Abstract
Background /Aim:
The symptoms of irritable bowel syndrome resemble those of small intestinal bacterial overgrowth (SIBO). The aim of this study was to determine the frequency of SIBO and lactose intolerance (LI) occurrence in patients with diarrhea-predominant irritable bowel syndrome (IBS-D) according to Rome III criteria.
Patients and Methods:
In this retrospective case-control study, patients over 18 years of age with altered bowel habit, bloating, and patients who had lactose Hydrogen breath test (H2BT) done were included. The “cases” were defined as patients who fulfill Rome III criteria for IBS-D, while “controls” were those having chronic nonspecific diarrhea (CNSD) who did not fulfill Rome III criteria for IBS-D. Demographic data, predominant bowel habit pattern, concurrent use of medications, etc., were noted.
Results:
Patients with IBS-D were 119 (51%) with a mean age of 35 ± 13 years, while those with CNSD were 115 (49%) with mean age 36 ± 15 years. Patients in both IBS-D and CNSD were comparable in gender, with male 87 (74%) and female 77 (64%). SIBO was documented by lactose H2BT in 32/234 (14%) cases. It was positive in 22/119 (19%) cases with IBS-D, while 10/115 (9%) cases had CNSD (P = 0.03). LI was positive in 43/234 (18%) cases. Of these, 25/119 (21%) cases had IBS-D and 18/115 (16%) cases had CNSD (P = 0.29).
Conclusion:
SIBO was seen in a significant number of our patients with IBS-D. There was no significant age or gender difference in patients with or without SIBO.
Keywords: Hydrogen breath test, IBS-diarrhea, lactose intolerance, Rome III criteria, small intestinal bacterial overgrowth, young age
Irritable bowel syndrome (IBS) is the most commonly diagnosed chronic functional gastrointestinal disorder worldwide including Asia.[1,2] IBS affects females approximately twice as often as males and is most frequently diagnosed in individuals between the ages of 30 and 50 years.[1,2] However, there is a lack of female dominance in Asian studies.[3] Symptoms of IBS can substantially impact patients’ quality of life, and the effect of IBS on physical and psychological health can negatively impact the workplace.[4–6] The economic burden of IBS is substantial and includes direct physicians’ costs as well as indirect costs in terms of employee absenteeism. IBS is characterized by abdominal discomfort associated with altered bowel habits including constipation, diarrhea, or alternating periods of constipation and diarrhea.
An emerging hypothesis suggests that small intestinal bacterial overgrowth (SIBO) may contribute to IBS pathophysiology.[7] SIBO is a condition characterized by abnormally high bacterial count (≥105 colony-forming units/ml) in the proximal small intestine, resembling distribution of bacteria normally found in the colon.[8,9] Symptoms of SIBO include abdominal pain, bloating, flatulence, and diarrhea, similar to those observed in patients with IBS.[10,11] SIBO was detected in 17 to 84% of patients who met Rome I or Rome II[12–16] criteria for IBS using Hydrogen breath testing (H2BT) with glucose or lactulose. Furthermore, treatment with antibiotics can reduce or eradicate SIBO[16–21] and improve symptoms of IBS[22,23] which supports the role of SIBO in IBS. As diagnosis of IBS is based on a combination of symptoms that are also common in patients with malabsorption disorders, it is possible that some of these patients may be misdiagnosed as IBS, particularly if specific tests are not performed.[7] This was supported by the reports of diagnosis of celiac disease in patients presenting as IBS .[19–21] As H2BT is simpler to administer, is more cost-effective, and does not expose the patient to radiation, it has become the noninvasive gold standard in the diagnosis of SIBO. Previously, SIBO in IBS was shown to be associated with positive lactose H2BT.[15] In a study of 98 consecutive IBS patients, a positive lactulose breath test was found in 64 of 98 (65%) subjects; these SIBO patients showed a significantly higher prevalence of positivity to the lactose breath test (P<0.05).[15] Clinical studies evaluating characteristics associated with SIBO in patients with IBS-diarrhea (IBS-D) according to Rome III criteria are lacking. Therefore, the purpose of this study was to determine the frequency of SIBO and lactose intolerance (LI) occurrence in patients with IBS-D according to Rome III criteria and determine the predictors of SIBO in these patients.
PATIENTS AND METHODS
Patients
In this retrospective case-controlled study, the medical records of patients over 18 years of age who attended gastroenterology clinic supervised by the investigators at the University Hospital with altered bowel habit and bloating, were included. They had lactose H2BT done between January 2007 and October 2010. The Ethics Review Committee at the University approved the study. The “cases” had, over the past three months or more, at least three bowel movements per day described as loose or watery stools associated with urgency, and fulfilled the Rome III criteria for IBS-D,[2] while “controls” were those having chronic nonspecific diarrhea (CNSD) defined as abdominal pain or discomfort associated with intermittent diarrhea with more than 3 stools a day for at least 4 weeks and less than 12 weeks and did not fulfill Rome III criteria for IBS-D.[24] Demographic data, predominant bowel habit pattern, and concurrent use of medications were noted. These patients underwent thorough history, physical examination, complete blood count, serum creatinine, electrolytes, and stool microscopy. All the patients had colonoscopy with rectal biopsy to exclude inflammatory bowel disease and microscopic colitis. Exclusion criteria were concurrent use of opiates analgesics, prokinetic drugs, and PPI, having infection with Giardia lamblia, Entameba histolytica, Blastocystis hominis, positive serology for celiac disease as determined by tissue transglutaminase antibody-IgA and transglutaminase antibody-IgG and abnormal thyroid activity as suggested by serum thyroxin and thyroid-stimulating hormone level. Patients with predisposing conditions for SIBO, e.g., prior small intestinal surgery, scleroderma, diabetes mellitus, liver cirrhosis, forms of autonomic neuropathy, history of peptic ulcer and therapy with H2 antagonists/PPIs during the preceding 8 weeks, hypothyroidism, intestinal pseudo-obstruction, colonoscopy within a week before H2BT, and drugs known to interfere with gastrointestinal motility, e.g., anticholinergics and antidepressants were also excluded.
Hydrogen breath test
All patients underwent a lactose H2BT at the beginning of the study. Immediately before the H2BT, patients used a mouthwash containing 40 ml of 1% chlorhexidine. The H2BT was done with patient fasting overnight for 12 hours. Patients were asked to refrain from smoking after the last meal till the end of the test and from exercise 30 minutes before and during the test. Antibiotics were stopped 4 weeks before administering the SIBO breath test. Patients also avoided laxatives, stool softeners, or stool bulking agents one week prior to the test. Food with high fiber content, e.g., beans, pasta, meats, fiber or bran cereals, cola drinks, butter, or margarine, was avoided 24 hours prior to the test. Samples of expired air were collected. Five samples were collected including one fasting sample (0 minute) after the dose of 50 g lactose for adult, and four samples at 30, 60, 90, and 120 minutes interval. Expiratory breath samples obtained were analyzed immediately after collection, using a commercial device (GaSampler; QuinTron Instrument Company, Milwaukee, Wisconsin). Gas chromatography (Model DP; QuinTron Instrument Company, Milwaukee, Wisconsin) was employed to measure the concentration in parts per million (ppm) of hydrogen in the air. The diagnostic criteria for a positive SIBO was a rise above baseline of 20 ppm in 30 or 60-minute breath sample, while isolated positive breath test at 90 or 120-minute samples corresponding to the passage of lactose into the colon was taken as LI rather than SIBO.[23,24] Definitions of normal and abnormal results of breath tests are variable and there is a lack of generally accepted definitions. The sensitivity and specificity of the lactulose H2BT in detecting SIBO has been reported to be only 68% and 44%, respectively.[25] However, sensitivity in general is acceptable, because of the low specificity for diagnosing SIBO compared with jejunal cultures.[26]
Sample size
The software EPI Info was utilised for sample size estimation. Assuming a 30% prevalence of SIBO in IBS-D and 8% in CNSD,[27] a two-sided alpha of 0.05 and odds ratio of 2 with an 80% power to detect a difference, the required sample size for this objective was 115 for the cases and 115 for control.
Statistical method
Results were expressed as mean ± standard deviation for continuous variables (e.g., age) and number (percentage) for categorical data (e.g., gender, diarrhea, etc.). Comparison of IBS-D patients and CNSD patients with other covariates like age, gender, hydrogen test, hemoglobin, and serology of celiac disease was done by using the independent sample t-test, Pearson Chi-square test, and Fisher Exact test where appropriate. The patients were further divided into SIBO positive and SIBO negative and a comparison was done with other covariates. A P value of <0.05 was considered as statistically significant. All P values were two sided. Statistical interpretation of data was performed by using the computerized software program SPSS version 17.0.
RESULTS
Patients with IBS-D (119, 51%) had a mean age of 35 ± 13 years and male : female ratio was 88 : 31, while those with CNSD (115, 49%) were 36 ± 15 years and male : female ratio of 77 : 38 [Table 1]. Patients with IBS-D were comparable in age with that of CNSD (mean age, 35 ± 13 years vs 36 ± 15 years, P = 0.38). Patients in both IBS-D and CNSD were also comparable in gender with more often male 88 (74%) and female 77 (67%) [Table 1].
Table 1.
Demographic, clinical, and laboratory parameters of IBS-D patients and chronic nonspecific diarrhea
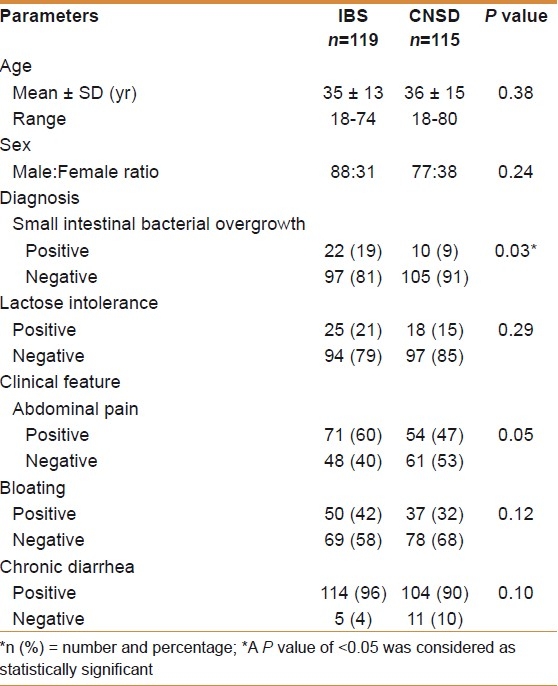
None of the patients in the two groups got excluded due to the diagnosis of celiac disease, thyroid diseases, or presence of gastrointestinal infection with Giardia lamblia, Blastocystis hominis, etc. Serology for celiac disease anti-TTG IgA and IgG and colonic biopsies were reported normal.
Comparison of symptoms in diarrhea-predominant irritable bowel syndrome and Chronic diarrhea
Patients in both groups had chronic diarrhea [Table 1]. Abdominal pain was significant in IBS-D (71, 60%) compared with chronic diarrhea (54, 47%) (P = 0.05) [Table 1]. Bloating was equally present in both, patients with chronic diarrhea (37, 32%) (P = 0.10).
Association of age and gender with small intestinal bacterial overgrowth
SIBO was positive in 32/234 (14%) cases. The mean age of patients with positive SIBO was 32 ± 14 years (age range, 18-61 years) and 36 ± 14 years (age range, 18-80 years) in those negative for SIBO [Table 2]. Of the 32 patients with positive SIBO, 22 (69%) were males, while 10 (31%) were females.
Table 2.
Comparison of patients with small intestinal bacterial overgrowth and lactose intolerance
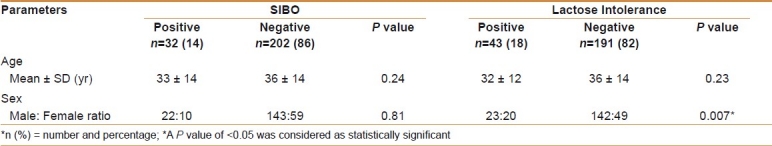
Comparison of small intestinal bacterial overgrowth in groups
SIBO was positive in 22/119 (19%) with IBS-D, while 10/115 (9%) had CNSD (P = 0.03) [Table 2]. Of 22 patients with SIBO in IBS-D, 16/22 (73%) were males and 6/22 (27%) were females, while in those with CNSD, 6/10 (60%) were males and 4/10 (40%) were females (P = 0.81) [Tables 1 and 2].
Association of age and gender with lactose intolerance
LI was present in 25/119 (21%) cases with IBS-D compared with 18/115 (16%) cases with CNSD (P = 0.29). The mean age of IBS-D patients with positive LI was 32 ± 13 years (age range, 18-62 years), while those with CNSD was 36 ± 14 years (age range, 18-80 years) (P = 0.23) [Table 2]. Of the 43 patients with positive LI, 23 (54%) were males and 20 (46%) were females (P = 0.007).
DISCUSSION
Previous studies reported contradictory results about the prevalence of SIBO in patients with IBS.[7,12,20,28–33] The variation in prevalence of SIBO in different studies could be attributed to their geographical location, studied population, criteria used for IBS diagnosis, and method used for the diagnosis of SIBO.
The present study showed that SIBO was more prevalent in patients with IBS-D (19%) compared with those with CNSD (9%). Patients with IBS-D and CNSD had similar mean age [Table 1]. SIBO was seen more commonly in male patients compared with female; however, in both IBS-D and CNSD, there was a preponderance of male patients. This is in keeping with previous observation that in Asian countries men are more likely to report symptoms of IBS as compared to women.[3] This is due to social attributes of the Asian society. LI was not more common in patients with IBS-D but patients were comparatively more of male gender [Tables 1 and 2]. We found that individuals with IBS-D had LI, in the absence of apparent complaints of lactose malabsorption.
The 19% frequency of SIBO in IBS-D patients in this study is similar to the one reported from India.[34] It is, however, much less than SIBO incidence of 78% reported in one study from USA.[16] Such a high frequency of SIBO was not reported in any subsequent study no matter which criteria was used for the diagnosis of IBS.[30–33] The unusually high frequency of SIBO was probably related to the criteria used to diagnose SIBO[16] which presumed that a rise in breath hydrogen 20 ppm above basal levels within 90 minutes after ingestion of test meal was diagnostic of SIBO.[16] This criterion presumed that mouth-to-cecum transit time was always greater than 90 minutes, so that a peak in breath hydrogen within 90 minutes after test meal ingestion must be due to bacterial fermentation in the small bowel. Mouth-to-cecum transit times in Asian population have been reported to be shorter than 90 minutes.[35,36] The median mouth-to-cecum transit time in healthy Indian subjects was 65 minutes with a range of 40 to 110 minutes.[37] Therefore, in the present study, the diagnostic criteria for a positive SIBO were a rise above baseline of 20 ppm up to the 60-minute breath sample collection. In this study, we used Rome III criteria for the diagnosis of IBS-D, as a recent study determined the degree of agreement between Rome III and Rome II criteria in diagnosing IBS, and reported that the severity of bowel habit did not agree among the four subtypes (P<0.005).[38] The limitations of this study include being retrospective and lack of lactose H2BT in healthy controls. However, it is interesting that SIBO was observed in comparatively young IBS-D patients, rather than in elderly patients, who are more likely to experience slow intestinal transit. Also, LI was frequent in male patients. These patients with SIBO were treated with ciprofloxacin 250 mg twice a day and folic acid 5 mg a day for 6 weeks and all patients experienced symptomatic relief.
The implications of this study are that in IBS-D, symptoms may be due to SIBO or LI that may need to be ruled out. Treatment with antibiotics may follow resolution of symptoms in patients with SIBO. However, revision of diagnosis of IBS-D did not follow in our patients with IBS-D as it has been reported in a previous study.[16] This may be attributed to a small number of patients in our study and need to be looked at in a prospective study with a larger sample size. A period of dairy product avoidance and/or lactose supplementation requesting a test for lactose malabsorption will be required for the documentation of LI. In conclusion, SIBO was seen in a significant number of our patients with IBS-D according to Rome III criteria. These patients were young and did not exhibit gender predisposition.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97(11 suppl):S1–5. doi: 10.1016/s0002-9270(02)05656-3. [DOI] [PubMed] [Google Scholar]
- 2.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 3.Jafri W, Yakoob J, Jafri N, Islam M, Ali QM. Irritable bowel syndrome and health seeking behavior in different communities of Pakistan. J Pak Med Assoc. 2007;57:285–7. [PubMed] [Google Scholar]
- 4.Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654–60. doi: 10.1053/gast.2000.16484. [DOI] [PubMed] [Google Scholar]
- 5.Cash B, Sullivan S, Barghout V. Total costs of IBS: Employer and managed care perspective. Am J Manag Care. 2005;11(1 suppl):S7–16. [PubMed] [Google Scholar]
- 6.Dean BB, Aguilar D, Barghout V, Kahler KH, Frech F, Groves D, et al. Impairment in work productivity and health-related quality of life in patients with IBS. Am J Manag Care. 2005;11(1 suppl):S17–26. [PubMed] [Google Scholar]
- 7.Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–80. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 8.Hulisz D. The burden of illness of irritable bowel syndrome: Current challenges and hope for the future. J Manag Care Pharm. 2004;10:299–309. doi: 10.18553/jmcp.2004.10.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin HC. Small intestinal bacterial overgrowth: A framework for understanding irritable bowel syndrome. JAMA. 2004;292:852–8. doi: 10.1001/jama.292.7.852. [DOI] [PubMed] [Google Scholar]
- 10.Husebye E. The pathogenesis of gastrointestinal bacterial overgrowth. Chemotherapy. 2005;51(suppl 1):1–22. doi: 10.1159/000081988. [DOI] [PubMed] [Google Scholar]
- 11.Simrén M, Stotzer PO. Use and abuse of hydrogen breath tests. Gut. 2006;55:297–303. doi: 10.1136/gut.2005.075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Stefano M, Corazza GR. Treatment of small intestine bacterial overgrowth and related symptoms by rifaximin. Chemotherapy. 2005;51(suppl 1):103–9. doi: 10.1159/000081996. [DOI] [PubMed] [Google Scholar]
- 13.Singh VV, Toskes PP. Small bowel bacterial overgrowth: Presentation, diagnosis, and treatment. Curr Treat Options Gastroenterol. 2004;7:19–28. doi: 10.1007/s11938-004-0022-4. [DOI] [PubMed] [Google Scholar]
- 14.Lupascu A, Gabrielli M, Lauritano EC, Scarpellini E, Santoliquido A, Cammarota G, et al. Hydrogen glucose breath test to detect small intestinal bacterial overgrowth: A prevalence case-control study in irritable bowel syndrome. Aliment Pharmacol Ther. 2005;22:1157–60. doi: 10.1111/j.1365-2036.2005.02690.x. [DOI] [PubMed] [Google Scholar]
- 15.Nucera G, Gabrielli M, Lupascu A, Lauritano EC, Santoliquido A, Cremonini F, et al. Abnormal breath tests to lactose, fructose and sorbitol in irritable bowel syndrome may be explained by small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2005;21:1391–5. doi: 10.1111/j.1365-2036.2005.02493.x. [DOI] [PubMed] [Google Scholar]
- 16.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome.a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–9. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 17.Walters B, Vanner SJ. Detection of bacterial overgrowth in IBS using the lactulose H2 breath test: Comparison with 14C-d-xylose and healthy controls. Am J Gastroenterol. 2005;100:1566–70. doi: 10.1111/j.1572-0241.2005.40795.x. [DOI] [PubMed] [Google Scholar]
- 18.Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503–6. doi: 10.1111/j.1572-0241.2000.03368.x. [DOI] [PubMed] [Google Scholar]
- 19.Di Stefano M, Malservisi S, Veneto G, Ferrieri A, Corazza GR. Rifaximin versus chlortetracycline in the short-term treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2000;14:551–6. doi: 10.1046/j.1365-2036.2000.00751.x. [DOI] [PubMed] [Google Scholar]
- 20.Di Stefano M, Strocchi A, Malservisi S, Veneto G, Ferrieri A, Corazza GR. Non-absorbable antibiotics for managing intestinal gas production and gas-related symptoms. Aliment Pharmacol Ther. 2000;14:1001–8. doi: 10.1046/j.1365-2036.2000.00808.x. [DOI] [PubMed] [Google Scholar]
- 21.Lauritano EC, Gabrielli M, Lupascu A, Santoliquido A, Nucera G, Scarpellini E, et al. Rifaximin dose-finding study for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2005;22:31–5. doi: 10.1111/j.1365-2036.2005.02516.x. [DOI] [PubMed] [Google Scholar]
- 22.Majewski M, McCallum RW. Results of small intestinal bacterial overgrowth testing in irritable bowel syndrome patients: Clinical profiles and effects of antibiotic trial. Adv Med Sci. 2007;52:139–42. [PubMed] [Google Scholar]
- 23.Majewski M, Reddymasu SC, Sostarich S, Foran P, McCallum RW. Efficacy of rifaximin, a nonabsorbed oral antibiotic, in the treatment of small intestinal bacterial overgrowth. Am J Med Sci. 2007;333:266–70. doi: 10.1097/MAJ.0b013e3180536784. [DOI] [PubMed] [Google Scholar]
- 24.Teo M, Chung S, Chitti L, Tran C, Kritas S, Butler R, et al. Small bowel bacterial overgrowth is a common cause of chronic diarrhea. J Gastroenterol Hepatol. 2004;19:904–9. doi: 10.1111/j.1440-1746.2004.03376.x. [DOI] [PubMed] [Google Scholar]
- 25.Corazza GR, Menozzi M G, Strocchi A, Rasciti L, Vaira D, Lecchini R, et al. The diagnosis of small bowel bacterial overgrowth. Reliability of jejunal culture and inadequacy of breath hydrogen testing. Gastroenterology. 1990;98:302–9. doi: 10.1016/0016-5085(90)90818-l. [DOI] [PubMed] [Google Scholar]
- 26.Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: A randomized trial. Ann Intern Med. 2006;145:557–63. doi: 10.7326/0003-4819-145-8-200610170-00004. [DOI] [PubMed] [Google Scholar]
- 27.Sharara AI, Aoun E, Abdul-Baki H, Mounzer R, Sidani S, Elhajj I. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am J Gastroenterol. 2006;101:326–33. doi: 10.1111/j.1572-0241.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 28.Rhodes JM, Middleton P, Jewell DP. The lactulose hydrogen breath test as a diagnostic test for small-bowel bacterial overgrowth. Scand J Gastroenterol. 1979;14:333–6. doi: 10.3109/00365527909179892. [DOI] [PubMed] [Google Scholar]
- 29.Kerlin P, Wong L. Breath hydrogen testing in bacterial overgrowth of the small intestine. Gastroenterology. 1988;95:982–8. doi: 10.1016/0016-5085(88)90173-4. [DOI] [PubMed] [Google Scholar]
- 30.Ghoshal UC, Kumar S, Mehrotra M, Lakshmi C, Misra A. Frequency of small intestinal bacterial overgrowth in patients with irritable bowel syndrome and chronic non-specific diarrhea. J Neurogastroenterol Motil. 2010;16:40–6. doi: 10.5056/jnm.2010.16.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pimentel M. The prevalence of small intestinal bacterial overgrowth in irritable bowel syndrome: IBS vs.healthy controls (not historical definitions) Gut. 2008;57:1334–5. [PubMed] [Google Scholar]
- 32.Vanner S. The small intestinal bacterial overgrowth. Irritable bowel syndrome hypothesis: Implications for treatment. Gut. 2008;57:1315–21. doi: 10.1136/gut.2007.133629. [DOI] [PubMed] [Google Scholar]
- 33.Rana SV, Sinha SK, Sikander A, Bhasin DK, Singh K. Study of small intestinal bacterial overgrowth in North Indian patients with irritable bowel syndrome: A case control study. Trop Gastroenterol. 2008;29:23–5. [PubMed] [Google Scholar]
- 34.Gupta D, Ghoshal UC, Misra A, Choudhuri G, Singh K. Lactose intolerance in patients with irritable bowel syndrome from northern India: a case-control study. J Gastroenterol Hepatol. 2007;22:2261–5. doi: 10.1111/j.1440-1746.2007.04986.x. [DOI] [PubMed] [Google Scholar]
- 35.Paik CN, Choi MG, Nam KW. The prevalence of small intestinal bacterial overgrowth in Korean patients with irritable bowel syndrome. Korean. Neurogastroenterol Motil. 2007;13:38–44. [Google Scholar]
- 36.Ghoshal UC, Ghoshal U, Ayyagari A, Ranjan P, Krishnani N, Misra A, et al. Tropical sprue is associated with contamination of small bowel with aerobic bacteria and reversible prolongation of oro-cecal transit time. J Gastroenterol Hepatol. 2003;18:540–7. doi: 10.1046/j.1440-1746.2003.03006.x. [DOI] [PubMed] [Google Scholar]
- 37.Lu CL, Chen CY, Chang FY, Lee SD. Characteristics of small bowel motility in patients with irritable bowel syndrome and normal humans: An oriental study. Clin Sci (Lond) 1998;95:165–9. [PubMed] [Google Scholar]
- 38.Wang AJ, Liao XH, Hu PJ, Liu SC, Xiong LS, Chen MH. A comparison between Rome III and Rome II criteria in diagnosing irritable bowel syndrome. Chinese J Intern Med. 2007;46:644–7. [PubMed] [Google Scholar]


