Abstract
BACKGROUND AND OBJECTIVE:
Published data on short-term outcomes of very low birth weight infants from Saudi Arabia are limited. In the present study, our objective was to describe and analyze the outcomes of very low birth weight infants admitted to our neonatal intensive care unit and to compare the results with data published by the National Institute of Child Health and Development.
DESIGN AND SETTING:
This study was a retrospective analysis of prospectively collected data from a single tertiary care center over a three years period.
PATIENTS AND METHODS:
Biodemographic data and data regarding multiple outcome measures were analyzed for infants with birth weight of 1500 g or less. Data were obtained from our neonatal intensive care unit database.
RESULTS:
Our results included a total of 186 infants with birth weights of 1500 g or less. Of these infants, 154 (82.8%) survived to discharge. Seventy-six (40.9%) were male, and mean (SD) gestational age (GA) was 29 (2.9) weeks with a range of 21 weeks, 6 days to 36 weeks, 2 days. Mean (SD) birth weight was 1062 (302) g with a range of 420 to 1495 g. Fifty-seven (30.6%) infants were characterized as small for gestational age. Antenatal steroids were given to 74.2% of mothers. Eighty-five percent of infants were born by cesarean section. The rate of bronchopulmonary dysplasia was 17.7%, patent ductus arteriosus 31.2%, intraventricular hemorrhage 12.9%, periventricular leukomalacia 3.8%, necrotizing enterocolitis 7.5%, retinopathy of prematurity 28.3%, and late-onset sepsis was 21.9%.
CONCLUSION:
In this population of very low birth weight infants, survival rates and complications of prematurity were comparable to international data.
Preterm birth affects almost 12% of all pregnancies.1,2 Prematurity is the second leading cause of infant mortality, after congenital anomalies, and a major determinant of neonatal and infant morbidity.1 Preterm infants are at risk for developing complications that result from anatomic or functional immaturity. The complications seen most frequently are hypothermia, respiratory abnormalities, patent ductus arteriosus (PDA), intracranial hemorrhage, hypoglycemia, necrotizing enterocolitis (NEC), infection, chronic lung disease, and retinopathy of prematurity (ROP). The risk of developing complications decreases with increasing gestational age and birth weight.3–5
Knowledge of the incidence of such complications is important for parent counseling, as well as anticipating and planning prior to preterm birth. In addition, accurate knowledge of trends in incidence is important for quality improvement.
Several studies have looked at the trends of mortality and morbidity of babies with birth weights of <1500 g (very low-birth-weight [VLBW] infants). In the last 20 years, survival of these VLBW infants has increased, but the incidence rates of the main neonatal complications remain unchanged.3–5 The increased survival is attributed mainly to improvements in obstetric and neonatal care, the widespread use of antenatal corticosteroids for women at risk for preterm birth,6 and the use of surfactant for prevention and treatment of respiratory distress syndrome (RDS).7
The long-term outcomes of preterm infants have been reported in several studies, as well as complications such as cerebral palsy, cognitive delay, learning difficulties, deafness, blindness, and behavioral problems.8–10 The frequency of such complications is inversely related to gestational age.11 In terms of predicting the long-term outcome as a result of the neonatal morbidities, a large study has shown that bronchopulmonary dysplasia (BPD), ultrasonographic signs of brain injury, and severe ROP are independently correlated with the 18-month outcome in extremely low-birth-weight (ELBW) infants (<1000 grams).12
Current available data on the incidence of short-term morbidities in preterm infants from Saudi Arabia are scarce. Almost all of the published studies from this region have been single center–based studies with small sample size and with no focus on VLBW infants; thus they were inadequate to outline the problem nationwide.13–19
The aim of the present study was to assess the short-term outcomes of infants with birth weights less than 1500 g born and treated at King Faisal Specialist Hospital and Research Centre in Riyadh, Saudi Arabia, over a 3-year period and to compare the results with outcomes documented by the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network.
PATIENTS AND METHODS
This study was a retrospective analysis of prospectively collected data on all live newborn infants with birth weights in the range of 400 to 1500 g who required life support immediately after birth and who were admitted to the neonatal intensive care unit (NICU) at the King Faisal Specialist Hospital and Research Centre, from January 2006 to December 2008. Infants were identified through the NICU database and then followed by a careful review of their medical records to obtain the required data. Demographic data included maternal history, maternal age, parity, use of antenatal steroids, gestational age, birth weight, sex, mode of delivery, Apgar score, as well as need for resuscitation, mechanical ventilation, and surfactant administration.
Complications of prematurity included intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), RDS, pneumothorax, PDA, NEC, spontaneous intestinal perforation, ROP, BPD, sepsis, and surgical intervention during NICU admission. The study was approved by the Research Advisory Council of King Faisal Specialist Hospital and Research Centre.
Gestational age was determined from the date of the mother's last menstrual period and/or from details of earliest available ultrasound scans (at least before 20 weeks). Immediate life support was defined as administration of respiratory support to infants immediately after birth; this support included suctioning, oxygen supply, continuous positive airway pressure, endotracheal intubation, mechanical ventilation, and surfactant therapy when appropriate. Small for gestational age was defined as birth weight below the 10th percentile for age, based on the United States Vital Statistics natality data sets for 2001 and 2002.20
IVH was detected routinely by head ultrasound performed during the first 4 weeks of life, and IVH was graded according to Papile's classification, from 0 to 4.21 If multiple ultrasounds were done in the first 4 weeks, then the worst grade was recorded. PVL refers to periventricular echogenecity detected on head ultrasound done at any time during the NICU stay. RDS was considered to be present if the infant needed supplemental oxygen and a chest radiograph was consistent with RDS. Pneumothorax was considered to be present if the infant had extrapleural air diagnosed by chest radiograph or needle aspiration. PDA was considered to be present if it was detected by echocardiography with evidence of left-to-right shunting. NEC was diagnosed clinically with abdominal distension and intolerance to food or bloody stool, in addition to an abdominal radiograph showing pneumatosis intestinalis, pneumoperitoneum, or gas in the biliary tree.22 Bell classifications were used for staging.23 Spontaneous intestinal perforation refers to gut perforation without NEC. In case of ROP, only the worst grade of ROP detected on retinal exam was recorded utilizing the International Classification of ROP.24 BPD was defined as requiring oxygen at 36 weeks corrected age, with total oxygen duration of 28 days or more.25,26 Sepsis was considered if a blood culture or a cerebrospinal fluid culture was positive with a bacterial pathogen.
Outcomes that included complications of prematurity, were assessed by descriptive analysis. Continuous variables are summarized by median and range; categorical variables are summarized as frequency and percentages.
The results were compared with data from the NICHD Neonatal Research Network published in 20075 after obtaining permission from the authors and the publisher. The NICHD data included short-term outcome data of infants weighing 501 to 1500 g who were born from January 1990 through December 2002 at 16 participating centers of the NICHD Neonatal Research Network. To facilitate the comparison, a range was created for our data by adding and subtracting the standard error.
RESULTS
Over our study period, 186 infants with a birth weight between 400 and 1500 g were admitted to our unit. Of these 186 infants, 154 (82.8 %) infants survived to discharge. Seventy-six (40.9%) infants were male. Mean (SD) gestational age (GA) was 29 (2.9) weeks with a range of 21 weeks, 6 days to 36 weeks, 2 days; mean birth weight was 1062 (302) g with a range of 420 to 1495 g; and 39.9% were less than 1000 g in birth weight. Fifty-seven (30.6%) infants were small for gestational age. Antenatal steroids were given to 74.2% of mothers. Eighty-five percent of infants were born by cesarean delivery. Among all mothers, 92.5% received prenatal care. Eighteen (9.7%) infants were diagnosed with major birth anomalies.
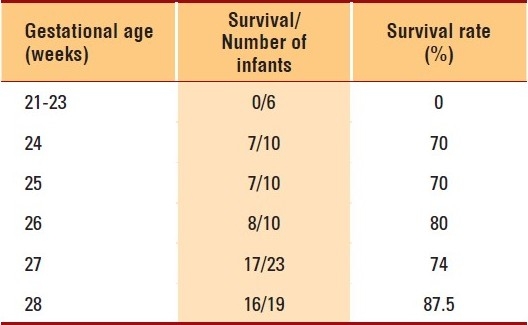
The overall mortality was 17.2%. In infants with birth weights below 1000 g, mortality was 23%, whereas 13.4% of infants with birth weights more than 1000 g died prior to discharge. Furthermore, 40% of the mortalities occurred in infants with birth weights below 700 g. Major congenital anomalies were present in almost one-third of the infants who died. The survival rate per gestational age is depicted in Table 1 for all infants born at a gestational age of 28 weeks or less. When infants born at a gestational age of 23 weeks or less were excluded, the survival to discharge ranged from 70% to 87.5%.
Table 1.
Survival by gestational age
RDS was present in 145 (78%) infants. Surfactant therapy was used in 124 (66.7%) infants; of these infants, 86 (46.2%) received surfactant in the labor room as a prophylactic therapy. Mechanical ventilation was used in 114 (61.3%) infants; of these, high-frequency ventilation was used in 55 (29.6%) infants. Continuous positive airway pressure was used in 98 (52.7%) infants. Pneumothorax was diagnosed in 17 (9.1%) infants. BPD was diagnosed in 33 (17.7%) infants, whereas 19 (10.2%) infants were discharged home on oxygen. Postnatal corticosteroids were used in 8 (4.3%) infants.
PDA was diagnosed in 58 (31.2%) infants; of these, 37 infants received indomethacin, 1 received ibuprofen, and 13 failed medical treatment and underwent surgical ligation. IVH was present in 24 (12.9%) infants; of these, 15 (8.1%) infants were diagnosed with severe IVH. PVL was present in 7 (3.8%) infants. Early bacterial sepsis (first 72 hours) was diagnosed in 3 (1.6%) infants, while 41 (21.9%) infants developed late bacterial sepsis. Coagulase-negative staphylococcus species were responsible for 71% of the cases of late-onset sepsis. Only 4 infants had invasive fungal infection. NEC was diagnosed in 14 (7.5%) infants; 8 of these infants required surgery. ROP of all stages was diagnosed in 53 (28.3%) infants; among these infants, only 7 (3.7 %) had severe ROP, with 5 requiring laser therapy.
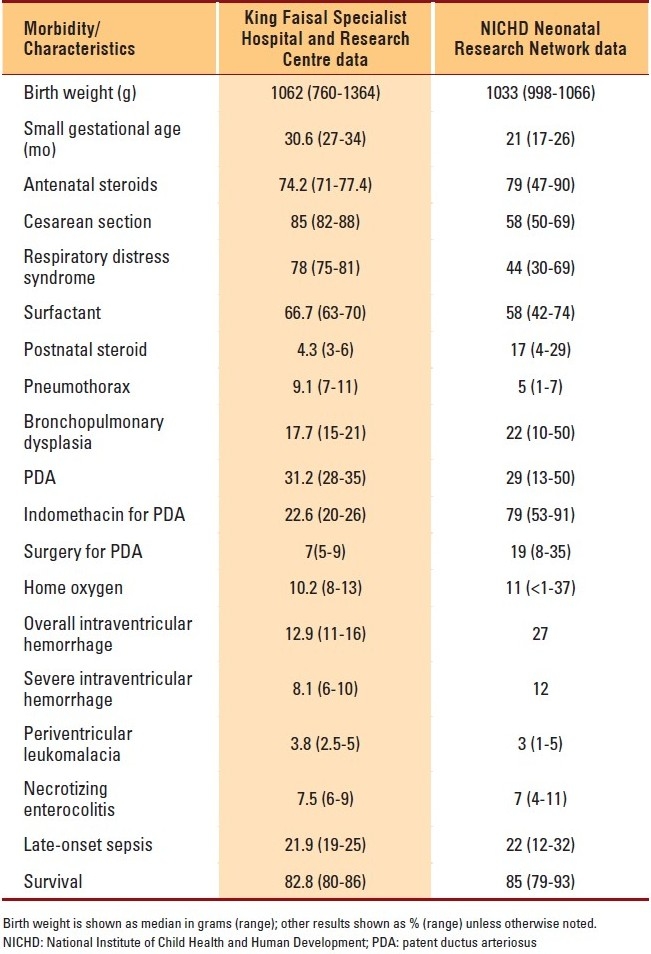
The mean (SD) birth weight of infants in our cohort was similar to that of the infants in the NICHD cohort: 1062 (302) vs. 1033 (289) g. However, the percentage of infants who were small for gestational age was higher in our cohort: 30.6% versus 21% (range, 17%-26%), respectively. The incidence of major neonatal morbidities was similar among the two cohorts except for IVH, where our cohort had a smaller incidence. Table 2 shows a comparison between our center and the NICHD centers in terms of the major morbidities and complications of prematurity.
Table 2.
Outcomes compared with NICHD data
DISCUSSION
Our findings indicate that the short-term outcomes of VLBW infants born at our hospital are comparable with outcomes of similar populations in developed countries. With the development of antenatal and neonatal intensive care during the last two decades, the survival of the VLBW infants has improved significantly.
In our study, we found 82.8% of our population survived to discharge, which is similar to the survival rate (85%) of infants in the NICHD study. Fifty-seven (30.6%) infants were small for gestational age. Antenatal steroids were given to 74.2% of mothers. Furthermore, 40% of the mortalities occurred in infants with birth weights below 700 g.
After excluding infants born at a gestational age of 23 weeks or less, we found that survival to discharge ranged from 70% to 87.5%. The survival to discharge among infants at a gestational age of 23 weeks or less was zero, reflecting the change in the attitude of our professional caregivers toward less aggressive treatment of infants with this gestational age after receiving life support immediately after birth and full evaluation in the NICU, as implemented according to our recent strategies.27–29 Previously, we reviewed the outcomes of 50 infants: 9 were born at 22 weeks gestational age, 17 at 23 weeks, and 24 at 24 weeks. Twenty-two (44%) infants survived to discharge, and 28 (56%) died during hospitalization. The survival rate varied according to the gestational age, with 58% survival for infants born at 24 weeks, 29% for those at 23 weeks and 33% for those at 22 weeks. The majority (89%) of infants died in the first week of life. One infant at 22 weeks, 4 at 23 weeks, and 9 at 24 weeks were found to be free of neurological abnormalities.30,31 Moreover, variations exist between regions in survival rates of infants of borderline viability,32 explained mainly by different attitudes to offering intensive care, both before and after birth. In a Swedish study comparing two population-based areas—one with proactive management and the other with selective management—the proactive strategy was associated with higher survival without an increase in neonatal morbidity than was selective management.33
Current available data on the incidence of short-term morbidities in preterm infants from the region are scarce. Almost all of the published studies from Saudi Arabia are single center–based studies with small sample size and with no focus on VLBW infants; thus they have been inadequate to outline the problem nationwide.13–19
The NICHD Neonatal Research Network study was undertaken to document the mortality and morbidity of infants weighing from 501 to 1500 g at birth, according to gestational age, birth weight, and sex, during two periods (1995-1996 and 1997-2002). When we compared our results with those of the NICHD Neonatal Research Network, we found that the median birth weights were comparable. However, we had slightly more infants of small gestational age and a higher rate of cesarean delivery, most probably due to the higher percentage of maternal risks. At our institution, perinatal services are directed toward pregnant women with complex tertiary care diseases, such as cancer, cardiac diseases, and liver disease. In addition, as a major perinatal referral center, we provide care to a large number of pregnant women with suspected or diagnosed fetal anomalies; this could explain the high rate of major congenital anomalies in our cohort. The rate of using antenatal steroids is almost the same. Despite a similar age group and rate of antenatal steroids use, our study showed a higher rate of incidence of RDS. The use of postnatal steroids was less in our population. The incidence of pneumothorax was 9.1% in our study but 5% in the NICHD Neonatal Research Network cohort. Our incidence of BPD was slightly less; however, the rate of infants that went home on oxygen was similar. We found a similar rate of incidence of PDA, but the use of indomethacin for PDA in our report was much less, as well as the rate of PDA ligation. The rate of incidence of IVH was much less and that of PVL was the same. Rates of NEC and late-onset sepsis were the same.
The limitation of our study is the small number of infants enrolled. This study represents outcomes in a single tertiary care center; thus our results cannot be generalized to all VLBW infants in Saudi Arabia. In conclusion, in our population of VLBW infants, survival rate and complications of prematurity were comparable to international data.
REFERENCES
- 1.Heron M, Sutton P, Xu J, Ventura S, Strobino D, Guyer B. Annual Summary of Vital Statistics: 2007. Pediatrics. 2010;125:4–15. doi: 10.1542/peds.2009-2416. [DOI] [PubMed] [Google Scholar]
- 2.Ventura SJ, Martin JA, Curtin SC, Menacker F, Hamilton BE. Births: Final data for 1999. Natl Vital Stat Rep. 2001;49:1–100. [PubMed] [Google Scholar]
- 3.Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, et al. Trends in mortality and morbidity for Very Low Birth Weight Infants, 1991-1999. Pediatrics. 2002;110:143–51. doi: 10.1542/peds.110.1.143. [DOI] [PubMed] [Google Scholar]
- 4.Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, et al. Very Low Birth Weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. Pediatrics. 2001;107:1–8. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 5.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. NICHD Neonatal Research Network.Trends in morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147.e1. doi: 10.1016/j.ajog.2006.09.014. 8. [DOI] [PubMed] [Google Scholar]
- 6.NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. Effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA. 1995;273:413–8. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 7.Hobe AH. Pulmonary surfactant therapy. N Eng J Med. 1993;328:861–8. doi: 10.1056/NEJM199303253281208. [DOI] [PubMed] [Google Scholar]
- 8.Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the national institute of child health and human development neonatal research network, 1993-1994. Pedistrics. 2000;105:1216–26. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 9.Marlow N. Neurocognitive outcome after preterm birth. Arch Dis Child Fetal Neonatal Ed. 2004;89:F224–8. doi: 10.1136/adc.2002.019752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Ananad KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm.A meta-analysis. JAMA. 2002;288:728–37. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 11.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–9. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt B, Asztalos EV, Roberts RS, Sauve RS, Whitfield MF. For the trial of Indomethacin Prophylaxis in Preterm (TIPP) Investigators.Impact of Bronchopulmonary Dysplasia, brain injury and sever Retinopathy of Prematurity on the outcome of extremely low-birth-weight infants at 18 months. Results from the trial of prophylaxis in preterms. JAMA. 2003;289:1124–9. doi: 10.1001/jama.289.9.1124. [DOI] [PubMed] [Google Scholar]
- 13.Dawodu AH, Al Umran K, Al Faraidy A. Neonatal vital statistics: A 5-year review in Saudi Arabia. Ann Trop Paediatr. 1988;8:187–92. doi: 10.1080/02724936.1988.11748567. [DOI] [PubMed] [Google Scholar]
- 14.Bassuni W, Abbag F, Asindi A, Al Barki A, Al Binali AM. Neonatal deaths in the Asir Region of Saudi Arabia: Experience in a referral neonatal intensive care unit. Ann Saudi Med. 1997;17:522–6. doi: 10.5144/0256-4947.1997.522. [DOI] [PubMed] [Google Scholar]
- 15.Nabi G, Karim MA. Predictors of neonatal mortality in the intensive care unit in Abha, Kingdom of Saudi Arabia. Saudi Med J. 2004;25:1306–7. [PubMed] [Google Scholar]
- 16.Arafa MA, Al Shehri MA. Predictors of neonatal mortality in the intensive care unit in Abha, Saudi Arabia. Saudi Med J. 2003;24:1374–6. [PubMed] [Google Scholar]
- 17.Abdelmoneim I. A study of determinants of low birth weight in Abha, Saudi Arabia. Afr J Med Sci. 2004;33:145–8. [PubMed] [Google Scholar]
- 18.Khashoggi TY. Outcome of pregnancies with preterm premature rupture of membranes. Saudi Med J. 2004;25:1957–61. [PubMed] [Google Scholar]
- 19.Abu-Heija AT. Maternal and neonatal outcome of high order gestation. Arch Gynecol Obstet. 2003;268:15–8. doi: 10.1007/s00404-002-0322-7. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Vital Statistics Natality data set for 2001 and 2002, Rockvill, MD: National Center for Health Statistics. 2004 [Google Scholar]
- 21.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and hemorrhage: A study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 22.Salama H, da Silva O. Images in clinical medicine.Neonatal necrotizing enterocolitis. N Engl J Med. 2001;344:108. doi: 10.1056/NEJM200101113440205. [DOI] [PubMed] [Google Scholar]
- 23.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis: Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An international classification of retinopathy of prematurity. Pediatrics. 1984;74:127. 33. [PubMed] [Google Scholar]
- 25.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcome in premature infants: Prediction from oxygen requirement in the neonatal period. Pediatrics. 1998;82:527–32. [PubMed] [Google Scholar]
- 26.Bancalari E, Calure N, Sosenko IR. Bronchopulmonary Dysplasia: Changes in pathogenesis, epidemiology and definition. Semin Neonatol. 2003;8:63–71. doi: 10.1016/s1084-2756(02)00192-6. [DOI] [PubMed] [Google Scholar]
- 27.Al-Alaiyan S. The decision not to resuscitate infants born at limit of viability in Saudi Arabia: An Islamic legal opinion. Paediatrics. 2009;14:8–10. [Google Scholar]
- 28.Al-Alaiyan S, Al-Hazzani F. The need for hospital-based neonatal palliative care programs in Saudi Arabia. Ann Saudi Med. 2009;29:337–41. doi: 10.4103/0256-4947.55161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Alaiyan S. Limit of viability in Saudi Arabia: An Islamic legal opinion. J Neonatal Perinatal Med. 2009;203:203. [Google Scholar]
- 30.Al-Alaiyan S. Call to establish a national lower limit of viability. Ann Saudi Med. 2008;28:1–3. doi: 10.5144/0256-4947.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aqeel A, Al-Deery M, Al-Alaiyan S. Outcome of infants born at the limit of viability: A local experience. Paediatrics. 2001;6:34–7. [Google Scholar]
- 32.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–9. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 33.Håkansson S, Farooqi A, Holmgren PA, Serenius F, Högberg U. Proactive management promotes outcome in extremely preterm infants: A population-based comparison of two perinatal management strategies. Pediatrics. 2004;114:58–64. doi: 10.1542/peds.114.1.58. [DOI] [PubMed] [Google Scholar]


