10 µM) caused little change in the expression of all three genes. However, SC significantly potentiated the ability of CDCA to increase BSEP (Figure 1C). This potentiating effect of SC exhibited larger inter-individual variation than the induction of BSEP by CDCA (Figure 1C).
Enhancement of FXR transactivation by SC
It has been established that the nuclear receptor FXR, a bile acid sensor (Fiorucci and Baldelli, 2009), supports CDCA induction of BSEP. Next we tested whether SC potentiates FXR transactivation of the BSEP promoter. The Huh7 hepatoma line was used to test this possibility. In an initial experiment we detected ∼20% potentiation of FXR transactivation by SC. This was unexpected because SC induced a higher potentiation of CDCA's activity in primary hepatocytes (Figure 1C). One possibility is that the hepatoma cell line has lower metabolizing capacity than hepatocytes. Therefore, we tested whether co-transfection of CYP3A4 or CYP1A2 increases the enhanced transactivation. CYP3A4 is known to mediate the metabolism of more than 50% of drugs and other xenobiotics, and CYP1A2 has been suggested to metabolize SC based on a study in rats (Mennes et al., 1991). As shown in Figure 2A, co-transfection of CYP1A2 but not CYP3A4 or the vector supported significant potentiation of the transactivation of the BSEP reporter. Interestingly, the reporter activity was significantly lower in CYP3A4-transfected cells even those responding to CDCA alone (Figure 2A). The precise mechanism for this decreased potency of CDCA remains to be established. It is likely that CDCA is metabolized by CYP3A4, and the metabolites antagonize the activating effect of CDCA on FXR. Bodin et al. (2005) reported that several bile acids including CDCA are metabolized by CYP3A4. To further establish the involvement of FXR in the SC-potentiated transactivation, the potentiation was determined with the BSEP promoter mutant that has the FXR elements disrupted. As shown in Figure 2B, this mutant was not activated by CDCA or the combination of CDCA and SC.
Figure 2.
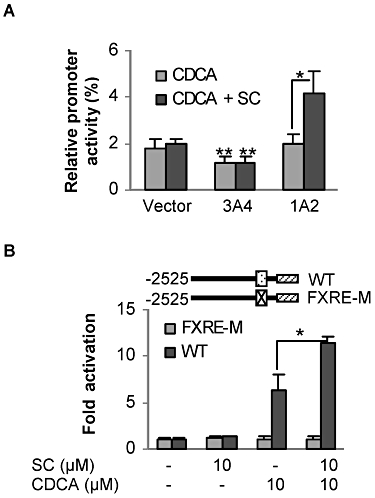
Enhanced CDCA activation of the human BSEP promoter. (A) Effect of CYP1A2 on the transactivation of BSEP. Huh7 cells were plated in 48-well plates at a density of 6 × 104 per well. After overnight incubation, cells were transiently transfected with FuGene HD by addition of a mixture containing 50 ng of BSEP(2.6)-Luc, 5 ng FXR, 5 ng of the null-Renilla luciferase plasmid along with 125 ng CYP1A2, CYP3A4 or the corresponding vector. After incubation at 37°C for 12 h, the transfected cells were treated with CDCA (10 µM) or CDCA plus SC for 24 h. Luciferase activities were determined with a Dual-Luciferase Reporter Assay System, and the reporter activity was normalized based on the Renilla luminescence signal. *Significant difference between CDCA and DE/CDCA co-treatment in CYP1A2 transfected cells; and **significant difference compared with the corresponding vector-transfected controls. (B) The FXR elements are required for the enhancement of BSEP promoter activation. Cells were plated and transfected with a mixture containing 50 ng BSEP(2.6)-Luc or its mutant (FXRE-M) along with 5 ng FXR, 5 ng of the null-Renilla luciferase plasmid and 125 ng CYP1A2 plasmid. Cells were then treated with SC (10 µM), CDCA (10 µM) or both. Luciferase activities were normalized based on the Renilla luminescence signal and repressed as fold activation. *Significant difference between CDCA and SC/CDCA co-treatment on the activation of the wild-type BSEP promoter reporter.
Involvement of phosphorylation in the potentiation of the effect of CDCA on BSEP
Several mechanisms probably account for the potentation of the effect of CDCA on BSEP induced by SC. Initially, we made an effort to determine whether SC increases the expression of FXR, which in turn supports the potentiation. However, neither RT-qPCR nor Western blotting detected an increase in this receptor in SC-treated cells (data not shown). Next, we tested whether phosphorylation of FXR is involved in the potentiation. Two experimental approaches were used, including FXR phosphorylation mutants and GF 109203X, a PKC inhibitor. Three FXR mutants were included: S135A (135A), S154A (154A) and S135A/S154A (AA). As shown in Figure 3A, SC potentiated the ability of CDCA to activate the wild-type FXR (WT) and the 154A mutant but not the 135A or AA mutants. The potentiation in the 154A mutant did not reach statistical significance (Figure 3A). The overall activation of the mutants by CDCA varied. Compared with FXR (WT), the 154A mutant supported higher, but 135A and AA mutants supported lower, activation in response to CDCA. This was interesting because the 135A and 154A mutants had similar reduction towards an FXR element reporter (Gineste et al., 2008). Nonetheless, the reduced or lack of potentiation with the mutants suggests that SC modulates the induction of BSEP by CDCA at least in part by enhancing FXR phosphorylation.
Figure 3.
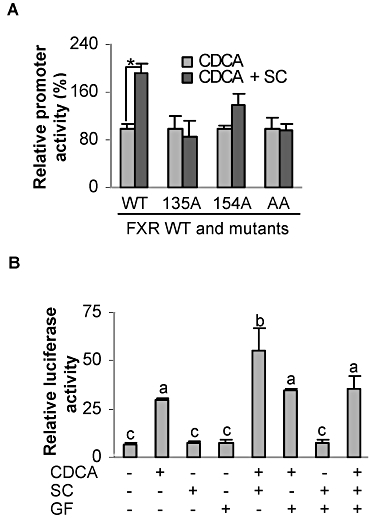
Involvement of FXR phosphorylation in the potentiatng effect of SC on the transactivation of BSEP. (A) FXR phosphorylation mutants reduced or eliminated the potentiation induced by SC. Huh7 cells were transfected with a mixture containing 50 ng BSEP(2.6)-Luc, 5 ng FXR or a mutant, 5 ng of the null-Renilla luciferase plasmid along with 125 ng CYP1A2. The transfected cells were treated with CDCA (10 µM) or CDCA plus SC (10 µM) for 24 h. Luciferase activities were determined. *Significant difference between CDCA and SC/CDCA co-treatment. (B) GF109203X Huh7 cells (in which the potentiating effect of SC on transactivation of BSEP was abolished) were transfected with a mixture containing 50 ng BSEP(2.6)-Luc, 5 ng FXR, 5 ng null-Renilla luciferase plasmid and 125 ng CYP1A2. The transfected cells were treated with CDCA (10 µM), SC (10 µM), GF109203X (1 µM) or in various combinations for 24 h. Luciferase activities were determined. Columns with different letters are statistically, significantly different.
To further establish the role of FXR phosphorylation in the potentiation, the reporter assay was performed in the presence of GF 109203X. The results are summarized in Figure 3B. As expected, CDCA significantly activated the BSEP reporter, and this activation was potentiated by SC co-treatment. However, GF109203X abolished this potentiation effect of SC. This inhibitor alone did not cause any changes in the activation by CDCA. These results further support the notion that SC potentiates the induction of BSEP by CDCA by enhancing the phosphorylation status of FXR.
In vivo activation of the human BSEP promoter
We next tested whether the enhanced transactivation of the human BSEP promoter occurs in vivo (mice). Instead of co-treatment with an FXR ligand such as CDCA, the in vivo study used SC alone because bile acids are normally produced in the liver. As shown in Figure 4A, mice treated with SC exhibited a strong bioluminescent signal (right). In contrast, mice receiving vehicle had no bioluminescent signal (left). Among the three mice treated with SC, two of them showed comparable bioluminescent intensity, whereas the third mouse had a much weaker signal (far right of Figure 4A). The weaker signal was due to a poor tail injection, as this mouse showed a comparable increase in bsep mRNA (endogenous bsep) (Figure 4B).
Figure 4.
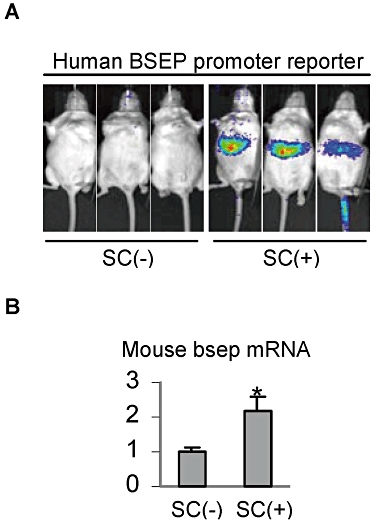
In vivo bioluminescent imaging analysis and induction of mouse bsep. (A) In vivo bioluminescent imaging analysis. Mice (∼22 g, n = 3 per group) were injected with the human BSEP promoter reporter (10 µg·mL−1) via the tail vein in a volume equivalent to 10% of the body weight in 5 s. Next day, mice were given an i.p. injection of SC at 10 mg·kg−1 in suspension (right) or the same volume of saline (left). The treatment was repeated twice 12 h apart. Four hours after the last injection, mice were injected i.p. with d-luciferin (∼200 µL of 15 mg·mL−1 depending on the weight). Thereafter, bioluminescent images were acquired by Xenogen IVIS 100 for 60 s at 15 min post d-luciferin injection. (B) Induction of mouse bsep. The livers from the above treated mice were collected, and total RNA was prepared. The level of mouse bsep mRNA was determined by RT-qPCR. *Significant difference from saline-treated mice.
Metabolism of SC
As shown in Figure 2A, SC exerted a stronger enhanced transactivation in cells transfected with CYP1A2, pointing to the importance of metabolism in the enhancement. We performed a study on SC metabolism with human liver microsomes and several recombinant CYPs including CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C18 and 3A4. The incubations were performed in the presence or absence of the NADPH-regenerating system. SC was assayed at 20 µM, and the reactions lasted for 60 min. The metabolic profiles were monitored by HPLC, and a purified major metabolite was analysed by LC-MS.
Figure 5 shows the representative HPLC chromatograms generated on a Chromolith SpeedROD column RP-18e at 345 nm (left) and the LC-MS spectra of the metabolite M1 (right). Among all recombinant CYPs tested, only CYP1A2 produced evident metabolites: M1, M2 and M3 (left of Figure 5). The M2 and M3 peaks (enlarged and arrowed) had a shorter retention time than the M1 peak. Another noticeable peak was present between the M1 and SC peaks. Human liver microsomes (HLM) produced a similar HPLC-UV metabolite profile: a predominant M1 peak and several smaller peaks (enlarged and arrowed). Some of the smaller peaks appeared to correspond to M2 and M3 produced by CYP1A2. We determined the identities of some of these metabolites. The M1 metabolite was purified and analysed by LC-MS and revealed to be at m/z 193, suggesting a loss of •CH3 (right of Figure 5). This demethylated product probably corresponds to isoscopoletin or scopoletin (Figure 6). The lack of metabolism by CYP2A6 was surprising as SC is structurally similar to coumarin. It is well established that CYP2A6 catalyses the hydroxylation of coumarin to produce 7-hydroxycoumarin (Li et al., 2011). On the other hand, demethylation is the major metabolic pathway of SC in humans (this study) and several animal species (Mennes et al., 1991; Meyer et al., 2001).
Figure 5.
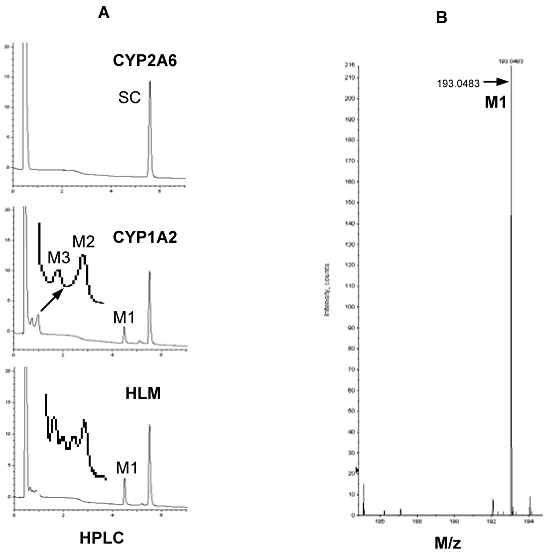
Analysis of SC metabolism by HPLC and mass spectrometry. (A) Metabolic profiles analysed by HPLC: SC (20 µM) was incubated with a CYP (1 pmol), or pooled human liver microsomes (20 µg) in a total volume of 100 µL in the presence or absence of the NADPH-regenerating system (NRS) and incubated at the same temperature for 60 min. The reactions were typically terminated by addition of 200 µL ice- cold acetonitrile and precipitated protein was removed by centrifugation (12 000×g for 15 min at 4°C). The metabolism was monitored by HPLC (Hitach-Elite LaChrom) with a Chromolith SpeedROD column RP-18e. The metabolic profiles were monitored by a diode array detector at 323 nm. (B) Analysis of purified M1 by mass spectrometry. The chemical identity of purified metabolite (M1) was determined in 60:40 acetonitrile by LC-MS.
Figure 6.
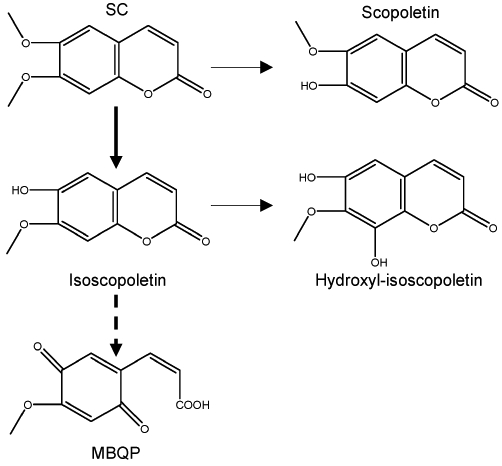
Putative metabolic pathways of SC by human liver microsomes.
Discussion
The Chinese herb Yin Chin has long been used to normalize liver functions including cholestasis and hyperbilirubinaemia (Huang et al., 2004; Tan et al., 2008; Murat Bilgin et al., 2011). SC is abundantly present in Yin Chin (2% based dry weight) and recognized as the principal active ingredient in this herb (Huang et al., 2004). This study reports a functional interplay that supports the action of SC in potentiating the expression of BSEP (Figure 7). Specifically, SC undergoes metabolism by CYP1A2, and the metabolite(s) has higher activation activity towards PKC, most likely the α and β1 isoforms. The activated PKC enhances the phosphorylation of FXR and leads to greater transactivation of the BSEP gene in response to the FXR ligand CDCA. This interplay provides a molecular explanation for the potent liver protective activity of Yin Chin against bile acid accumulation. In addition, we showed that the human BSEP promoter was robustly activated in SC-treated mice and the endogenous mouse bsep gene was also induced. These findings suggest that the protection against cholestasis induced by SC is species-conserved.
Figure 7.
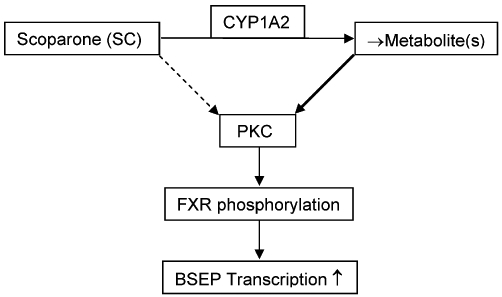
Functional interplay among CYP1A2, PKC and FXR in the potentiation of BSEP expression by SC.
On the other hand, there is an apparent species difference in the induction of UGT1A1, a critical enzyme that glucronidates bilirubin and plays a determinant role in bilirubin clearance. In mice, both SC at 10 µM and Yin Chin-containing decoction Yin Zhi Huang markedly increased the expression of UGT1A1 (Huang et al., 2004). In contrast, in cultured primary hepatocytes and Huh7 cells this concentration did not increase human UGT1A1 mRNA (Figure 1A). Even at 100 µM, the level of UGT1A1 mRNA was increased by only 30% (Figure 1A). Another species difference is the relative ability of SC to increase the expression of UGT1A1 and CYP2B6 among CAR targets. For example, mouse cyp2b10 and UGT1A1 were comparably increased by SC (Huang et al., 2004), whereas the human counterparts, CYP2B6 and UGT1A1, were differentially increased. CYP2B6 was evidently increased by 50 µM and significantly increased by 100 µM SC (Figure 1A). In contrast, neither concentration caused a significant increase in the expression of UGT1A1 (Figure 1A). The precise mechanism remains to be determined. Given the fact that SC caused evident translocation of CAR in human hepatocytes, it is likely that CYP2B6 is a more sensitive target than UGT1A1 in humans. Nevertheless, SC is an activator (ligand) of CAR in both mice and humans, although whether SC is equally potent towards both mouse and human CAR remains to be determined.
The species-dependent effect of SC is probably due to differences in its metabolism. Mouse liver microsomes reportedly produced two primary metabolites: isoscopoletin and scopoletin (Meyer et al., 2001). Both metabolites have the same molecular weight, and isoscopoletin is formed by 6-demethylation, whereas scopoletin is formed by 7-demethylation (Figure 6). Mouse microsomes produced isoscopoletin and scopoletin at a ratio of 5 to 1. The ratio was increased to 12 to 1 in microsomes from mice treated with phenytoin, a potent inducer of Cyp2c29 (Meyer et al., 2001). In this study, we showed that CYP1A2 was the primary CYP enzyme that catalysed the metabolism of SC among all the CYPs tested. Mass spectrometry revealed that one of the major metabolites (M1) produced by CYP1A2 was at m/z 193, suggesting a loss of •CH3 (Right of Figure 5). An earlier study found that incubation of SC in human hepatocytes produced isoscopoletin but not scopoletin (Mennes et al., 1991). These findings collectively suggest that isoscopoletin is a dominant primary metabolite in humans if not the only one. It should be emphasized that the use of seven recombinant CYPs and pooled microsomes represented only an initial study on the metabolism of SC in humans. A comprehensive approach is needed to fully establish whether CYP1A2 is actually the only human enzyme that metabolizes SC.
The significance of the different metabolite profiles between humans and mice remains to be established in terms of the induction of CAR-regulated genes. Given the observation that SC is a weaker inducer of CYP2B6 (human) than Cyp2b10 (mouse), isoscopoletin is probably a weaker activator of CAR than SC or scopoletin. Alternatively, isoscopoletin represents a less stable metabolite than scopoletin and undergoes further metabolism (Figure 6). Indeed, it was reported that mouse liver microsomes rapidly convert isoscopoletin into a propenoate: 3-[4-methoxy-p-(3,6)-benzoquinone]-2-propenoate (MBQP) (Meyer et al., 2001). In mice, the formation of isoscopoletin and MBQP was catalysed by the same enzyme, namely Cyp2c29 (Meyer et al., 2001). It is not clear whether humans can metabolically produce MBQP. Nevertheless, MBQP represents profound changes in the structure with an opening lactone ring. As a result, MBQP is much more hydrophilic than SC, isoscopoletin and scopoletin. It is known that the ligand binding pocket of CAR is highly hydrophobic (Jyrkkärinne et al., 2005); thus, MBQP cannot interact with this pocket as well as SC and its primary metabolites.
The metabolites of SC, on the other hand, may have higher activating activity towards PKC. In this study, we have shown that SC underwent metabolism by CYP1A2 and co-transfection of this CYP enzyme enhanced the activation of the BSEP promoter reporter (Figure 2A). Importantly, the enhanced activation was abolished by GF109203X, a potent PKC inhibitor (Figure 3B). Several studies have shown that SC stimulates PKC activity in cell lines with limited metabolic activity (Yang et al., 2008; 2009;); however, these studies used high concentrations of SC (200 µM). In addition to modulating gene expression, the stimulation of PKC activity by SC, or more so by its metabolites, may have a direct effect on the catalytic activity of enzymes, notably UGT1A1. Basu et al. (2003) reported that UGT1A1 is a PKC substrate and phosphorylation led to increased catalytic activity of this conjugation enzyme. Therefore, SC enhances the overall catalytic activity in both mice and humans, but the mechanisms vary markedly. In mice, SC increases UGT1A1 activity by induction, and probably enhanced phosphorylation as well (Huang et al., 2004), whereas in humans, enhanced phosphorylation probably plays a major role.
Interestingly, the PKC activity, particularly PKCα, is involved in both cholestatic and anticholestatic processes. For example, oestradiol 17β-d-glucuronide [E(2)17G], a cholestatic agent, decreased the bile flow by 61% within 10 min, and the presence of the PKC inhibitor Gö6976 (PKCα > β1) completely abolished the decrease (Crocenzi et al., 2008). In contrast, tauroursodeoxycholic acid, an anti-cholestatic agent, has been shown to protect against ischaemia-induced cholestasis (Baiocchi et al., 2008). Likewise, Gö6976 abolished this protection (Baiocchi et al., 2008). The precise mechanisms of the conflicting observations remain to be determined. It is generally accepted that PKCs play different roles depending on the models and duration of the induced cholestasis (Kubitz et al., 2004; Fiorucci et al., 2005; Wang et al., 2005; Crocenzi et al., 2008; Wimmer et al., 2008). PKCα supports the initiation of cholestasis from normal conditions, but in cholestatic conditions, PKCα activation improves bile flow and thus has anti-cholestatic activity.
The FXR and PKC are probably the major players that support the hepatic protective action of SC against the accumulation of bile acids. In addition to the export transporter BSEP, the liver expresses bile acid uptake transporters including the high-affinity Na+/taurocholate co-transporter (NTCP) and two members of the family of multispecific organic anion transporters (OATPs) (Wagner et al., 2010). Activation of PKC has been shown to increase NTCP endocytosis accompanied by decreased bile acid uptake (Stross et al., 2010). FXR is a major transactivator of the small heterodimer partner gene (SHP). In rodents, induction of SHP via FXR constitutes a negative feedback loop that down-regulates the uptake transporters, thus protecting the liver against bile acid accumulation (Wagner et al., 2010). However, this negative feedback loop has not been established in humans. Patients with progressive familial intrahepatic cholestasis express much lower protein levels of NTCP, OATP1B1 and 1B3 (Keitel et al., 2005), suggesting the existence of such a negative feedback loop in humans. However, other studies have not shown a clear inverse expression relationship between SHP and the uptake transporters (Jung et al., 2007; Schaap et al., 2009). Very recently, FXR has been shown to directly up-regulate the OATP1B1 gene (Mayer Zu Schwabedissen et al. 2010). Nevertheless, collectively these findings suggest that the action of SC on the FXR–PKC pathway has a broad effect on the hepatic homeostasis of bile acids. Further studies are warranted to specify whether SC actually modulates the uptake transporters and whether the modulation varies depending on the cholestatic stage and severity.
In summary, several important conclusions can be deduced from our results. Firstly, we have shown that SC potentiates the induction of BSEP and established that SC is anti-cholestatic. Secondly, we found that SC causes significant enhancement of the activation of the BSEP promoter in CYP1A2-transfected cells, suggesting that metabolism of SC represents a functional switch, namely, from a relatively potent CAR activator to relatively strong PCK stimulator. Thirdly, we demonstrated that SC significantly induces mouse bsep and the human BSEP promoter is robustly stimulated in SC-treated mice, suggesting that the anti-cholestatic activity is species-conserved. The Chinese herb Yin Chin has long been used to normalize liver functions including cholestasis. SC is abundantly present in Yin Chin and recognized as the principal active ingredient in this herb. This study provides a molecular explanation for the therapeutic benefits of Yin Chin and SC and indicates how these benefits can be maximized.
Acknowledgments
We thank Dr Bart Staels of Institut National de la Santé et de la Recherche Médicale for providing the FXR mutant constructs. This work was supported by NIH grants R01GM61988, R01ES07965, R01DK087755 and F05AT003019. The analysis of metabolites was determined through the core facility supported by National Institutes of Health Grant P20RR01645.
Glossary
Abbreviation
- BSEP
bile salt export pump
- CAR
constitutive androstane receptor
- CDCA
chenodeoxycholic acid
- CYP
cytochrome P450
- CYP1A2
cytochrome P4501A2
- EMEM
Eagle's minimum Essential medium
- FXR
farnesoid X receptor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- ITS
insulin–transferrin–selenium
- MRP2
multidrug resistance-related protein 2
- NTCP
Na+/taurocholate co-transporter
- OATP
organic anion transporter; RT-qPCR, reverse transcription quantitative-PCR
- SC
scoparone
- UGT1A1
uridine diphosphate-5′-glucuronosyltransferase-1A1
- WME
William's medium E
Conflict of interest
The authors indicate no potential conflict of interest.
References
- Baiocchi L, Tisone G, Russo MA, Longhi C, Palmieri G, Volpe A, et al. TUDCA prevents cholestasis and canalicular damage induced by ischemia-reperfusion injury in the rat, modulating PKCalpha-ezrin pathway. Transpl Int. 2008;21:792–800. doi: 10.1111/j.1432-2277.2008.00682.x. [DOI] [PubMed] [Google Scholar]
- Basu NK, Kole L, Owens IS. Evidence for phosphorylation requirement for human bilirubin UDP-glucuronosyltransferase (UGT1A1) activity. Biochem Biophys Res Commun. 2003;303:98–104. doi: 10.1016/s0006-291x(03)00241-9. [DOI] [PubMed] [Google Scholar]
- Bock KW, Bock-Hennig BS. UDP-glucuronosyltransferases (UGTs): from purification of Ah-receptor-inducible UGT1A6 to coordinate regulation of subsets of CYPs, UGTs, and ABC transporters by nuclear receptors. Drug Metab Rev. 2010;42:5–12. doi: 10.3109/03602530903205492. [DOI] [PubMed] [Google Scholar]
- Bodin K, Lindbom U, Diczfalusy U. Novel pathways of bile acid metabolism involving CYP3A4. Biochim Biophys Acta. 2005;1687:84–93. doi: 10.1016/j.bbalip.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Brites D, Rodrigues CM, van-Zeller H, Brito A, Silva R. Relevance of serum bile acid profile in the diagnosis of intrahepatic cholestasis of pregnancy in a high incidence area: Portugal. Eur J Obstet Gynecol Reprod Biol. 1998;80:31–38. doi: 10.1016/s0301-2115(98)00086-4. [DOI] [PubMed] [Google Scholar]
- Calderon RM, Cubeddu LX, Goldberg RB, Schiff ER. Statins in the treatment of dyslipidemia in the presence of elevated liver aminotransferase levels: a therapeutic dilemma. Mayo Clin Proc. 2010;85:349–356. doi: 10.4065/mcp.2009.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- Chen SP, Tian LL, Liu FL. Clinical observation of Yinzhihuang Oral Liquid on prevention of the premature infantile jaundice. Chin J Integr Med. 2009;15:299–302. doi: 10.1007/s11655-009-0299-1. [DOI] [PubMed] [Google Scholar]
- Crocenzi FA, Sánchez Pozzi EJ, Ruiz ML, Zucchetti AE, Roma MG, Mottino AD. Ca(2+)-dependent protein kinase C isoforms are critical to estradiol 17beta-D-glucuronide-induced cholestasis in the rat. Hepatology. 2008;48:1885–1895. doi: 10.1002/hep.22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuperus FJ, Hafkamp AM, Hulzebos CV, Verkade HJ. Pharmacological therapies for unconjugated hyperbilirubinemia. Curr Pharm Des. 2009;15:2927–2938. doi: 10.2174/138161209789058219. [DOI] [PubMed] [Google Scholar]
- Deng R, Yang D, Yang J, Yan B. Cholesterol oxylsterol transcriptionally induces the expression of the bile salt efflux pump through the farnesoid X receptor but not the liver X receptor. J Pharmacol Exp Ther. 2006;317:317–325. doi: 10.1124/jpet.105.097758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng R, Yang D, Radke A, Yang J, Yan B. Hypolipidemic agent guggulsterone regulates BSEP expression: dominance of transactivation over FXR-antagonism. J Pharmacol Exp Ther. 2007;320:1277–1286. doi: 10.1124/jpet.106.113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolz C, Xiol X, Abad A, Cabré E, González-Huix F, Giné JJ, et al. Changes in liver function tests in patients with inflammatory bowel disease on enteral nutrition. JPEN J Parenter Enteral Nutr. 1989;13:401–405. doi: 10.1177/0148607189013004401. [DOI] [PubMed] [Google Scholar]
- Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy – definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- Ferri C, Govoni M, Calabrese L. The A, B, Cs of viral hepatitis in the biologic era. Curr Opin Rheumatol. 2010;22:443–450. doi: 10.1097/BOR.0b013e328338f6df. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Baldelli F. Farnesoid X receptor agonists in biliary tract disease. Curr Opin Gastroenterol. 2009;25:252–259. doi: 10.1097/MOG.0b013e328324f87e. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Clerici C, Antonelli E, Orlandi S, Goodwin B, Sadeghpour BM, et al. Protective effects of 6-ethyl chenodeoxycholic acid, a farnesoid X receptor ligand, in estrogen-induced cholestasis. J Pharmacol Exp Ther. 2005;313:604–612. doi: 10.1124/jpet.104.079665. [DOI] [PubMed] [Google Scholar]
- Gineste R, Sirvent A, Paumelle R, Helleboid S, Aquilina A, Darteil R, et al. Phosphorylation of farnesoid X receptor by protein kinase C promotes its transcriptional activity. Mol Endocrinol. 2008;22:2433–2447. doi: 10.1210/me.2008-0092. [DOI] [PubMed] [Google Scholar]
- Hirouchi M, Suzuki H, Sugiyama Y. Treatment of hyperbilirubinemia in Eisai hyperbilirubinemic rat by transfecting human MRP2/ABCC2 gene. Pharm Res. 2005;22:661–666. doi: 10.1007/s11095-005-2502-1. [DOI] [PubMed] [Google Scholar]
- Ho RH, Leake BF, Kilkenny DM, Meyer Zu Schwabedissen HE, Glaeser H, Kroetz DL, et al. Polymorphic variants in the human bile salt export pump (BSEP; ABCB11): functional characterization and interindividual variability. Pharmacogenet Genomics. 2010;20:45–57. doi: 10.1097/FPC.0b013e3283349eb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zhang J, Moore DD. A traditional herbal medicine enhances bilirubin clearance by activating the nuclear receptor CAR. J Clin Invest. 2004;113:137–143. doi: 10.1172/JCI200418385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieiri I, Suzuki H, Kimura M, Takane H, Nishizato Y, Irie S, et al. Influence of common variants in the pharmacokinetic genes (OATP-C, UGT1A1, and MRP2) on serum bilirubin levels in healthy subjects. Hepatol Res. 2004;30:91–95. doi: 10.1016/j.hepres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- IPA. Top 20 causes of mortality throughout the world. 2007. http://www.infoplease.com/ipa/A0779147.html.
- Jung D, Elferink MG, Stellaard F, Groothuis GM. Analysis of bile acid-induced regulation of FXR target genes in human liver slices. Liver Int. 2007;27:137–144. doi: 10.1111/j.1478-3231.2006.01393.x. [DOI] [PubMed] [Google Scholar]
- Jyrkkärinne J, Windshügel B, Mäkinen J, Ylisirniö M, Peräkylä M, Poso A, et al. Amino acids important for ligand specificity of the human constitutive androstane receptor. J Biol Chem. 2005;280:5960–5971. doi: 10.1074/jbc.M411241200. [DOI] [PubMed] [Google Scholar]
- Kagawa T, Watanabe N, Mochizuki K, Numari A, Ikeno Y, Itoh J. Phenotypic differences in PFIC2 and BRIC2 correlate with protein stability of mutant Bsep and impaired taurocholate secretion in MDCK II cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G58–G67. doi: 10.1152/ajpgi.00367.2007. [DOI] [PubMed] [Google Scholar]
- Kaimal R, Song X, Yan B, King R, Deng R. Differential modulation of farnesoid X receptor signaling pathway by the thiazolidinediones. J Pharmacol Exp Ther. 2009;330:125–134. doi: 10.1124/jpet.109.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitel V, Burdelski M, Warskulat U, Kühlkamp T, Keppler D, Häussinger D, et al. Expression and localization of hepatobiliary transport proteins in progressive familial intrahepatic cholestasis. Hepatology. 2005;41:1160–1172. doi: 10.1002/hep.20682. [DOI] [PubMed] [Google Scholar]
- Kis E, Ioja E, Nagy T, Szente L, Herédi-Szabó K, Krajcsi P. Effect of membrane cholesterol on BSEP/Bsep activity: species specificity studies for substrates and inhibitors. Drug Metab Dispos. 2009;37:1878–1886. doi: 10.1124/dmd.108.024778. [DOI] [PubMed] [Google Scholar]
- Kubitz R, Saha N, Kühlkamp T, Dutta S, vom Dahl S, Wettstein M, et al. Ca2+-dependent protein kinase C isoforms induce cholestasis in rat liver. J Biol Chem. 2004;279:10323–10330. doi: 10.1074/jbc.M306242200. [DOI] [PubMed] [Google Scholar]
- Lee TY, Chang HH, Chen JH, Hsueh ML, Kuo JJ. Herb medicine Yin-Chen-Hao-Tang ameliorates hepatic fibrosis in bile duct ligation rats. J Ethnopharmacol. 2007;109:318–324. doi: 10.1016/j.jep.2006.07.042. [DOI] [PubMed] [Google Scholar]
- Li W, Shen J, Liu G, Tang Y, Hoshino T. Exploring coumarin egress channels in human cytochrome P450 2A6 by random acceleration and steered molecular dynamics simulations. Proteins. 2011;79:271–281. doi: 10.1002/prot.22880. [DOI] [PubMed] [Google Scholar]
- Liver Foundation. Liver awareness month. 2009. http://www.liverfoundation.org/chapters/lam2009.
- Ma Y, Song X, Sachdeva K, Liu J, Li Y, Yang D, et al. Clofibrate and perfluorodecanoate both up-regulate the expression of the pregnane X receptor but only clofibrate enhances its ligand-dependent induction of cytochrome P4503A23. Biochem Pharmacol. 2005;69:1 363–1 371. doi: 10.1016/j.bcp.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Mennes WC, van Holsteijn CW, Timmerman A, Noordhoek J, Blaauboer BJ. Biotransformation of scoparone used to monitor changes in cytochrome P450 activities in primary hepatocyte cultures derived from rats, hamsters and monkeys. Biochem Pharmacol. 1991;41:1203–1208. doi: 10.1016/0006-2952(91)90659-s. [DOI] [PubMed] [Google Scholar]
- Meyer RP, Hagemeyer CE, Knoth R, Kurz G, Volk B. Oxidative hydrolysis of scoparone by cytochrome p450 CYP2C29 reveals a novel metabolite. Biochem Biophys Res Commun. 2001;285:32–39. doi: 10.1006/bbrc.2001.5111. [DOI] [PubMed] [Google Scholar]
- Meyer Zu Schwabedissen HE, Böttcher K, Chaudhry A, Kroemer HK, Schuetz EG, Kim RB. Liver X receptor α and farnesoid X receptor are major transcriptional regulators of OATP1B1. Hepatology. 2001;52:1797–1807. doi: 10.1002/hep.23876. [DOI] [PubMed] [Google Scholar]
- Murat Bilgin H, Atmaca M, Deniz Obay B, Ozekinci S, Taşdemir E, Ketani A. Protective effects of coumarin and coumarin derivatives against carbon tetrachloride-induced acute hepatotoxicity in rats. Exp Toxicol Pathol. 2011;63:325–330. doi: 10.1016/j.etp.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Paul IM, Phillips TA, Widome MD, Hollenbeak CS. Cost-effectiveness of postnatal home nursing visits for prevention of hospital care for jaundice and dehydration. Pediatrics. 2004;114:1015–1022. doi: 10.1542/peds.2003-0766-L. [DOI] [PubMed] [Google Scholar]
- Paumgartner G. Biliary physiology and disease: reflections of a physician-scientist. Hepatology. 2010;51:1095–1106. doi: 10.1002/hep.23472. [DOI] [PubMed] [Google Scholar]
- Pérez Fernández T, López Serrano P, Tomás E, Gutiérrez ML, Lledó JL, Cacho G, et al. Diagnostic and therapeutic approach to cholestatic liver disease. Rev Esp Enferm Dig. 2004;96:60–73. doi: 10.4321/s1130-01082004000100008. [DOI] [PubMed] [Google Scholar]
- Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677–1689. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrangelo A. Inherited metabolic disease of the liver. Curr Opin Gastroenterol. 2009;25:209–214. doi: 10.1097/MOG.0b013e328329e13d. [DOI] [PubMed] [Google Scholar]
- Rodrigues CM, Solá S, Brito MA, Brites D, Moura JJ. Bilirubin directly disrupts membrane lipid polarity and fluidity, protein order, and redox status in rat mitochondria. J Hepatol. 2002;36:335–341. doi: 10.1016/s0168-8278(01)00279-3. [DOI] [PubMed] [Google Scholar]
- Romero-Gómez M, Boza F, García-Valdecasas MS, García E, Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol. 2001;96:2718–2723. doi: 10.1111/j.1572-0241.2001.04130.x. [DOI] [PubMed] [Google Scholar]
- Santoro A, Mancini E, Ferramosca E, Faenza S. Liver support systems. Contrib Nephrol. 2007;156:396–404. doi: 10.1159/000102130. [DOI] [PubMed] [Google Scholar]
- Schaap FG, van der Gaag NA, Gouma DJ, Jansen PL. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology. 2009;49:1228–1235. doi: 10.1002/hep.22771. [DOI] [PubMed] [Google Scholar]
- Stieger B. Role of the bile salt export pump, BSEP, in acquired forms of cholestasis. Drug Metab Rev. 2010;42:437–445. doi: 10.3109/03602530903492004. [DOI] [PubMed] [Google Scholar]
- Stross C, Helmer A, Weissenberger K, Görg B, Keitel V, Häussinger D, et al. Protein kinase C induces endocytosis of the sodium taurocholate cotransporting polypeptide. Am J Physiol Gastrointest Liver Physiol. 2010;299:G320–G328. doi: 10.1152/ajpgi.00180.2010. [DOI] [PubMed] [Google Scholar]
- Tan XJ, Li Q, Chen XH, Wang ZW, Shi ZY, Bi KS, et al. Simultaneous determination of 13 bioactive compounds in Herba Artemisiae Scopariae (Yin Chen) from different harvest seasons by HPLC-DAD. J Pharm Biomed Anal. 2008;47:847–853. doi: 10.1016/j.jpba.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Tandon BN. Hepatic encephalopathy syndromes. Indian J Gastroenterol. 2003;22(Suppl 2):S4–S6. [PubMed] [Google Scholar]
- Trauner M, Fickert P, Halilbasic E, Moustafa T. Lessons from the toxic bile concept for the pathogenesis and treatment of cholestatic liver diseases. Wien Med Wochenschr. 2008;158:542–548. doi: 10.1007/s10354-008-0592-1. [DOI] [PubMed] [Google Scholar]
- Villarroya F, Domingo P, Giralt M. Drug-induced lipotoxicity: lipodystrophy associated with HIV-1 infection and antiretroviral treatment. Biochim Biophys Acta. 2010;1801:392–399. doi: 10.1016/j.bbalip.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Vítek L, Ostrow JD. Bilirubin chemistry and metabolism; harmful and protective aspects. Curr Pharm Des. 2009;15:2869–2883. doi: 10.2174/138161209789058237. [DOI] [PubMed] [Google Scholar]
- Wagner M, Zollner G, Trauner M. Nuclear receptor regulation of the adaptive response of bile acid transporters in cholestasis. Semin Liver Dis. 2010;30:160–177. doi: 10.1055/s-0030-1253225. [DOI] [PubMed] [Google Scholar]
- Wang H, Faucette S, Moore R, Sueyoshi T, Negishi M, LeCluyse E. Human constitutive androstane receptor mediates induction of CYP2B6 gene expression by phenytoin. J Biol Chem. 2004;279:29295–29301. doi: 10.1074/jbc.M400580200. [DOI] [PubMed] [Google Scholar]
- Wang JM, Wang H, Xu LN, Zou SQ. Hepatic injury in rats with obstructive jaundice: roles of the protein kinase C signal pathway and cytoprotection of fructose. Hepatobiliary Pancreat Dis Int. 2005;4:577–581. [PubMed] [Google Scholar]
- Wang N, Li P, Wang Y, Peng W, Wu Z, Tan S, et al. Hepatoprotective effect of Hypericum japonicum extract and its fractions. J Ethnopharmacol. 2008;116:1–6. doi: 10.1016/j.jep.2007.08.031. [DOI] [PubMed] [Google Scholar]
- Wimmer R, Hohenester S, Pusl T, Denk GU, Rust C, Beuers U. Tauroursodeoxycholic acid exerts anticholestatic effects by a cooperative cPKC alpha-/PKA-dependent mechanism in rat liver. Gut. 2008;57:1448–1454. doi: 10.1136/gut.2007.140871. [DOI] [PubMed] [Google Scholar]
- Yang J, Shi D, Yang D, Song X, Yan B. Interleukin-6 suppresses the expression of carboxylesterases HCE1 and HCE2 through transcriptional repression. Mol Pharmacol. 2007;72:686–694. doi: 10.1124/mol.107.036889. [DOI] [PubMed] [Google Scholar]
- Yang YJ, Lee HJ, Choi DH, Huang HS, Lim SC, Lee MK. Effect of scoparone on neurite outgrowth in PC12 cells. Neurosci Lett. 2008;440:14–18. doi: 10.1016/j.neulet.2008.05.051. [DOI] [PubMed] [Google Scholar]
- Yang YJ, Lee HJ, Huang HS, Lee BK, Choi HS, Lim SC, et al. Effects of scoparone on dopamine biosynthesis and L-DOPA-induced cytotoxicity in PC12 cells. J Neurosci Res. 2009;87:1929–1937. doi: 10.1002/jnr.22009. [DOI] [PubMed] [Google Scholar]
- Yu ZG, Wang Q, Li K, Li YQ, Gao XX. Determination and pharmacokinetics of 6,7 dimethoxycoumarin in rat plasma after intragastric administration of different decoctions of yinchenhao tang. J Chromatogr Sci. 2007;45:544–548. doi: 10.1093/chromsci/45.8.544. [DOI] [PubMed] [Google Scholar]
- Zhu W, Song L, Zhang H, Matoney L, LeCluyse E, Yan B. Dexamethasone differentially regulates expression of carboxylesterase genes in humans and rats. Drug Metab Dispos. 2000;28:186–191. [PubMed] [Google Scholar]


