Abstract
BACKGROUND AND OBJECTIVES:
The effects of vitamin D on bone mass remain to be understood. This study was conducted with the objective of evaluating the influence of 25-hydroxyvitamin D (25OHD) levels on bone mineral density (BMD) among Saudi nationals.
DESIGN AND SETTING:
Cross-sectional study carried out at university hospital from 1 February 2008 to 31 May 2008.
SUBJECTS AND METHODS:
Healthy Saudi men and women in the peak bone mass (PBM) age group and those aged ≥50 years were recruited from the outpatient department of King Fahd University Hospital, Al Khobar, Saudi Arabia, between February 1, 2008, and May 31, 2008. Patient age and sex were documented, and body mass index was calculated. Hematological, biochemical, and serum 25OHD tests were performed. BMD was determined by dual-energy x-ray absorptiometry of the upper femur and lumbar spine. Patients were divided into three groups, based on their 25OHD level.
RESULTS:
Data from 400 patients were analyzed. Among individuals with a normal 25OHD level, 50% of women and 7% of men in the PBM age group and 26.4% of women and 49.2% of men aged ≥50 years had low bone mass. In patients with 25OHD insufficiency, 84.2% of women and 88.9% of men in the PBM age group and 83.3% of women and 80% of men aged ≥50 years had low bone mass. Results for patients with 25OHD deficiency revealed that none of the men and women in the PBM age group or ≥50 years old had normal BMD. Significant positive correlations between 25OHD level and BMD and significant negative correlations with parathyroid hormone were shown in most of the groups.
CONCLUSIONS:
This study showed that the vitamin D level significantly influences BMD reading among Saudi individuals. Evaluation and treatment of hypovitaminosis D should be considered during management of low bone mass.
Adequate levels of vitamin D have an important effect on bone mass in the young and old. Hypovitaminosis D adversely affects calcium metabolism, osteoblastic activity, matrix ossification, bone remodeling, and hence bone density.1,2 Low 25-hydroxyvitamin D (25OHD) was also reported to be associated with secondary hyperparathyroidism and increased bone turnover.3 Vitamin D deficiency can be an important risk factor for osteoporosis.4,5 On the other hand, an adequate vitamin D level has been shown to prevent osteoporotic fractures.6,7
Bone mineral density (BMD), which measures the quantity of the calcified bone, at present is the gold standard technique for the diagnosis of osteopenia and osteoporosis. Unfortunately, BMD does not differentiate between osteomalacia and osteoporosis, which means patients with osteomalacia or osteoporosis may be misdiagnosed, one for the other, and thus mismanaged, if the vitamin D level is not measured. In general, serum 25OHD is a robust and reliable marker of vitamin D status,8 and although there is no consensus on the definition of an optimal serum 25OHD level, vitamin D deficiency is defined by most experts as a serum 25OHD level <50 nmol/L (<20 ng/mL), whereas a serum 25OHD level of >75 nmol/L (>30 ng/mL) is considered to be normal, and a level of 50-75 nmol/L (20-30 ng/mL) defines vitamin D insufficiency.9
Ethnically, Saudi Arabians are known to have low vitamin D levels,10-13 and the incidence of osteoporosis among healthy Saudi individuals has been reported to be between 23% and 31%.14,15 In light of the high prevalence of both a vitamin D deficiency and low bone mass among Saudi nationals, we hypothesized that vitamin D deficiency contributes to low bone mass among Saudi Arabs. This study was carried out with the objective of evaluating the relationship between vitamin D levels and bone mass among Saudi individuals. To our knowledge, the relationship between vitamin D and bone mass among both the male and female Saudi population has not been evaluated. Also, there are a scarcity of reports from the Middle East on this topic.
SUBJECTS AND METHODS
This cross-sectional observational study was carried out at the King Fahd University Hospital, Al Khobar, located in the eastern province of Saudi Arabia. This study was performed from February 1 to May 31, 2008. We recruited 400 healthy Saudi Arabian men and women: 200 subjects (100 men and 100 women) were at the age of peak bone mass (PBM) (between 25 and 35 years) and 200 subjects (100 men and 100 women) were ≥50 years of age. The study was approved by the Ethical and Research Committees of King Fahd University Hospital and King Faisal University, Dammam. Informed verbal consent was obtained. None of the participants received any form of remuneration for participation.
Physical examination was performed and history was compiled for all subjects. Data collected included age, sex, and lifestyle. Weight and height measurements were taken while patients wore light clothes, using a Detecto scale to the nearest 0.1 kg and 0.5 cm. Body mass index was calculated using the formula “weight in kilograms divided by the square of the height in meters.” Exclusion criteria included the presence of organ dysfunction and chronic medical illnesses or being on medications that can alter the level of vitamin D or affect bone mass. Pregnant, lactating, and postpartum females were also excluded. Blood was drawn in the morning between 7 am and 10 am in a fasting state for serum calcium, serum phosphorous, serum albumin, alkaline phosphatase, serum parathyroid hormone (PTH), and serum 25OHD. Serum 25OHD levels were measured by radioimmunoassay using Wallac1470 Gamma Counter (Wallac Inc., Gaithersburg, MD, USA). In this study, the 25OHD level was considered to be normal if it was ≥30 ng/mL (≥75 nmol/L), insufficient if it was between 20 and 30 ng/mL (50 and 75 nmol/L), and deficient if it was <20 ng/mL (<50 nmol/L). Based on 25OHD levels, subjects were divided into three groups (group 1, with vitamin D sufficiency; group 2, with vitamin D insufficiency; and group 3, with vitamin D deficiency). Intact PTH was determined by an immunoradiometric assay. The normal range for PTH at our laboratory is 1.3-7.6 pmol/L. Serum calcium, serum phosphorous, serum albumin, and alkaline phosphatase were determined according to standard laboratory procedures. BMD was measured using dual-energy x-ray absorptiometry (DXA) scan (Hologic, Waltham, MA, USA) at the hip region and the lumbar spine. Hip BMD included trochanter, femoral neck, and intertrochanteric regions; lumbar spine BMD included lumbar vertebrae L1-L4. Both T and Z scores were obtained. The reference value of T and Z scores was entered in the DXA machine with software for the Asian reference value. We considered osteopenia when the T score of total lumbar spine or total hip was between -1 and -2.5, and osteoporosis was considered when the T score was <-2.5.16
Data were analyzed using the Statistical Package for Social Sciences (SPSS), version 14.0 (Chicago, IL, USA). Data are expressed as mean and standard deviation (SD). Statistically significant differences between groups were determined with a Student t test. P values less than .05 and a CI of 95% were used to indicate statistical significance.
RESULTS
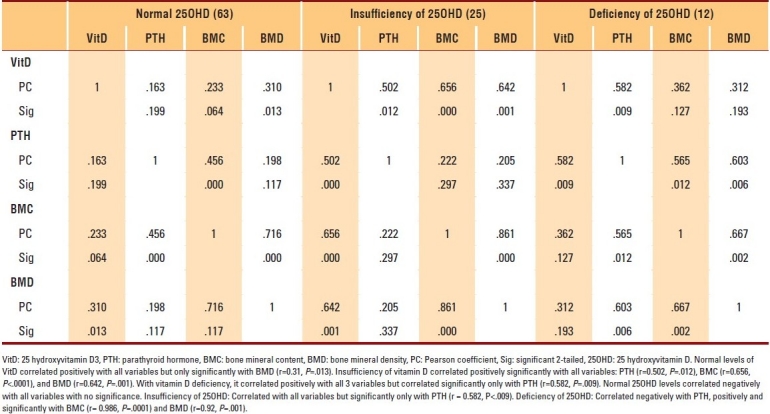
The data of 400 subjects were analyzed. Men and women with vitamin D deficiencies were significantly older than those with normal vitamin D levels (P=.01 and P=.03, respectively). Among subjects with normal 25OHD levels, only 7.2% of women and 2.8% of men in the PBM age group had BMD readings consistent with osteoporosis, whereas more than 42.9% of women had BMD readings in the range of osteopenia compared to approximately 4.2% of men (P≤.001). The results of individuals aged ≥50 years with a normal 25OHD level revealed that 17.6% of women and 11.1% of men had BMD readings consistent with osteoporosis, whereas 38.1% of men and 8.8% of women had BMD readings in the range of osteopenia (P≤.001) (Table 1). The majority of individuals with 25OHD insufficiency had low BMD. Only 15.8% of women and 11.1% of men in the PBM age group and 16.7% of women and 20% of men aged ≥50 years had normal BMD, with more men in the PBM age group and more women at older age having BMD readings in the range of osteopenia (Table 2). As shown in Table 3, none of the subjects in both age groups had normal BMD, and the majority had BMD readings consistent with osteoporosis. Female and male subjects aged ≥50 years were found to be at greater risk of having BMD readings consistent with osteoporosis than subjects in the PBM age group. Tables 4–7 show the correlations between vitamin D level, BMD, and PTH among male and female subjects in the PBM and ≥50 years of age groups. With few exceptions, there was a significant positive correlation between vitamin D level and BMD and a significant negative correlation between vitamin D status and PTH level.
Table 1.
Results of men and women with normal 25OHD (>30 pg/mL) (group 1)
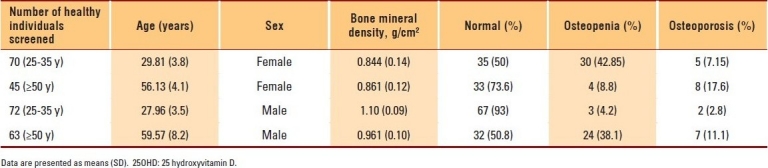
Table 2.
Results of men and women with insufficiency of 25OHD (21-29 pg/mL) (group 2)
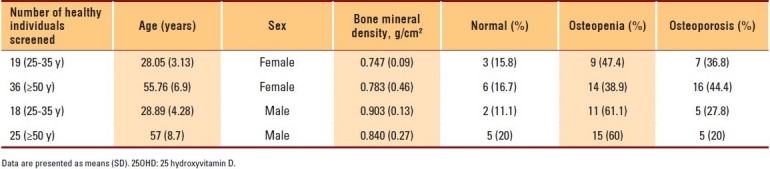
Table 3.
Results of men and women with deficiency of 25OHD (<20 pg/mL) (group 3)
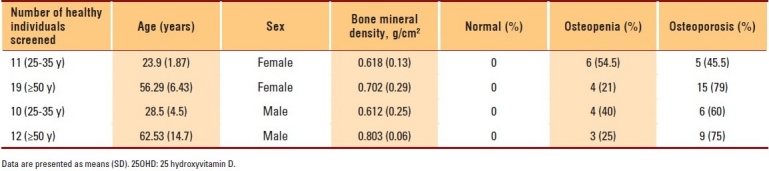
Table 4.
Correlation of 25OHD to other assessed parameters in postmenopausal women aged ≥50 years
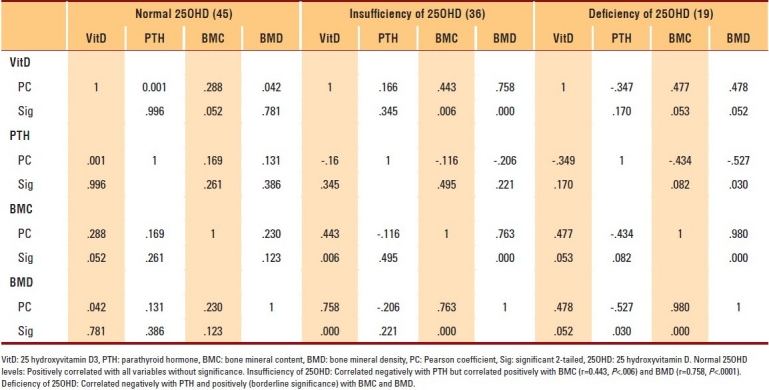
Table 7.
Correlation of 25OHD to other assessed parameters in men aged ≤35 years
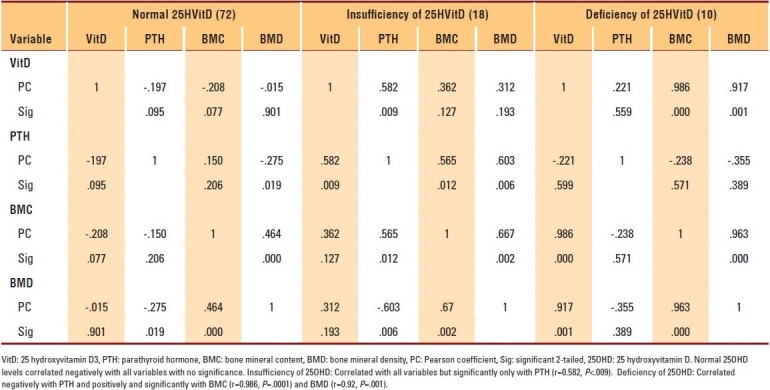
Table 5.
Correlation of 25OHD to other assessed parameters in women aged ≤35 years
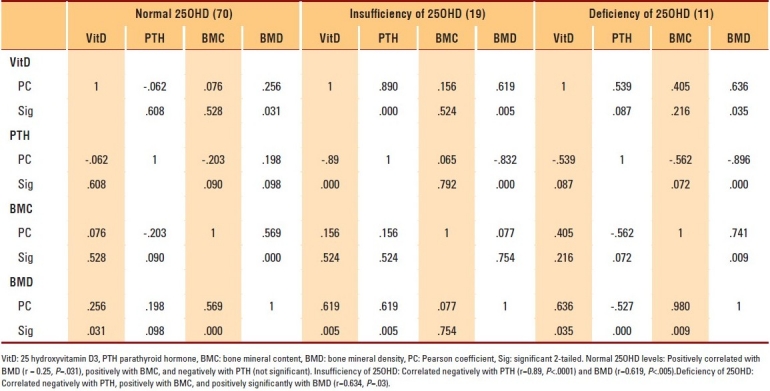
Table 6.
Correlation of 25OHD to other assessed parameters in men aged ≥50 years
DISCUSSION
An evaluation of vitamin D status in patients with osteoporosis is essential for two main reasons. First, vitamin D deficiency causes defective bone mineralization and leads to low bone mass.1,2 Second, optimal vitamin D repletion in patients with osteoporosis is important to maximize the response to anti-resorptive therapy in terms of both BMD changes and anti-fracture efficacy.17 Our study revealed that the majority of subjects with an insufficiency of 25OHD had low bone mass, whereas 100% of subjects with 25OHD deficiency had BMD readings in the range of osteopenia or consistent with osteoporosis. This study also showed a positive correlation between BMD and 25OHD in most subjects, particularly in the insufficiency and deficiency groups. On the basis of our findings, we emphasize that it is important to measure 25OHD levels in Saudi patients with low bone mass, rather than relying on BMD alone.
The association between 25OHD and BMD is still debatable. Some studies suggest that a low serum 25OHD level is associated with low BMD.18–21 In fact, the initial results of the study by Bischoff-Ferrari et al18 showed a strong positive relationship between 25OHD and BMD among white young and older males. However, no such association has been found in other studies.22–24 The above heterogeneity in the results of the relationship between vitamin D status and BMD can be partially explained by differences in populations, differences in age groups, and differences in the sites of the body studied. For example, Garnero et al in the Os des Femmes de Lyon (OFELY) study25 and Allali et al26 failed to show any significant correlation between 25OHD levels and BMD after adjusting for age. However, Rassouli et al27 found a positive correlation with spine BMD, but not with hip BMD.More important is the fact that different vitamin D levels were used to define vitamin D deficiency and insufficiency during such studies.23–25
On the other hand, we found a significant negative correlation between 25OHD and PTH levels. The elevated PTH level in subjects with low 25OHD levels possibly contributed to low bone mass.3,28 Age could also be another contributing factor, since men and women with vitamin D deficiencies are significantly older than individuals with normal vitamin D levels.
This study has some limitations, including the fact that only a single measurement of vitamin D was done. In addition, apart from alkaline phosphatase, no other bone markers were studied. Also, no multivariate analysis was carried out to evaluate the effects of other confounding factors on bone mass, such as age, sex, and lifestyle. Despite the above limitations, this study supported our initial hypothesis that vitamin D deficiency can be an important contributing factor to low bone mass among the Saudi population.
In conclusion, our study showed a positive association between vitamin D levels and low bone mass in Saudi men and women in both the PBM and older age groups. Because prevention of low bone mass can be achieved by nonpharmacological means, adequate intake of vitamin D and calcium becomes imperative and should be encouraged. There is still a paucity of data on hypovitaminosis D and its effects on bone mass and PTH among Saudi citizens, but we strongly emphasize that proper evaluation and treatment of hypovitaminosis D should be considered during the management of low bone mass.
REFERENCES
- 1.Lukert B, Higgins J, Stoskopf M. Menopausal bone loss is partially regulated by dietary intake of vitamin D. Calcif Tissue Int. 1992;51:173–9. doi: 10.1007/BF00334543. [DOI] [PubMed] [Google Scholar]
- 2.Villareal DT, Civitelli R, Chines A, Avioli LV. Subclinical vitamin D deficiency in Postmenopausal women with low vertebral bone mass. J Clin Endocrinol Metab. 1991;72:628–34. doi: 10.1210/jcem-72-3-628. [DOI] [PubMed] [Google Scholar]
- 3.Khaw KT, Sneyd MJ. Compston J BMD, parathyroid hormone and 25-Hydroxyl vitamin D concentrations in middle aged women. BMJ. 1992;305:273–7. doi: 10.1136/bmj.305.6848.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heaney RP. Nutritional factors in osteoporosis. Annu Rev Nutr. 1993;13:287–316. doi: 10.1146/annurev.nu.13.070193.001443. [DOI] [PubMed] [Google Scholar]
- 5.Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006;260:245–54. doi: 10.1111/j.1365-2796.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 6.Washington DC: National Osteoporosis Foundation; 1998. National Osteoporosis Foundation. Pocket guide to the prevention and treatment of osteoporosis. [Google Scholar]
- 7.Chapuy MC. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–42. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 8.Seamans KM, Cashman KD. Existing and potentially novel functional markers of vitamin D status: a systematic review. Am J Clin Nutr. 2009;89:1997S–2008S. doi: 10.3945/ajcn.2009.27230D. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–8. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Turki H, Sadat-Ali M, Al-Elq A, Al-Mulhim F, Al-Ali M. 25- Hydroxy vitamin D levels among healthy Saudi Arabian Women. Saudi Med J. 2008;29:1765–8. [PubMed] [Google Scholar]
- 11.Sadat-Ali M, Al-Elq AM, Al-Turki H, Al-Mulhim F, Al-Ali A. Vitamin D levels among Healthy Saudi Arabian Men. Annals of Saudi Med. 2009;29:378–82. doi: 10.4103/0256-4947.55168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ardawi MS, Qari MH, Rouzi AA, Maimani AA, Raddadi RM. Vitamin D status in relation to obesity, bone mineral density, bone turnover markers and vitamin D receptor genotypes in healthy Saudi pre- and postmenopausal women. Osteoporos Int. 2010 Apr 30; doi: 10.1007/s00198-010-1249-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Sedrani SH, El Arabi KM, Abanmy A, Elidrissy AWTH. Vitamin D Status of Saudis II.Effect of regional and environmental location. Saudi Med J. 1992;13:206–13. [Google Scholar]
- 14.Al-Habdan IM, Sadat-Ali M, Al-Muhanna FA, Al-Elq AH, Al-Mulhim AA. Bone mass measurement using quantitative ultrasound in healthy Saudi women. A cross-sectional screening. Saudi Med J. 2009;30:1426–31. [PubMed] [Google Scholar]
- 15.Ardawi MS, Maimany AA, Bahksh TM, Nasrat HA, Milaat WA, Al-Raddadi RM. Bone mineral density of the spine and femur in healthy Saudis. Osteoporosis Int. 2005;16:43–55. doi: 10.1007/s00198-004-1639-9. [DOI] [PubMed] [Google Scholar]
- 16.Washington DC: National Osteoporosis Foundation; 2002. National Osteoporosis Foundation. American's Bone Health. The state of Osteoporosis and Low Bone Mass in Our Nation. [Google Scholar]
- 17.Adami S, Giannini S, Bianchi G, Sinigaglia L, Di Munno O, Fiore CE. Vitamin D status and response to treatment in post-menopausal osteoporosis. Osteoporos Int. 2009;20:239–44. doi: 10.1007/s00198-008-0650-y. [DOI] [PubMed] [Google Scholar]
- 18.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study on younger and older adults. Am J Med. 2004;1:634–9. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 19.Fradinger EE, Zanchetta JR. Vitamin D and bone mineral density in ambulatory women living in Buenos Aires, Argentina. Osteoporos Int. 2001;12:24–7. doi: 10.1007/s001980170153. [DOI] [PubMed] [Google Scholar]
- 20.Hannan MT, Litman HJ, Araujo AB, McLennan CE, McLean RR, McKinlay JB, et al. Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab. 2008;93:40–6. doi: 10.1210/jc.2007-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuchuk NO, Pluijm SM, van Schoor NM, Looman CW, Smit JH, Lips P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009;94:1244–50. doi: 10.1210/jc.2008-1832. [DOI] [PubMed] [Google Scholar]
- 22.Ghannam NN, Hammami MM, Bakheet SM, Khan BA. Bone mineral density of the spine and femur in healthy Saudi females: Relation to vitamin D status, pregnancy, and lactation. Calcif Tissue Int. 1999;65:23–8. doi: 10.1007/s002239900652. [DOI] [PubMed] [Google Scholar]
- 23.Hosseinpanah F, Rambod M, Hossein-nejad A, Larijani B, Azizi F. Association between vitamin D and bone mineral density in Iranian postmenopausal women. J Bone Miner Metab. 2008;26:86–92. doi: 10.1007/s00774-007-0791-7. [DOI] [PubMed] [Google Scholar]
- 24.Akhter N, Sinnott B, Mahmood K, Rao S, Kukreja S, Barengolts E. Effects of vitamin D insufficiency on bone mineral density in African American men. Osteoporos Int. 2009;20:745–50. doi: 10.1007/s00198-008-0746-4. [DOI] [PubMed] [Google Scholar]
- 25.Garnero P, Munoz F, Sornay-Rendu E, Delmas PD. Associations of vitamin D status with bone mineral density, bone turnover, bone loss and fracture risk in healthy postmenopausal women. The OFELY study. Bone. 2007;40:716–22. doi: 10.1016/j.bone.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 26.Allali F, El Aichaoui S, Khazani H, Benyahia B, Saoud B, El Kabbaj S, et al. High prevalence of hypovitaminosis D in Morocco: Relationship to lifestyle, physical performance, bone markers, and bone mineral density. Semin Arthritis Rheum. 2009;38:444–51. doi: 10.1016/j.semarthrit.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Rassouli A, Milanian I, Moslemi-Zadeh M. Determination of serum 25-hydroxyvitamin D (3) levels in early postmenopausal Iranian women: relationship with bone mineral density. Bone. 2001;29:428–30. doi: 10.1016/s8756-3282(01)00591-9. [DOI] [PubMed] [Google Scholar]
- 28.Jesudason D, Need AG, Horowitz M, O’Loughlin PD, Morris HA, Nordin BE. Relationship between serum 25-hydroxyvitamin D and bone resorption markers in vitamin D insufficiency. Bone. 2002;31:626–30. doi: 10.1016/s8756-3282(02)00866-9. [DOI] [PubMed] [Google Scholar]


