Abstract
BACKGROUND AND OBJECTIVES:
Congenital toxoplasmosis is associated with significant morbidity and mortality. This study investigates the prevalence of toxoplasmosis among pregnant women.
DESIGN AND SETTING:
A retrospective study at King Khalid University Hospital, Riyadh from September 2009 to August 2010.
PATIENTS AND METHODS:
Laboratory data of 2176 pregnant women screened for Toxoplasma gondii in the antenatal care unit were assessed during the study period. The mean (SD) age of the women and the duration of pregnancy were 25 (7.3) years and 18 (7.7) weeks, respectively. Data were extracted for the presence or absence of anti-T gondii immunoglobulin G (IgG) and IgM antibodies.
RESULTS:
Of 2176 sera tested, 1351 (62%) did not show any evidence of exposure to T gondii. The remaining 825 (38%) samples tested positive for anti-T gondii IgG antibodies, and none was found to have anti-T gondii IgM antibodies in the serum. These data reveal that a significantly high number of women in the antenatal care unit at King Khalid University Hospital in Riyadh had been exposed to T gondii.
CONCLUSION:
A high prevalence of toxoplasmosis among pregnant women warrants multicenter community-based investigations for assessment of T gondii infection and identification of risk factors for transmission of toxoplasmosis in general, and particularly during pregnancy.
Congenital toxoplasmosis affects between 1 and 10 in 10 000 newborn babies in Europe.1 Among them, 1% to 2% develop learning difficulties or die, and 4% to 27% develop ocular lesions leading to permanent impairment of vision in the affected eye.2–5 The main source of infection is by ingestion of viable tissue cysts in meat or oocysts excreted by cats.6 The exact mode of acquisition of infection is not clear, thus making it difficult to identify the risk factors.7,8 Prevention of congenital toxoplasmosis therefore depends on avoidance of infection during pregnancy. To cut down the risk of congenital toxoplasmosis, it is therefore imperative to identify and avoid local sources of infections, especially during pregnancy.
Acute primary maternal toxoplasmosis if acquired during the first trimester of pregnancy can cause significant morbidity and mortality in developing fetuses.9 Congenital infection of the fetus in women infected just before conception is extremely rare; and even during the first few weeks of pregnancy, the maternal-fetal transmission rate is low.10 The risk of fetal infection rises from 6% at 13 weeks to 72% at 36 weeks. Fetuses acquiring infections between 24 and 30 weeks of gestation have been shown to carry the highest risk of suffering from long-term complications.11 The aim of prenatal serological screening for toxoplasmosis is to identify and treat maternal infection as soon as possible in order to prevent transmission of the parasite to the fetus. However, despite widespread provision of prenatal toxoplasma screening, the effectiveness of prenatal treatment is uncertain.12
Various histological and serological techniques, such as the Sabin-Feldman dye test,13 direct hemagglutination test,14 enzyme-linked immunosorbent assay (ELISA),15 and indirect fluorescent antibody tests,16 are commonly applied to detect Toxoplasma gondii infection. For screening purposes, detection of antibodies against T gondii is generally believed to be the most acceptable test. Different assays have been developed to detect anti-toxoplasma antibodies (immunoglobulin M [IgM], IgG, IgA, and IgE) in the sera of pregnant women with a history of repeated abortions and women suspected of being infected with T gondii.17 The prolonged presence of specific IgM in the peripheral blood makes it difficult to differentiate acute from chronic infection.18,19 Detection of Toxoplasma-specific IgA and IgE antibodies in sera of pregnant women appears to be more sensitive than IgM detection during acute infection, but this is awaiting evaluation in a larger study.20
Seroprevalence studies are useful means of gathering estimates of a population at risk and the number of infected individuals. Screening for T gondii infection by detection of specific IgG and IgM in the antenatal care unit is routinely practiced at King Khalid University Hospital. This study examines the seroprevalence of T gondii during pregnancy.
PATIENTS AND METHODS
This was a retrospective analysis of laboratory data for T gondii screening in the antenatal care unit at the King Khalid University Hospital in Riyadh. Between September 2009 and August 2010, sera from a total of 2176 pregnant women with mean (SD) age of 25 (7.3) years and mean duration of pregnancy of 18 (7.7) weeks were screened in the Immunology Division. Data were extracted for the presence or absence of anti-T gondii IgG and IgM antibodies. Specific IgG antibodies against T gondii were detected by indirect hemagglutination test (Wampole Laboratories, Princeton, NJ). The test consists of stabilized sheep erythrocytes sensitized with T gondii antigen, which reacts with the antibodies present in the serum. A titer of 1:64 and above indicates a positive test. Although variation in titer may occur, the assay has never been reported to yield a single false-positive or false-negative reaction. Anti-T gondii IgM antibodies were assessed by ELISA (Vircell SL, Santa Fe, Granada, Spain). The assay is based upon the capture of IgM in the sample, with anti-IgM antibodies adsorbed on the polystyrene surface. When compared with other ELISA kits, this kit has been shown to have a sensitivity of 98% and specificity of 100% with virtually no cross-reactivity.
RESULTS
The mean (SD) age of the pregnant females assessed was 25 (7.3) years, and duration of gestation was 18 (7.7) weeks at the time of screening for T gondii infection. Figure 1 shows the seroprevalence of T gondii in 2176 women screened during pregnancy. Of 2176 sera tested, 1351 (62%) did not show any evidence of exposure to T gondii. The rest of the group, comprising 825 (38%) women, tested positive for anti-T gondii IgG antibodies, and none was found to have anti-T gondii IgM antibodies in the serum. These data reveal that a significantly high number of women in the antenatal care unit at King Khalid University Hospital in Riyadh had been exposed to T gondii.
Figure 1.
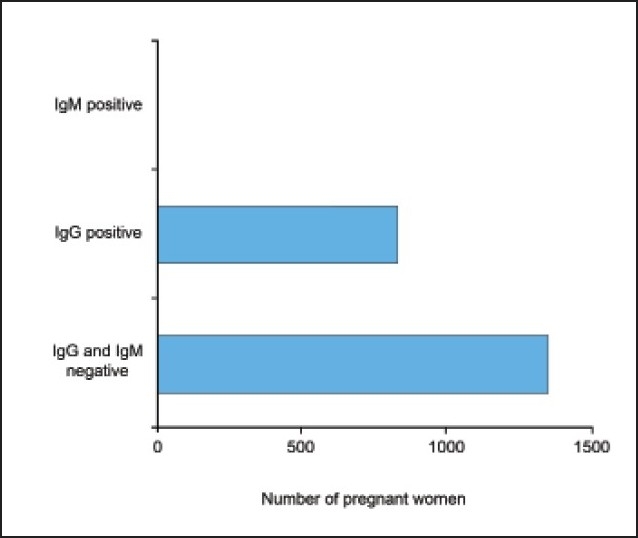
Seroprevalence of Toxoplasma gondii: Assessment of specific IgG and IgM during pregnancy (n=2176).
DISCUSSION
The mortality and morbidity associated with congenital toxoplasmosis have prompted several studies involving serological screening for T gondii infection during pregnancy.21–25 This study shows that, although none of the women screened during pregnancy had anti-T gondii IgM, 38% tested positive for anti-T gondii IgG, indicating exposure in the past. Similar findings have already been reported in a study from Turkey, in which none of the women during their pregnancy tested positive for anti-T gondii IgM antibodies.25 On the contrary, there are studies reporting the presence of anti-T gondii-specific IgM antibodies in sera of pregnant women.21,24,26 The presence of specific IgM is generally believed to be associated with acute infection. However, this may not be true in toxoplasmosis, as the existence of IgM for several months in the sera may interfere in calculating the time of exposure.17 Differentiation of acute from chronic infection is now being recommended by performing anti-T gondii IgG avidity tests.20 The presence of low-avidity–specific IgG is believed to be associated with acute infection, whereas the presence of high-avidity IgG indicates exposure in the distant past.
Seroprevalence of T gondii assessed by the presence of specific IgG antibodies in this study (38%) when compared with the seroprevalence rate of 3.7% among pregnant Korean women27 appears to be alarmingly high. The lower rate of seroprevalence in Korean women, however, seems to be unique, as a number of other studies have reported seroprevalence rates between 20% and 43% among pregnant women in other parts of the world.22–27 Based on the IgG avidity test and polymerase chain reaction for detection of toxoplasma infection in pregnant women, a separate study from Kuwait has reported toxoplasma prevalence of 53.1%.28 These data suggest that T gondii seroprevalence among pregnant women exhibits a trend of regional variation. The higher seroprevalence observed in the present study and the study from Kuwait may suggest a tendency towards a higher T gondii seroprevalence in the Middle East.
Regional variation has been attributed to climate, cultural differences in the amount and type of raw meat consumed, and the increased consumption of meat from animals farmed indoors and frozen meat.6,29–31 A multicenter study has identified eating raw or undercooked lamb, beef, or other meat as the most important single risk factor for acquiring toxoplasma infection, apart from some association with travel outside Europe, United States, and Canada.32 There is sufficient evidence to believe that—compared with beef and chicken—lamb, goat or other meats are more commonly infected and may contain viable cysts.33–36 Undercooked lamb but not beef has been identified as a risk factor in Norway,37 whereas in northern France beef and lamb have been shown to be potent risk factors.38 Similarly, contact with soil has also been regarded as an independent risk factor for T gondii seroconversion during pregnancy, while contact with cat litter may also pose a risk in certain situations.39 Identification of regional lifestyle risk factors for toxoplasmosis is of utmost importance for creating awareness for avoidance of toxoplasmosis in general and specifically during pregnancy.
Many pregnant women lack knowledge of the risk factors involved for the transmission of toxoplasmosis.39 Counseling of pregnant women about risk factor reduction may reduce the risk of congenital toxoplasmosis. The issue is further complicated by the observation that obstetricians, internists, and family practitioners lack relevant education of the risk factors for toxoplasmosis transmission.39 Studies focusing on education of pregnant women have proven to be effective in increasing general knowledge about toxoplasmosis and potentially decreasing the incidence of congenital toxoplasmosis.40,41
This study describes the seroprevalence of T gondii among pregnant women at a single tertiary care hospital, and the findings may not be applicable to the community on the whole. It is therefore recommended that a multicenter study investigating the seroprevalence of T gondii across Saudi Arabia be conducted to assess the actual prevalence of T gondii among pregnant women. In addition, attempts should be made for identification and avoidance of the local sources of infection.
REFERENCES
- 1.Gilbert RE. Epidemiology of infection in pregnant women. In: Petersen E, Amboise-Thomas P, editors. Congenital toxoplasmosis: Scientific background, clinical management and control. Paris: Springer-Verlag France; 1999. [Google Scholar]
- 2.Guerina NG, Hsu HW, Meissner HC, Maguire JH, Lynfield R, Stechenberg B, et al. Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. The New England Regional Toxoplasma Working Group. N Engl J Med. 1994;330:1858–63. doi: 10.1056/NEJM199406303302604. [DOI] [PubMed] [Google Scholar]
- 3.Koppe JG, Loewer Sieger DH, de Roever Bonnet H. Results of 20-year follow-up of congenital toxoplasmosis. Lancet. 1986;1:254–6. doi: 10.1016/s0140-6736(86)90785-3. [DOI] [PubMed] [Google Scholar]
- 4.Lappalainen M, Koskiniemi M, Hiilesmaa V, Ammälä P, Teramo K, Koskela P, et al. Outcome of children after maternal primary Toxoplasma infection during pregnancy with emphasis on avidity of specific IgG. Pediatr Infect Dis J. 1995;14:354–61. doi: 10.1097/00006454-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Lebech M, Andersen O, Christensen NC, Hertel J, Nielsen HE, Peitersen B, et al. Feasibility of neonatal screening for toxoplasma infection in the absence of prenatal treatment. Lancet. 1999;353:1834–7. doi: 10.1016/s0140-6736(98)11281-3. [DOI] [PubMed] [Google Scholar]
- 6.Remington JS, McLeod R, Desmonts G. Toxoplasmosis. In: Remington JS, Klein JO, editors. Infectious diseases of the fetus and newborn. 4th ed. Pennsylvania: WB Saunders; 1995. pp. 140–267. [Google Scholar]
- 7.Hall SM. Congenital toxoplasmosis. BMJ. 1992;305:291–7. doi: 10.1136/bmj.305.6848.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newton LH, Hall SM. A survey of health education material for the primary prevention of congenital toxoplasmosis. Commun Dis Rep CDR Rev. 1995;5:R21–7. [PubMed] [Google Scholar]
- 9.Thiebaut R, Leproust S, Chene G, Gilbert R. Effectiveness of prenatal treatment for congenital toxoplasmosis: A meta-analysis of individual patients’ data. SYROCOT (Systematic Review on Congenital Toxoplasmosis) Study Group. Lancet. 2007;369:115–22. doi: 10.1016/S0140-6736(07)60072-5. [DOI] [PubMed] [Google Scholar]
- 10.Emna S, Karim A, Mohammed K, Aida B. Difficulty in dating primary infections by Toxoplasma gondii in pregnant women in Tunisia. Tunis Med. 2006;84:85–7. [PubMed] [Google Scholar]
- 11.Dunn D, Wallon M, Peyron F, Petersen E, Peckham C, Gilbert R. Mother-to-child transmission of toxoplasmosis: Risk estimates for clinical counseling. Lancet. 1999;353:1829–33. doi: 10.1016/S0140-6736(98)08220-8. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert RE, Gras L, Wallon M, Peyron F, Ades AE, Dunn DT. Effect of prenatal treatment on mother to child transmission of Toxoplasma gondii: Retrospective cohort study of 554 mother-child pairs in Lyon, France. Int J Epidemiol. 2001;30:1303–8. doi: 10.1093/ije/30.6.1303. [DOI] [PubMed] [Google Scholar]
- 13.Sabin AB. Dyes as microchemical indicators of a new phenomenon affecting a protozoan parasite toxoplasma. Science. 1948;108:660–3. doi: 10.1126/science.108.2815.660. [DOI] [PubMed] [Google Scholar]
- 14.Desmonts G, Remington JS. Direct agglutination test for diagnosis of Toxoplasma infection: Method for increasing sensitivity and specificity. J Clin Microbiol. 1980;11:562–8. doi: 10.1128/jcm.11.6.562-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naot Y, Remington JS. An enzyme-linked immunosorbent assay for detection of IgM antibodies to Toxoplasma gondii: Use for diagnosis of acute acquired toxoplasmosis. J Infect Dis. 1980;142:757–66. doi: 10.1093/infdis/142.5.757. [DOI] [PubMed] [Google Scholar]
- 16.Remington JS, McLeod R. Infectious diseases. In: Gorbach SL, Bartlett JG, Blacklow NR, editors. Infectious diseases. Philadelphia: WB Saunders Company; 1992. pp. 1328–43. [Google Scholar]
- 17.Pelloux H, Fricker-Hidalgo H, Goullier-Fleuret A, Ambroise-Thomas P. Detection of anti-toxoplasma immunoglobulin M in pregnant women. J Clin Microbiol. 1997;35:2187. doi: 10.1128/jcm.35.8.2187-2187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liesenfeld O, Press C, Montoya JG, Gill R, Isaac-Renton JL, Hedman K, et al. False-positive results in immunoglobulin M (IgM) toxoplasma antibody tests and importance of confirmatory testing: The Platelia Toxo IgM test. J Clin Microbiol. 1997;35:174–8. doi: 10.1128/jcm.35.1.174-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turunen H, Vuorio KA, Leinikki PO. Determination of IgG, IgM and IgA antibody responses in human toxoplasmosis by enzyme-linked immunosorbent assay (ELISA) Scand J Infect Dis. 1983;15:307–11. doi: 10.3109/inf.1983.15.issue-3.12. [DOI] [PubMed] [Google Scholar]
- 20.Ashburn D, Joss AW, Pennington TH, Ho-Yen DO. Do IgA, IgE and IgG avidity tests have any value in the diagnosis of toxoplasma infection in pregnancy? J Clin Pathol. 1998;51:312–5. doi: 10.1136/jcp.51.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nissapatorn V, Noor Azmi MA, Cho SM, Fong MY, Init I, Rohela M, et al. Toxoplasmosis: Prevalence and risk factors. J Obstet Gynaecol. 2003;23:618–24. doi: 10.1080/01443610310001604376. [DOI] [PubMed] [Google Scholar]
- 22.Koskiniemi M, Lappalainen M, Koskela P, Hedman K, Ammälä P, Hiilesmaa V, et al. The program for antenatal screening of toxoplasmosis in Finland: A prospective cohort study. Scand J Infect Dis. 1992;84(Suppl):70–4. [PubMed] [Google Scholar]
- 23.Lappalainen M, Koskela P, Hedman K, Teramo K, Ammälä P, Hiilesmaa V, et al. Incidence of primary toxoplasma infections during pregnancy in southern Finland: A prospective cohort study. Scand J Infect Dis. 1992;24:97–104. doi: 10.3109/00365549209048407. [DOI] [PubMed] [Google Scholar]
- 24.Morris A, Croxson M. Serological evidence of Toxoplasma gondii infection among pregnant women in Auckland. N Z Med J. 2004;117:U770. [PubMed] [Google Scholar]
- 25.Ertug S, Okyay P, Turkmen M, Yuksel H. Seroprevalence and risk factors for toxoplasma infection among pregnant women in Aydin province, Turkey. BMC Public Health. 2005;5:66. doi: 10.1186/1471-2458-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowakowska D, Slaska M, Kostrzewska E, Wilczyński J. Anti - T.gondii antibody concentration in sera of pregnant women in the sample of Lódź population. Wiad Parazytol. 2001;47:83–9. [PubMed] [Google Scholar]
- 27.Han K, Shin DW, Lee TY, Lee YH. Seroprevalence of Toxoplasma gondii infection and risk factors associated with seropositivity of pregnant women in Korea. J Parasitol. 2008;94:963–5. doi: 10.1645/GE-1435.1. [DOI] [PubMed] [Google Scholar]
- 28.Iqbal J, Khalid N. Detection of acute Toxoplasma gondii infection in early pregnancy by IgG avidity and PCR analysis. J Med Microbiol. 2007;56:1495–9. doi: 10.1099/jmm.0.47260-0. [DOI] [PubMed] [Google Scholar]
- 29.Decavalas G, Papapetropoulou M, Giannoulaki E, Tzigounis V, Kondakis XG. Prevalence of Toxoplasma gondii antibodies in gravidas and recently aborted women and study of risk factors. Eur J Epidemiol. 1990;6:223–6. doi: 10.1007/BF00145798. [DOI] [PubMed] [Google Scholar]
- 30.Dupouy Camet J, Gavinet MF, Paugam A, Tourte Schaeffer C. Transmission, incidence and prevalence of toxoplasmosis. Med Mal Infect. 1993;23:139–47. [Google Scholar]
- 31.Gilbert RE, Tookey PA, Cubitt WD, Ades AE, Masters J, Peckham CS. Prevalence of toxoplasma IgG among pregnant women in west London according to country of birth and ethnic group. BMJ. 1993;306:185. doi: 10.1136/bmj.306.6871.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook AJ, Gilbert RE, Buffolano W, Zufferey J, Petersen E, Jenum PA, et al. Sources of toxoplasma infection in pregnant women: European multicentre case-control study. BMJ. 2000;321:142–7. doi: 10.1136/bmj.321.7254.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubey JP. Strategies to reduce transmission of Toxoplasma gondii to animals and humans. Vet Parasitol. 1996;64:65–70. doi: 10.1016/0304-4017(96)00961-2. [DOI] [PubMed] [Google Scholar]
- 34.Dubey JP. Toxoplasmosis in sheep, goats, pigs and cattle. In: Dubey J, Beattie C, editors. Toxoplasmosis in animals and man. Boca Raton Florida: CRC Press; 1988. pp. 61–114. [Google Scholar]
- 35.Desmonts G, Couvreur J, Alison F, Baudelot J, Gerbeaux J, Lelong M. Epidemiological study on toxoplasmosis: The influence of cooking slaughter-animal meat on the incidence of human infection. Rev Fr Etud Clin Biol. 1965;10:952–8. [PubMed] [Google Scholar]
- 36.Smith JL. Foodborne toxoplasmosis. J Food Safety. 1991;12:17–57. [Google Scholar]
- 37.Kapperud G, Jenum PA, Stray Pedersen B, Melby KK, Eskild A, Eng J. Risk factors for Toxoplasma gondii infection in pregnancy.Results of a prospective case-control study in Norway. Am J Epidemiol. 1996;144:405–12. doi: 10.1093/oxfordjournals.aje.a008942. [DOI] [PubMed] [Google Scholar]
- 38.Baril L, Ancelle T, Thulliez P, Goulet V, Tirard V, Carme B. Facteurs de risque d’aquisition de la toxoplasmose chez les femmes enceintes en 1995 (France) Bulletin Epidemiologique Hebdomadaire. 1996;16:73–5. [Google Scholar]
- 39.Kravetz JD, Federman DG. Prevention of toxoplasmosis in pregnancy: Knowledge of risk factors. Infect Dis Obstet Gynecol. 2005;13:161–5. doi: 10.1080/10647440500068305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pawlowski ZS, Gromadecka-Sutkiewicz M, Skommer J, Paul M, Rokossowski H, Suchocka E, et al. Impact of health education on knowledge and prevention behavior for congenital toxoplasmosis: The experience of Poznan, Poland, Health. Educ Res. 2001;16:493–502. doi: 10.1093/her/16.4.493. [DOI] [PubMed] [Google Scholar]
- 41.Foulon W, Naessens A, Ho-Yen D. Prevention of congenital toxoplasmosis. J Perinat Med. 2000;28:337–45. doi: 10.1515/JPM.2000.043. [DOI] [PubMed] [Google Scholar]


