Abstract
Myocardial perfusion single photon emission-computed tomography (MPS) has been one of the most important and common non-invasive diagnostic cardiac test. Gated MPS provides simultaneous assessment of myocardial perfusion and function with only one study. With appropriate attention to the MPS techniques, appropriate clinical utilization and effective reporting, gated MPS will remain a useful diagnostic test for many years to come. The aim of this article is to review the basic techniques of MPS, a simplified systematic approach for study interpretation, current clinical indications and reporting. After reading this article the reader should develop an understanding of the techniques, interpretation, current clinical indications and reporting of MPS studies.
Myocardial perfusion single photon emission-computed tomography (MPS) is one of the most important and commonly performed non-invasive cardiac imaging tests. MPS plays a key role in diagnosis of cardiovascular disease, establishing prognosis, assessing the effectiveness of therapy, and evaluating myocardial viability. The success of this imaging modality has, in large part, been due to advanced technology that continues to improve and expand the field. This include progressive improvement of single photon emission-computed tomography (SPECT) technologies, new radiopharmaceuticals and new software.1
This report reviews different aspects of MPS including techniques, methods of interpretation, current clinical applications and reporting. Currently, most stress MPS is performed with ECG-gated SPECT for evaluation of both myocardial perfusion and cardiac function simultaneously. Several stress techniques are available, but the exercise stress test is the preferred method because it provides several important prognostic data in addition to increasing coronary blood flow. In case of contraindication to exercise stress testing, pharmacological stress must be considered. Thalium-201 (201Tl- chloride) and technetium-99m (99mTC) sestamibi and tetrofosmin are the currently used radiopharmaceuticals. There are several artifacts and interpretation pitfalls that can potentially compromise MPS. It is essential for both the technologist and the interpreting physician to be aware of these potential sources of error, take appropriate steps to limit them beforehand, wherever possible correct them if they do occur, and when they cannot be eliminated, recognize their potential impact on the interpretation of the study. The major indications for MPS are diagnosis of coronary artery disease (CAD), risk stratification in patients with known CAD, assessment of therapy and intervention and myocardial viability before percutaneous intervention or bypass surgery. Therefore, close collaboration between the referring and interpreting physicians is essential to ensure appropriate indication for the study, optimal patient preparation and effective use of the test result in patient clinical management. Currently, there are several appropriate indications for stress MPS; familiarity with appropriate indications is crucial to ensure proper utilization of the test and avoidance of inappropriate use with subsequently excessive cost and radiation.2
After reading this review the readers will understand (1) techniques of MPS in term of patient preparation, radiopharmaceuticals, methods of stress testing and different imaging protocols, (2) interpretation of normal and abnormal MPS with special attention on artifacts and pitfalls, (3) current clinical applications and (4) reporting of MPS as per current guidelines.
Techniques
Patient preparation
Patients should have nothing orally (expect oral medication or water) after midnight before the examination to limit gut activity that may interfere with the evaluation of the inferior wall of the left ventricle (LV). Patients should wear comfortable clothing for the exercise portion of the exam. Medications that contain methylxanthines or caffeine and food beverage with caffeine must be avoided for 12-24 hours if vasodilator stress test is anticipated.3 Before imaging, metal or other potential attenuators must be removed if they project on the imaging field so as to avoid attenuation artifacts. If the study is being done for primary diagnosis of CAD, certain cardiac medication such as nitroglycerine or β-blockers must be avoided to increase the sensitivity.
Cardiac stressing
Stress MPS assesses the physiological significance of coronary stenosis by inducing heterogeneity in coronary flow.4 Resting coronary flow is maintained until there is an approximately 90% reduction of coronary arterial flow. However, the ability to maintain the maximum flow (termed coronary flow reserve) is impaired with approximately 50% coronary stenosis. An increase in coronary flow can be achieved by increased oxygen demand with exercise (treadmill or bicycle), b-adrenergic agonist (dobutamine) or by direct vasodilator (adenosine, dipyridamole).
Treadmill exercise
Graded exercise testing is preferred over pharmacological testing in patients able to exercise adequately because it provides an assessment of well-validated prognostic markers such as workload, ST segment depression, anginal symptoms, heart rate and blood pressure response. Maximum exercise results in a 3-4 fold increase in coronary blood flow secondary to (1) an increase in myocardial oxygen consumption due to increase in heart rate and contractility and (2) flow-mediated vasodilatation from the release of endothelial-derived factor from normal endothelial cell in response to stress from increased flow. The diagnostic accuracy of the examination is dependent on the ability of the patient to exercise to induce maximum vasodilatation, a frequently used index, to determine if the patient has exercised adequately to attain his target heart rate (THR). THR is 85% of the predicted maximum heart rate (PMHR) where PMHR=(220-age) beat per minute.
Pharmacological stress test
Pharmacological vasodilatation with adenosine or dipyridamole is indicated for patients who are unable to exercise or unable to increase their heart rate (rate-limiting medications) and patients with left bundle branch block (LBBB) or paced ventricular rhythm.5 Vasodilator stress with adenosine or dipyridamole creates flow heterogeneity by causing a great increase in coronary blood flow in normal coronary arteries compared with coronary arteries with significant stenosis. Vasodilator stress is contraindicated in patients with significant reactive airway disease. Methylxanthines such as caffeine and theophylline competitively block adenosine receptors and must be avoided for 24 hours prior examination.
Dobutamine stress test
Dobutamine is a β-adrenergic agonist with both positive chronotropic and inotropic effects resulting in coronary artery dilatation secondary to an increase in myocardial oxygen demand.6 Dobutamine is less potent than either exercise or a vasodilator for maximizing coronary blood flow (2-3-fold increase in the coronary flow) and may be associated with a lower sensitivity in the detection of CAD. It is primary used for patients with reactive airways disease and when adenosine or dipyridamole are contraindicated. Contraindications for dobutamine include acute coronary syndrome (recent MI or unstable anima), significant LV outflow obstruction (hypertropic cardiomypathy and severe aortic stenosis), arrhythmia (atrial tachycardia with uncontrolled ventricular response), complex ventricular ectopy, Wolff-Parkinson-White syndrome and severe hypertension.
Radiopharmaceuticals
In stress MPS, the injected radiopharmaceuticals are delivered to the myocardium in proportion to flow. There is reduced tracer uptake in the regions with reduced flow and incensed tracer uptake in the region with increase blood flow. The currently used radiotracers in MPS are 201Tl-chloride, 99mTc- sestamibi and 99mTc- tetrofosmin. 201Tl-chloride: The uptake of 201Tl-chloride is primarily an active process involving the Na-K ATPase pump. The extraction efficiency of 201Tl-chloride is 85%.7 In the physiological flow range, uptake is proportional to flow rate; at higher flow rates the extraction efficiency decreases modestly. While the initial distribution of 201Tl-chloride is proportional to flow, over the next several hours there is a net washout of activity from the heart, so that the late distribution of 201Tl-chloride reflects the viable myocytes. It has a half-life of 72 hours and decays by electron capture. Characteristic X-rays (range 63-80 keV) produced during electron capture in addition to low-abundance 135-167 keV γ-rays are imaged. 201Tl-chloride has relatively poor imaging characteristics.
99mTc-labeled radionuclides (99mTc sestamibi and 99mTc-tetrofosmin) are the most commonly used radiopharmaceuticals in MPS.8 The extraction efficiency is approximately 65% in the physiological flow range, the uptake is proportional to flow, and the deposition of the tracer does not increase linearly with flow, but rather tends to level off at higher flow rate. The distribution of 99mTc-related radionuclides remains relatively fixed over several hours. This allows imaging to be delayed up to several hours after injection, thereby facilitating the evaluation of patients presenting with acute chest pain. Hepatobiliary excretion of 99mTc may result in liver and/or gut activity obscuring the inferior wall of the LV. To immunize adjacent infradiaphragmatic activity imaging is delayed for at least 30 minutes after a stress injection and 60 minutes after a rest injection. The hepatobiliary excretion of 99mTc tetrofosmin is less compared to sestamibi and evaluation of the inferior wall is less problematic.
Imaging protocols
The traditional protocol of 201Tl-chloride involves stress imaging followed by redistribution imaging 3 or 4 hours later and when necessarily delayed, imaging is obtained 24 hours later to evaluate fixed defects (mainly to assess the myocardium viability). The second 201Tl-chloride protocol involves performance of the optional delayed images on the same day only following an injection of a small amount of 201Tl-chloride in patients with a fixed defect. The third 201Tl-chloride protocol involves obtaining the 3 or 4-hour images only after injection of small does of 201Tl-chloride, and delayed images at 24 hours is still performed when needed.
For 99mTc agents there are four protocols. The original protocol involves two injections of the radiopharmaceuticals on two different days. For patient convenience the original protocol was modified to use a low dose and high dose on the same day starting with either rest and then stress (2nd protocol and most commonly used, or stress then rest (3rd protocol). The fourth protocol is the so-called dual isotopes, in which 201Tl-chloride images is first performed following resting injection and 99mTc radiopharmaceutical is then injected during stress.9 Any of these protocols has its proponents, which usually emphasizes the practical logistical consequences, as available scientific evidence has not established the clear superiority of any one of these protocols.10 In patients with established CAD and severe LV dysfunction, only resting imaging may be acquired in order to demonstrate normal or near normal uptake of either 99mTc agents or 201Tl-chloride and therefore myocardial viability.
Interpretation of MPS
Approach to interpretation
The interpretation of MPS SPECT images should be performed in a systematic and consistent fashion to include: (1) evaluation of the raw images in cine mode to determine the presence of potential sources of image artifact and the distribution of extracardiac tracer activity; (2) proper alignment of the post-stress and rest images; (3) interpretation of images with respect to the location, size, severity and reversibility of perfusion defects as well as cardiac chamber sizes, and especially for Tl-201, presence or absence of increased pulmonary uptake; (4) incorporation of the results of quantitative perfusion analysis; (5) consideration of functional data obtained from the gated images; and (6) consideration of clinical factors that may influence the final interpretation of the study. All of these factors contribute to the production of a final clinical report.11
Normal MPS and variants
Both rest and stress images must be evaluated carefully for any recognized artifacts before visual interpretation. In normal myocardial perfusion study, there is homogenous radiotracer distribution in both stress and rest images (Figure 1). There is, however, slightly diminished activity in the apex accounted for by physiological apical thinning, which is usually localized to the apex and does not extend to the anterior wall. Normal thinning of the basal membranous septum and basal inferior wall causes perfusion defect in the corresponding segments. Focal increase activity in and at insertion of papillary muscle (about 2 and 6 o’clock positions) may give a false impression of a defect adjacent to or between them; reviewing these in long-axis images will demonstrate homogenous normal distribution in these region.
Figure 1.
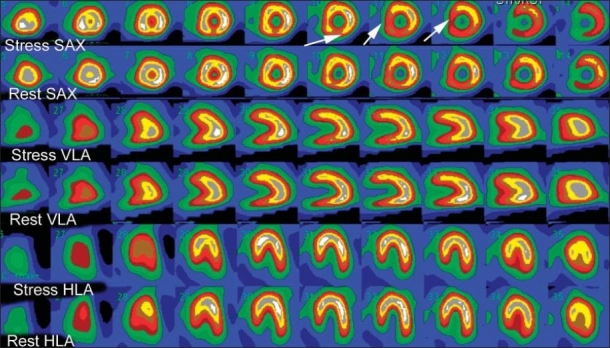
Normal myocardial perfusion SPECT (MPS) in a 55-year-old man referred because of positive exercise stress test. Both stress and rest images demonstrate normal radiotracer distribution. A mild decreased uptake in basal inferior wall and septum is normal due to membranous septum (white arrows). The top two rows are short-axis (SAX) images of the left ventricle at stress and the second row the corresponding SAX images at rest. The next two rows are vertical long-axis images (VLA) at stress and rest. The bottom two rows are horizontal long axis (HLA) at stress and rest.
Normal appearance of attenuation correction MPS
In general, image appearance following attenuation correction is superior with regard to contrast and resolution.12,13 The right ventricle appears more prominent on corrected images but should not be confused with the presence of right ventricular hypertrophy (Figure 2). The septum intensity appears greater than the lateral wall. Also, the physiological apical thinning is more frequently present on attenuation-corrected images and might resemble a true perfusion defect. These findings may reflect a more accurate assessment of tracer distribution and improved perfusion images with attenuation correction.
Figure 2.
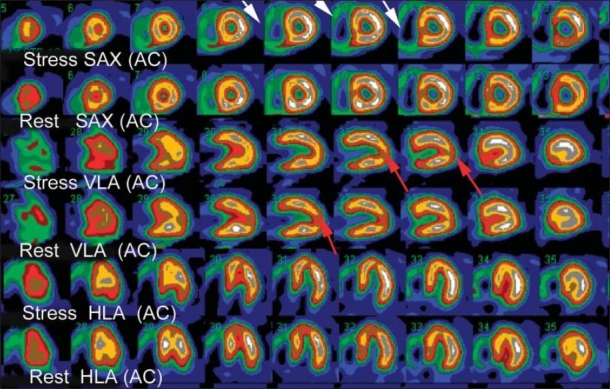
Normal attenuation correction (AC) MPS in 62-year-old women was performed for risk assessment before vascular surgery. In the AC images the right ventricle is clearly visualized, not to be confused with right ventricular hypertrophy (white arrows). The normal apical thing is more severe in AC images, and sometimes appears as a true perfusion defect (red arrows).
Abnormal MPS
True myocardial perfusion defect should be described with reference to (1) the defect size or extent (small, medium and large), (2) severity of perfusion defect (mild, moderate,and severe), (3) extent of reversibility (reversible, irreversible or reverse redistribution) and (4) location (based on 17 segment model and coronary artery territory if possible). Initial interpretation is usually visual (quantitative) analysis followed by semiquantitative and quantitative analysis.14,15
Visual Analysis
The perfusion defect size may be quantitatively described as small, medium or large. Small defect represents less than 10%, medium 10-20% and large represents greater than or equal 20% of the LV myocardium (Figures 3a,b,c). Alternatively, the defect size may be estimated as a fraction such as the apical half of the anterior wall. The defect severity is expressed visually as mild, moderate or severe. A mild defect is identified by a decrease count compared to the adjacent wall with preserved thickness, moderate defect demonstrate wall thinning and severe defect appears similar to background activity (Figure 4).
Figure 3a.
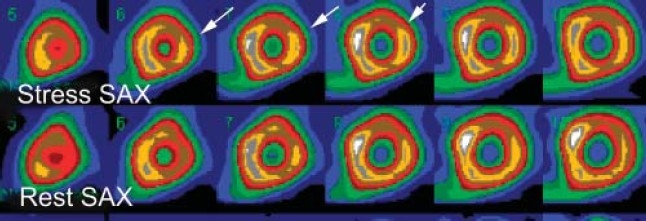
The size or extent of the myocardial perfusion in three different patients. Selected short-axis images (SAX) at stress and rest. Small size (less than 10% of the LV myocardium) reversible anterolateral wall perfusion defect (arrows).
Figure 3b.
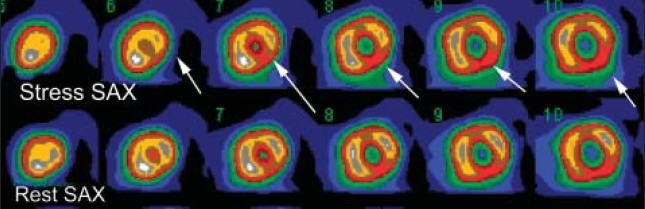
The size or extent of the myocardial perfusion in three different patients. Selected short-axis images (SAX) at stress and rest. Medium size (less than 20% of the LV myocardium) reversible perfusion defect involving inferior and inferolateral (arrows).
Figure 3c.
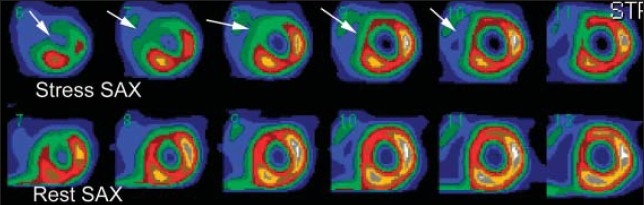
The size or extent of the myocardial perfusion in three different patients. Selected short-axis images (SAX) at stress and rest. Large sized (more than 20%) of the LV myocardium involving almost entire LAD distribution (arrows).
Figure 4.
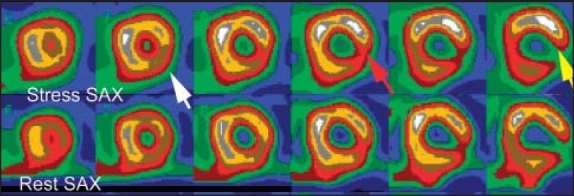
The severity of the perfusion defect in 71-year-old male with typical anginal pain. The perfusion defect in the apical lateral wall is mild (white arrow), moderate in the mid lateral wall (red arrow) and severe in the basal lateral wall (yellow arrow). For details please refer to the text.
The degree of reversibility of a perfusion defect is identified on post-stress images as an area of decreased radiopharmaceutical activity that improves or disappears on rest or redistribution images. Non-reversible defect (fixed) shows no significant changes in activity between post-stress or rest images. Severe fixed defect most likely represents scarring or fibrosis from prior MI, but a mild or moderate fixed defect may indicate hibernating myocardium or prior nontransmural MI. Reverse redistribution has been reported after myocardial infarction especially after revascularization or thrombolytic therapy. Some postulate that a regional hyperemic response to exercise may mask hypoperfusion in this region. Location of the perfusion defect can be characterized as they are located to specific myocardial wall's segment based on 17-segment model. Standardization of segment nomenclature is highly recommended.15
Semiquantitative and Quantitative Analysis
After visual interpretation of MPS, it is recommended that physicians also apply semiquantitative and quantitative analysis. One of the most frequently used approaches is 17 to 20 myocardial segments. Scoring is based on dividing the short-axis slices (apical, mid and basal) to represent the entire LV into small regions plus an additional apical segment. A five-point scoring per myocardial segment allows for the calculation of a summed score, which can be used to represents global indices of myocardial perfusion.16 Quantitative perfusion is usually performed with polar maps or a bull's eye to display the processed data and compare it with pooled data based on gender-matched controls. A difference of more than 2.5 standard deviations bellow the mean is usually considered abnormal.17
Gated MPS
MPS can be gated to the patient's EKG to obtain eight or 16 sets of tomographic data spanning the RR interval.18,19 The key component in a functional analysis software program is the algorithm used to find the endocardium and epicardial sufaces of the LV. The surface detection algorithm has to be robust in defining the LV surface even in the presence of large and severe perfusion abnormalities; abnormalities that provide little, if any, information to locate the myocardial wall. Gated MPS is helpful in many clinical situations and includes identification of suspected artifacts, risk stratification of patients with known or suspected CAD, myocardial viability, distinguish ischemic from non-ischemic cardiomyopathy and enhanced detection of multivessels disease.
Common MPS Artifacts and Pitfalls
Several artifacts and pitfalls can affect MPS and limit its clinical utility and accuracy.20 By anticipating and recognizing such artifacts, the nuclear medicine technologist and interpreting physicians can increase test specificity and avoid unnecessary additional tests. These artifacts may be related to the patient, the patient's heart, nuclear medicine equipment or the action of the nuclear medicine technologist. However, there is a considerable overlap among these factors. Some problems related to the heart such as balanced ischemia or left bundle branch block (LBBB) are better considered as imaging pitfalls because they are not related to limitations of the technique itself.
Artifacts Related to the Patients
Motion artifact
It is virtually impossible to correct for horizontal motion, and it is necessary to repeat the acquisition. Vertical movement and upward creep can be assessed by viewing a cineloop display of the acquired projection images with a cursor placed at the upper edge of the LV.12,21 In some instances it is possible to correct motion artifact by using correction algorithm.
Soft-tissue attenuation
Excessive subdiaphragmatic activity adjacent to the heart can interfere with evaluation of the perfusion of the inferior wall by decreasing the activity in the adjacent myocardium or by increasing activity in the inferior wall as result of scatter and volume averaging.22 To overcome the subdiaphragmatic activity one must consider prone imaging (Figure 5), gated images for thickening (helpful only in fixed defect) and attenuation correction. Breast attenuation results in a perfusion defect along the anterior wall or anteroseptal wall, typically in women.23 A fixed defect along the anterior wall with normal motion and thickening on gated images does favor attenuation artifact. Shifting breast attenuation is difficult to recognize with gated images (Figure 6). Reviewing attenuation correction images, gated images and cine images are very important in suspect breast attenuation.
Figure 5.
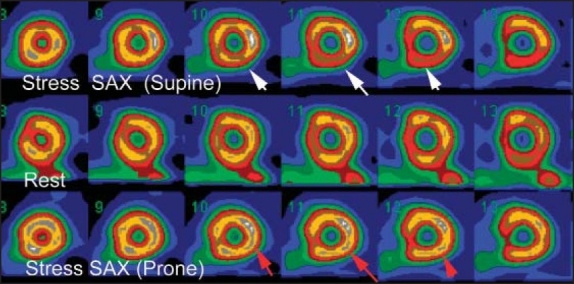
A 50-year-old male with atypical chest pain. The stress SAX images show mild inferior wall perfusion defect (white arrows) that nearly normalized on rest images. Stress-prone imaging shows normal perfusion along the inferior wall (red arrows), this finding is consistent with attenuation artifact by subdiaphragmatic tissue.
Figure 6.
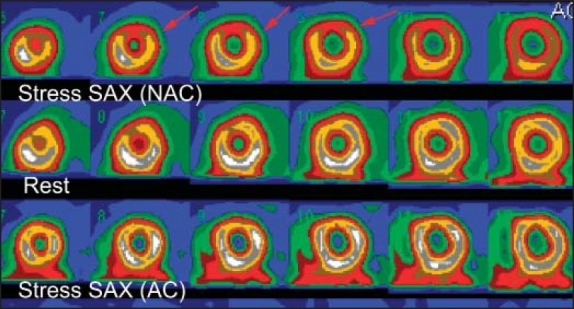
Shifting breast attenuation in 60-year-old women came with chest pain, the MPS was performed with dipyridmale because patient could not exercise owing to severe arthritis. Stress NAC images show anterior wall perfusion defect (red arrows) with normalization at rest. In the AC images (lower row) the perfusion defect disappears. Based on this finding the study was interpreted as normal with breast attenuation on NAC images.
There are several factors related to the patient that may cause an artifact and impede the quality of the study and final interpretation. Poor patient preparation such as recent heavy meals causes intense uptake in the adjacent gut and inferior wall attenuation. Inadequate exercise and cardiac arrhythmia are potential source of image artifacts.
Pitfalls Related to the Heart
LBBB produces a septal defect that can mimic ischemia or infarction.24 Examining the ECG for LBBB and reviewing gated study for paradoxical septal motion are clues for LBBB-induced artifacts. A stress vasodilator test may minimize the occurrence of abnormal septal perfusion, but does not eliminate it entirely. In some patients abnormal MPS cannot be excluded and other imaging techniques must be considered to role out CAD. From our experience we found that cardiac-computed coronary angiography is an excellent alternative test in patients with LBBB.
In hypertrophic cardiomyopathy, asymmetrical septal thickness may create false widespread perfusion defect because of normalization to the hottest pixel in the septum.25 In balanced ischemia, MPS can only measure relative perfusion, not absolute quantification. If there is decreased perfusion to all walls, the perfusion abnormalities may not be recognized. The clues for possible balanced ischemia include decreased post-stress EF and new regional wall motion abnormalities compared to rest scan, abnormal TID and abnormal increased lung uptake with TL-201.
Artifact Related to Nuclear Medicine Equipment
Flood field nonuniformity may be secondary to a defect in the photomultiplier tube, damage in collimator or faulty camera electronics.26 To detect such abnormalities a daily intrinsic and weekly extrinsic flood field should be obtained. SPECT acquisition using a camera with one or more source of nonuniformity typically produces ring artifact. A ring artifact may crate a false myocardial ischemia.Other sources of instrumentation artifacts include errors in center rotation, camera head tilt and increased detector-to-patient distance. Both technologists and physicians should be familiar with these potential artifacts and be able to detect and correct them.
Clinical Applications of MPS
Diagnosis of CAD
The excellent accuracy in detection of coronary artery disease (CAD) in patients with undiagnosed chest pain and no known CAD with MPS has been established in a number of large studies.27,28 The sensitivity was 87% in meta-analysis of 33 studies with specificity somewhat lower than 73%. The normalcy rate in this meta-analysis was 91%. The normalcy rate is defined as the percent of patients with less than 5% pretest likelihood of CAD who have normal MPS studies, but who do not have cardiac catheterization. This variable eliminates the referral bias of having predominately patients with abnormal scans (e.g. false positives) being referred for coronary angiography.
Indications for MPS for detection of CAD:
Intermediate pretest probability of CAD
Abnormal resting EKG: non-specific ST-T changes, left ventricular hypertrophy and conduction abnormalities
Non-diagnostic treadmill stress test; inability to reach 85% of PMHR or patients on digoxin.
Clinical Role in Prognosis and Risk Stratification
Patients with known or suspect CAD
Normal MPS in patients with suspected CAD indicates a low-risk group with less than 1% cardiovascular death or non-fatal myocardial infarction per year.29 On the other hand, patients with high-risk scintigraphic findings have a higher incidence of multivessel disease. High-risk scintigraphic findings include multiple ischemic perfusion defects in more than one coronary artery territory, low LV ejection (LVEF) of less than 40%, and increased end-diastolic and end-systolic volumes and increased lung uptake with TL 201 (Figure 7). Transient ischemic dilatation from rest to stress resulting from subendocardial hypoperfusion at stress causes an apparent increase in LV cavity at stress. This has been reported with high-risk scan.30
Figure 7.
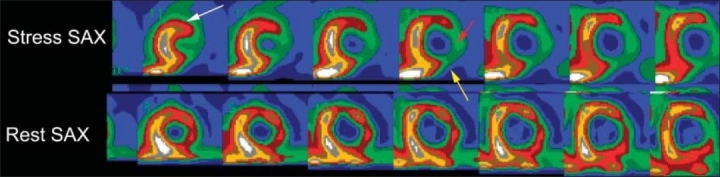
High-risk MPS in 63-years-old referred for cardiac risk stratification before vascular surgery. The stress images show extensive ischemia in the anterior (white arrows), lateral wall (red arrow) and inferior wall (yellow arrow). The gated images (not shown) demonstrate dilated LV with severe LV dysfunction, LV EF 24%.
Risk stratification following myocardial infarction
Post-MI MPS can identify myocardial ischemia, viable myocardium and lVEF. Post-MI MPS with submaximum exercise or vasodilators stress test stratifies patient into low, intermediate and high risk.31 Patient identified as low-risk scan can be considered for early discharge, whereas those at higher risk scan can be referred for early coronary angiography and possible intervention.32
Pre-operative risk stratification
Stress MPS can be used to evaluate cardiac status before non-cardiac surgical procedures.33 Patients undergoing vascular surgery including repair of abdominal aortic aneurysm have a high prevalence of CAD and at an increased risk for perioperative cardiac complication. Normal MPS or a small fixed defect confirms a low risk for adverse cardiac events. Patients with significant reversible stress-induced ischemia should be considered for coronary angiography.
Assessment of Therapeutic Intervention
After coronary bypass grafting, 10% to 20% of venous graft was occluded by 1 year and up to 50% graft occluded by 10 years. Stress MPS is superior to both clinical findings and stress ECG in predicting graft patency.34 Reversible perfusion abnormalities not identified on preoperative study suggest progression of disease in the native vessel, whereas a new fixed defect may indicate perioperative myocardial injuries. Although many patients have stress MPS prior to revascularization, this imaging is not routinely performed after coronary bypass surgery and is, according to guidelines, only indicated when symptoms reoccurs.
A percutaneous coronary intervention (PCI) is associated with a 30% to 40% restenosis by 6 months after the procedure.35 The optimal timing for imaging after PCI remains unclear. There is a high incidence of false positive after PCI most likely due to post-traumatic changes at the site of coronary dilation seen after PCI (including elastic recoil, spasm, internal hemorrhage and intraluminal debris). At approximately 4 weeks after PCI a good correlation has been demonstrated between stress-induced myocardial perfusion abnormalities and the presence or obscene [absence] of restenosis, independent of symptoms. MPS is performed in patients who develop symptoms after PCI to exclude restenosis or at 6 months in asymptomatic patients. MPS imaging allows determination of whether clinical ischemia is caused by restenosis at the site of angioplasty or by progression of disease in other coronary artery segments.
Monitoring medical therapy has been proved by several MPS imaging studies. Some investigators have recommended holding anti-anginal therapy prior to stress MPS imaging. Patient symptoms have proved to be unreliable for guiding management of patients with established CAD. MPS is a useful tool for optimizing medical management and may even lead to improvement in long-term outcome.36 Several studies in which MPS was performed to monitor medical therapy include nitroglycerine, lipid lowering therapy and β-adrenergic receptors blockade.
Myocardial Viability Determination
MPS that shows a fixed perfusion defect could represent myocardial scarring or a viable non-functional hibernating myocardium.37 Hibernating myocardium is the result of severe coronary artery stenosis producing chronic hypoperfusion and resting ischemia. Typical segment of hibernating myocardium demonstrate diminished contractility even on resting images. Stunned myocardium is a more acute and temporary state that results from acute ischemia and reperfusion injury secondary to an acute coronary occlusion that has reopened spontaneously or by thrombolytic therapy. Distinguishing hibernating myocardium form myocardial scarring is crucial in patient management.38 Revascularization of an area of myocardial scarring provides no potential for improving cardiac function and is associated with higher morbidity. However, revascularization of hibernating myocardium is associated with improves cardiac function, heart failure symptoms and overall survival.
201Tl-chloride assesses not only perfusion but also myocytes cell membrane integrity. 201Tl-chloride imaging using the rest and stress protocol had a sensitivity of 86% and a specificity of 59% in predicting functional recovery following revascularization. The relatively low specificity may reflect the criteria use classifying viability as for protocol for data acquisition. 201Tl-chloride redistribution pattern at 24 hours is more predictive for tissue recovery than 3-4 hours. As the quality of 24 hours is poor as a result of low counts, a new method for viability was sought in patients with fixed defect or redistribution images immediately following acquisition of delayed images, and additional dose of 201Tl-chloride reinjection imaging has much greater concordance with fluorine-18- (FDG) PET imaging than does conventional 201Tl-chloride redistribution.
The role of 99Tc sestamibi and tetrofosmin for the detection of viable myocardium has been a matter of debate, since several studies observed underestimation of viable myocardium as compared to other imaging modalities, whereas others reported excellent agreement between 99mTC agents and other imaging modalities. To improve the detection of viability with TC-labeled agents, several modifications of the imaging protocol have been proposed. Modified protocols with TC-related agents include stress redistribution-reinjection, quantitative analysis of the tracer in the fixed defect, and administration of dobutamine or nitroglycerin. Gated SPECT provides a strong functional data, fixed defect or resting perfusion defect with normal wall motion or thickness on gated images consistent with viable myocardium.39
Reporting of MPS Scan Result
The report should contain the patient age, gender, height, weight and body surface area. These data may directly affect the image result and final interpretation.11 Study-related information should be clearly known to help the interpreting physician focus on the clinical question. Imaging protocols should be specified, including the radiopharmaceuticals, dose and imaging protocol. Information about attenuation correction, whether it was performed or not, must be reported. The quality of the study, whether optimum or suboptimum, must be included. A suboptimumal study may affect the accuracy of the study, and a repeat study or other alternative diagnostic test must be considered.
Certain ECG findings that might have a direct effect on the study interpretation should be included, such as the presence of LBBB and resting ECG abnormalities. Type of stress (Bruce, modified Bruce, etc.) should be identified and the reason why pharmacological stress was performed instead of exercise test. The reason for test termination and symptoms experienced during the test are integral components of stress reporting. A combined report for both exercise and the pharmacological stress test and perfusion results is recommended. Adequacy of stress test should be noted. The perfusion defect should be described in terms of extent (small, medium and large), severity (mild, moderate and severe), location based on 17 segments and reversibility (reversible, fixed or mixed). Results of function should be identified as normal, hyperdynamic, or with mild, moderate and severe systolic dysfunction. Regional wall motion abnormalities should be also noted based on a 17-segment model for localization. The quantitative left ventricular function should be included.
The final interpretation of the scan should identify the readers impression as to whether the scan is normal or abnormal. Other terms such as possible, probably and equivocal should be avoided as much as possible. The likelihood of CAD must be reported based on the prescan probability of CAD and post-test probability of CAD. The final impression must address the referring physician's clinical question.
In conclusion, this article has demonstrated the basics of myocardial perfusion scinitigraphy techniques, current clinical applications, a systematic approach for interpretation and reporting. Understanding and familiarity of these aspects are important for optimum and efficient utilization of the test. This will ensure MPS will retain its essential role in the management of patients with cardiovascular disease.
REFERENCES
- 1.Russe RR, Zaret BL. Nuclear cardiology: Present and future. Curr Probl Cardiol. 2006;31:557–629. doi: 10.1016/j.cpcardiol.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomograph the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine, endorsed by the American College of Emergency Physicians. Circulation. 2009;119:e561–87. doi: 10.1161/CIRCULATIONAHA.109.192519. [DOI] [PubMed] [Google Scholar]
- 3.Lapeyre AC, 3rd, Goraya TY, Johnston DL, Gibbons RJ. The impact of Caffeine on vasodilator stress perfusion studies. J Nucl Cardiol. 2004;11:506–11. doi: 10.1016/j.nuclcard.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Kharabsheh SM, Al-Sugair A, Al-Buraiki J, Al-Farhan J. Overview of exercise stress testing. Ann Saudi Med. 2006;26:1–6. doi: 10.5144/0256-4947.2006.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albro PC, Gould KL, Westcott RJ, Hamilton GW, Ritchie JL, Williams DL, et al. Noninvasive assessment of coronary stenoses by myocardial imaging during pharmacologic coronary vasodilatation.III. Clinical trial. Am J Cardiol. 1978;42:751–60. doi: 10.1016/0002-9149(78)90094-2. [DOI] [PubMed] [Google Scholar]
- 6.Sonneblick EH, Fishman WH, LeJemetel TH. Dobutamine a new synthetic cardiaoactive sympathetic amines. N Eng J Med. 1979;300:17–22. doi: 10.1056/NEJM197901043000105. [DOI] [PubMed] [Google Scholar]
- 7.Weich HF, Strauss HW, Pitt B. The Extraction of TL-201 by the myocardium. Circulation. 1977;56:188–91. doi: 10.1161/01.cir.56.2.188. [DOI] [PubMed] [Google Scholar]
- 8.Beller GA, Watson DD. Physiological basis of myocardial perfusion imaging with technetium99m agents. Semin Nucl Med. 1991;12:173. doi: 10.1016/s0001-2998(05)80038-8. [DOI] [PubMed] [Google Scholar]
- 9.Taillefer R, Gagnon A, Laflamme L, Grégoire J, Léveillé J, Phaneuf DC. Same day injections of tc99m methoxy isobutyl isonitrile (hexamibi) for myocardial tomographic imaging: Comparison between rest-stress and stress-rest injection sequences. Eur J NuclMed. 1989;15:113–7. doi: 10.1007/BF00254621. [DOI] [PubMed] [Google Scholar]
- 10.Husain SS. Myocardial perfusion imaging protocols: Is there an ideal protocol? J Nucl Med Technol. 2007;35:3–9. [PubMed] [Google Scholar]
- 11.Holly TA, Abbott BG, Al-Mallah M, Calnon DA, Cohen MC, DiFilippo FP, et al. Imaging guidelines for nuclear cardiology procedures. Menu: Manage your practice: Guidelines and standards. [Last accessed on 2010 Jun]. Available from: http://www.asnc.org .
- 12.Hendel RC, Corbett JR, Cullom SJ, DePuey EG, Garcia EV, Bateman TM. The value and practice of attenuation correction for myocardial perfusion SPECT imaging: A joint position statement from the American Society of Nuclear Cardiology and the Society of Nuclear Medicine. J Nucl Med. 2002;43:273–80. doi: 10.1067/mnc.2002.120680. [DOI] [PubMed] [Google Scholar]
- 13.Hendel RC. Attenuation correction: Eternal dilemma or real improvement? Q J Nucl Med Mol Imaging. 2005;49:30–42. [PubMed] [Google Scholar]
- 14.Mettler FA, Guiberteau MJ. 5th ed. Amsterdam: Saunders-Elsevier; 2006. Essential of Nuclear Medicine Imaging; pp. 101–40. [Google Scholar]
- 15.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. J Nucl Cardiol. 2002;9:240–5. doi: 10.1067/mnc.2002.123122. [DOI] [PubMed] [Google Scholar]
- 16.Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: Differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–43. doi: 10.1161/01.cir.97.6.535. [DOI] [PubMed] [Google Scholar]
- 17.Berman DS, Kang X, Van Train KF, Lewin HC, Cohen I, Areeda J, et al. Comparative prognostic value of automatic quantitative analysis versus Semiquantitative visual analysis of exercise myocardial perfusion single-photon emission computed tomography. J Am Coll Cardiol. 1998;32:1987–95. doi: 10.1016/s0735-1097(98)00501-4. [DOI] [PubMed] [Google Scholar]
- 18.Cullom SJ, Case JA, Bateman TM. Electrocardiographically gated myocardial perfusion SPECT: Technical principles and quality control considerations. J Nucl Cardiol. 1998;5:418–25. doi: 10.1016/s1071-3581(98)90148-9. [DOI] [PubMed] [Google Scholar]
- 19.Smanio PE, Watson DD, Segalla DL, Vinson EL, Smith WH, Beller GA. Value of gating of technetium-99m sestamibi singlephoton emission computed tomographic imaging. J Am Coll Cardiol. 1997;30:1687–92. doi: 10.1016/s0735-1097(97)00363-x. [DOI] [PubMed] [Google Scholar]
- 20.Burrell S, MacDonald A. Artifacts and pitfalls in myocardial perfusion imaging. J Nucl Med Technol. 2006;34:193–211. [PubMed] [Google Scholar]
- 21.Cooper JA, Neumann PH, McCandless BK. Effect of patient motion on tomographic myocardial perfusion imaging. J Nucl Med. 1992;33:1566–71. [PubMed] [Google Scholar]
- 22.van Dongen AJ, van Rijk PP. Minimizing liver, bowel, and gastric activity in myocardial perfusion SPECT. J Nucl Med. 2000;41:1315–7. [PubMed] [Google Scholar]
- 23.Miles J, Cullom SJ, Case JA. An introduction to attenuation correction. J Nucl Cardiol. 1999;6:449–57. doi: 10.1016/s1071-3581(99)90011-9. [DOI] [PubMed] [Google Scholar]
- 24.Burns RJ, Galligan L, Wright LM, Lawand S, Burke RJ, Gladstone PJ. Improved specificity of myocardial thallium-201 single-photon emission computed tomography in patients with left bundle branch block by dipyridamole. Am J Cardiol. 1991;68:504–8. doi: 10.1016/0002-9149(91)90786-k. [DOI] [PubMed] [Google Scholar]
- 25.DePuey EG, Guertler-Krawczynska E, Perkins JV, Robbins WL, Whelchel JD, Clements SD. Alteration of myocardial thallium-201 in patients with chronic hypertension undergoing single-photon emission computed tomography. Am J Cardiol. 1988;62:234–8. doi: 10.1016/0002-9149(88)90218-4. [DOI] [PubMed] [Google Scholar]
- 26.Galt JR, Germano G. Advances in instrumentation for cardiac SPECT. In: Depuey EG, Berman DS, Garcia EV, editors. Cardiac SPECT imaging. New York: Raven Press; 1995. pp. 91–102. [Google Scholar]
- 27.Maddahi J. Myocardial perfusion imaging for the detection and evaluation of coronary artery disease. In: Marcus ML, Schelbert HR, Skorton DJ, Marcus ML, Schelbert HR, Skorton DJ, Wolf GL, et al., editors. Cardiac imagingprinciples and practice. 2nd ed. Philadelphia: WB Saunders; 1996. [Google Scholar]
- 28.Frans J, wackers HT. SPECT detection of coronary artery disease. In: Dlisizian V, Narula J, Braunwald E, editors. Atals of Nuclear cardiology. Philadelphia: Current Medicine inc; 2006. pp. 35–66. [Google Scholar]
- 29.Brown KA. Prognostic value of thallium-201 myocardial perfusion imaging: A diagnostic tool comes of age. Circulation. 1991;83:363–81. doi: 10.1161/01.cir.83.2.363. [DOI] [PubMed] [Google Scholar]
- 30.Hachamovitch R, Berman DS, Kiat H, Cohen I, Cabico JA, Friedman J, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease.Incremental prognostic value and use in risk stratification. Circulation. 1996;93:905–14. doi: 10.1161/01.cir.93.5.905. [DOI] [PubMed] [Google Scholar]
- 31.Gibson RS, Watson DD, Craddock GB, Crampton RS, Kaiser DL, Denny MJ, et al. Prediction of cardiac events after uncomplicated myocardial infarction: A prospective study comparing predischarge exercise thallium-201 scintigraphy and coronary angiography. Circulation. 1983;68:321–36. doi: 10.1161/01.cir.68.2.321. [DOI] [PubMed] [Google Scholar]
- 32.Mahmarian JJ, Mahmarian AC, Marks GF, Pratt CM, Verani MS. Role of adenosine thallium-201 totmography for defrining long term risk in patients after acute myocardial infarction. J am coll cardiology. 1994;25:1333–40. doi: 10.1016/0735-1097(95)00016-W. [DOI] [PubMed] [Google Scholar]
- 33.Brown KA, Rowen M. Extent of jeopardized viable myocardium determined by myocardial perfusion imaging best predicts perioperative cardiac events in patients undergoing noncardiac surgery. J Am Coll Cardiol. 1993;21:325–30. doi: 10.1016/0735-1097(93)90670-v. [DOI] [PubMed] [Google Scholar]
- 34.Zellweger MJ, Lewin HC, Lai S, Dubois EA, Friedman JD, Germano G, et al. When to stress patients after coronary artery bypass surgery? Risk stratification in patients early and late post-CABG using stress myocardial perfusion SPECT: Implications of appropriate clinical strategies. J Am Coll Cardiol. 2001;37:144–52. doi: 10.1016/s0735-1097(00)01104-9. [DOI] [PubMed] [Google Scholar]
- 35.Beygui F, Le Feuvre C, Maunoury C, Helft G, Antonietti T, Metzger JP, et al. Detection of coronary restenosis by exercise electrocardiography thallium-201 perfusion imaging and coronary angiography in asymptomatic patients after percutaneous transluminal coronary angioplasty. Am J Cardiol. 2000;1(86):35–40. doi: 10.1016/s0002-9149(00)00825-0. [DOI] [PubMed] [Google Scholar]
- 36.Shaw L, Berman DS. Sequential single-photon emission computed tomography myocardial perfusion imaging. Am J Cardiol. 2005;17(96):28J–39. doi: 10.1016/j.amjcard.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 37.Wijns W, Vatner SF, Camici PG. Hibernating myocardium. N Engl J Med. 1998;339:173–81. doi: 10.1056/NEJM199807163390307. [DOI] [PubMed] [Google Scholar]
- 38.Beller GA. Noninvasive assessment of myocardial viability. N engl J Med. 2000;343:1488–90. doi: 10.1056/NEJM200011163432011. [DOI] [PubMed] [Google Scholar]
- 39.Friedman J, Berman DS, Van Train K, Garcia EV, Bietendorf J, Prigent F, et al. Patient motion in thallium-201 myocardial SPECT imaging.An easily identified frequent source of artifactual defect. Clin Nucl Med. 1988;13:321–4. doi: 10.1097/00003072-198805000-00001. [DOI] [PubMed] [Google Scholar]


