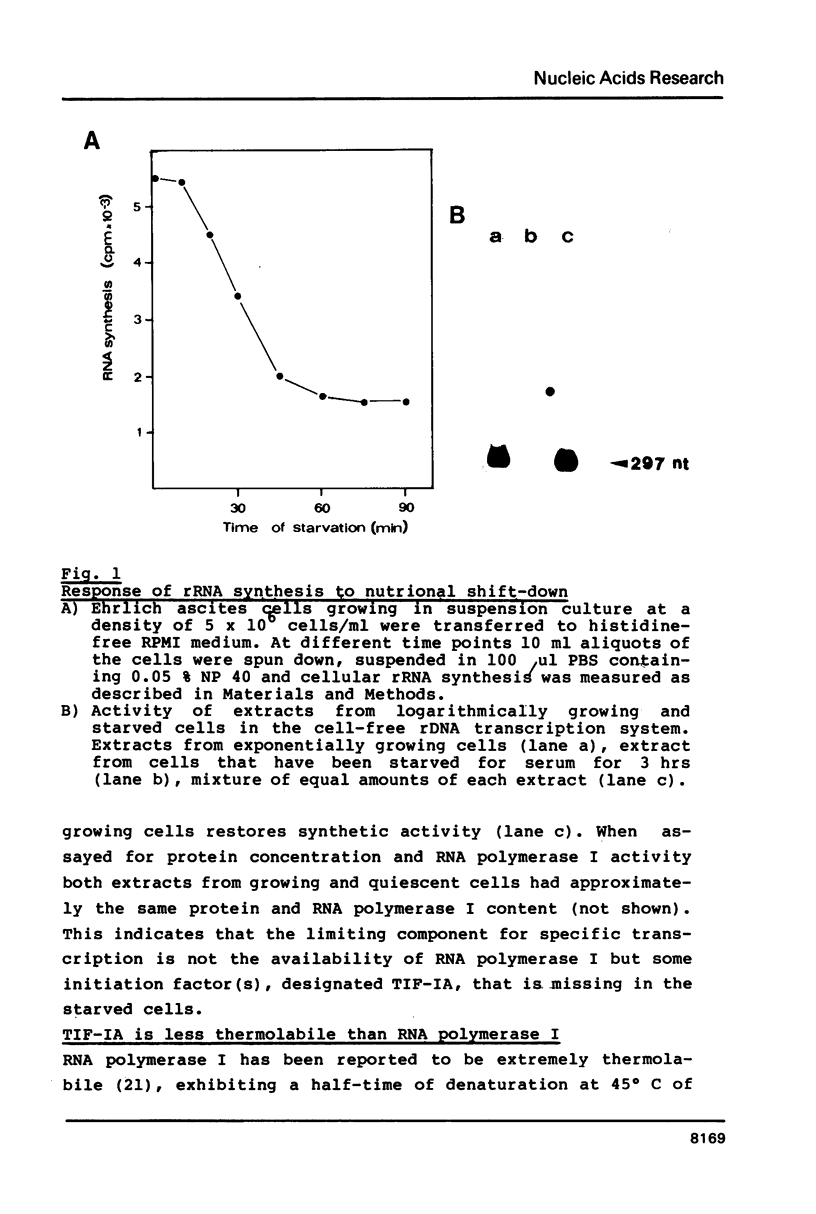
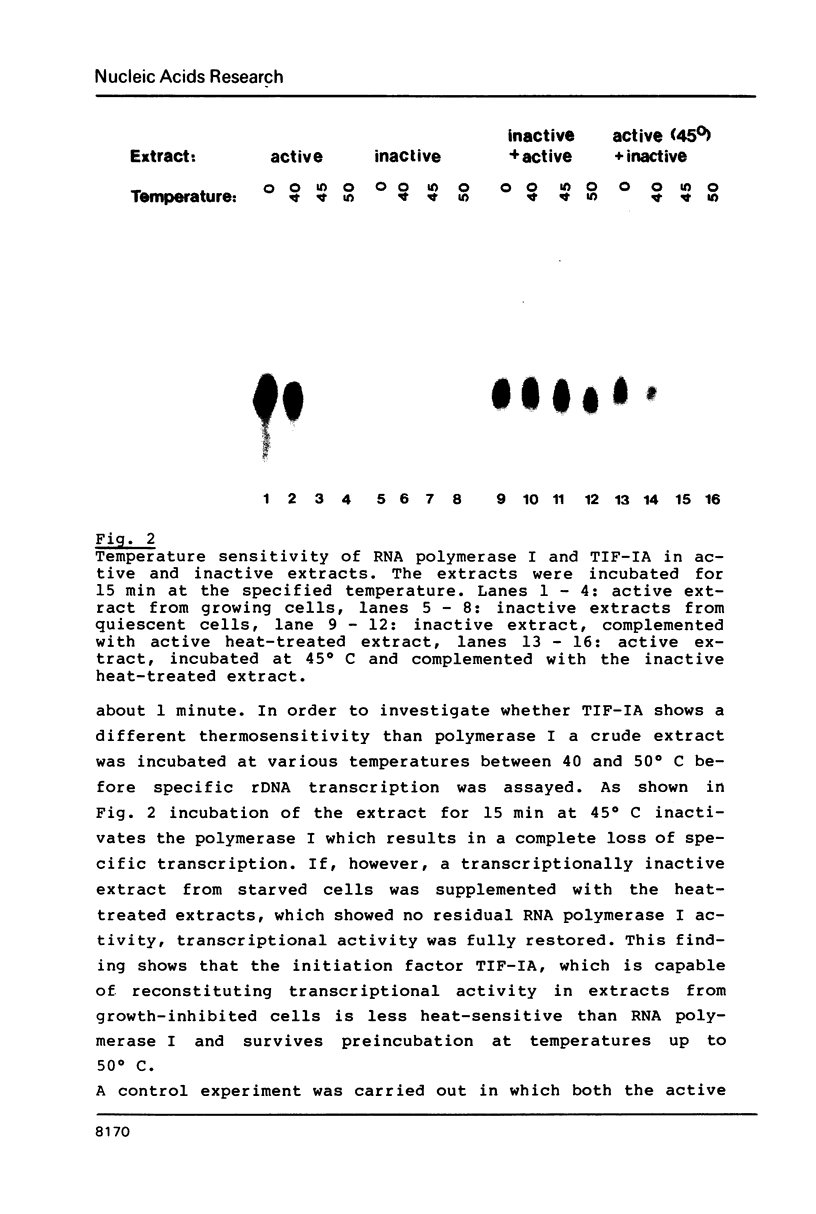
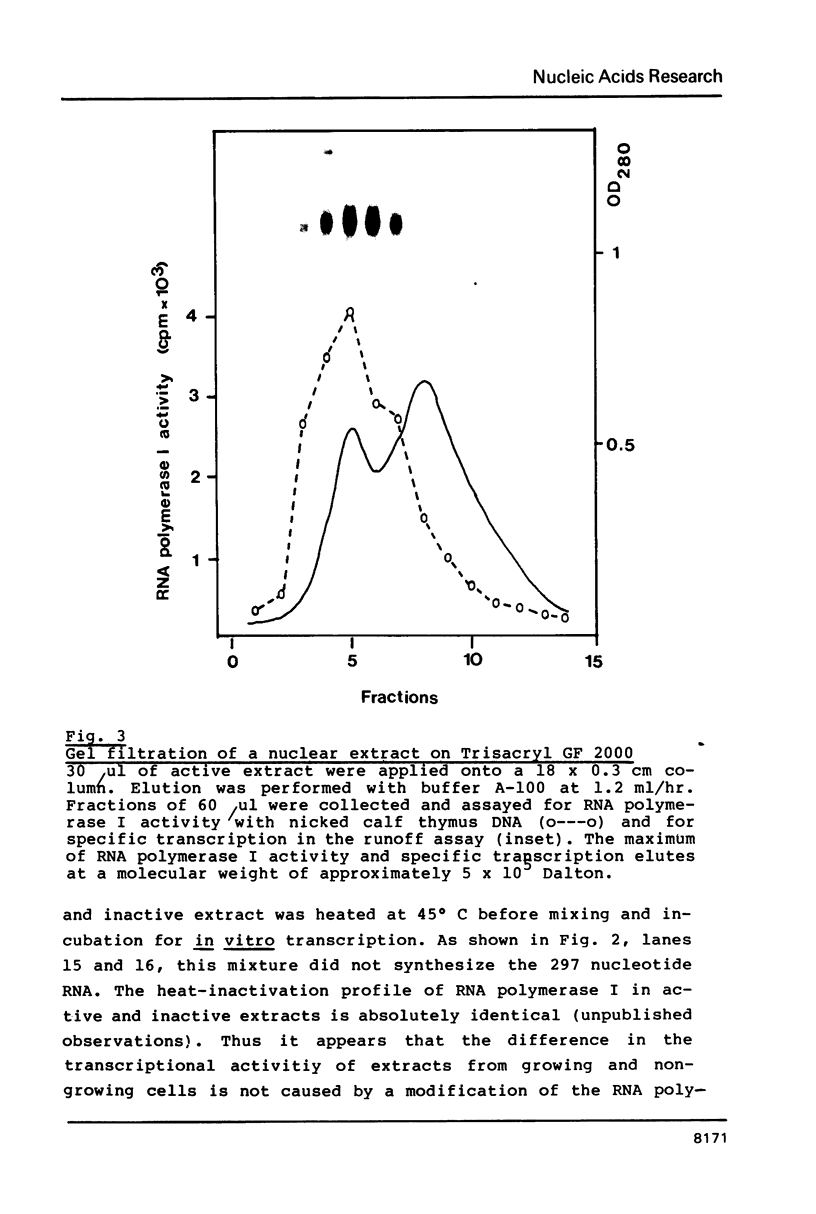
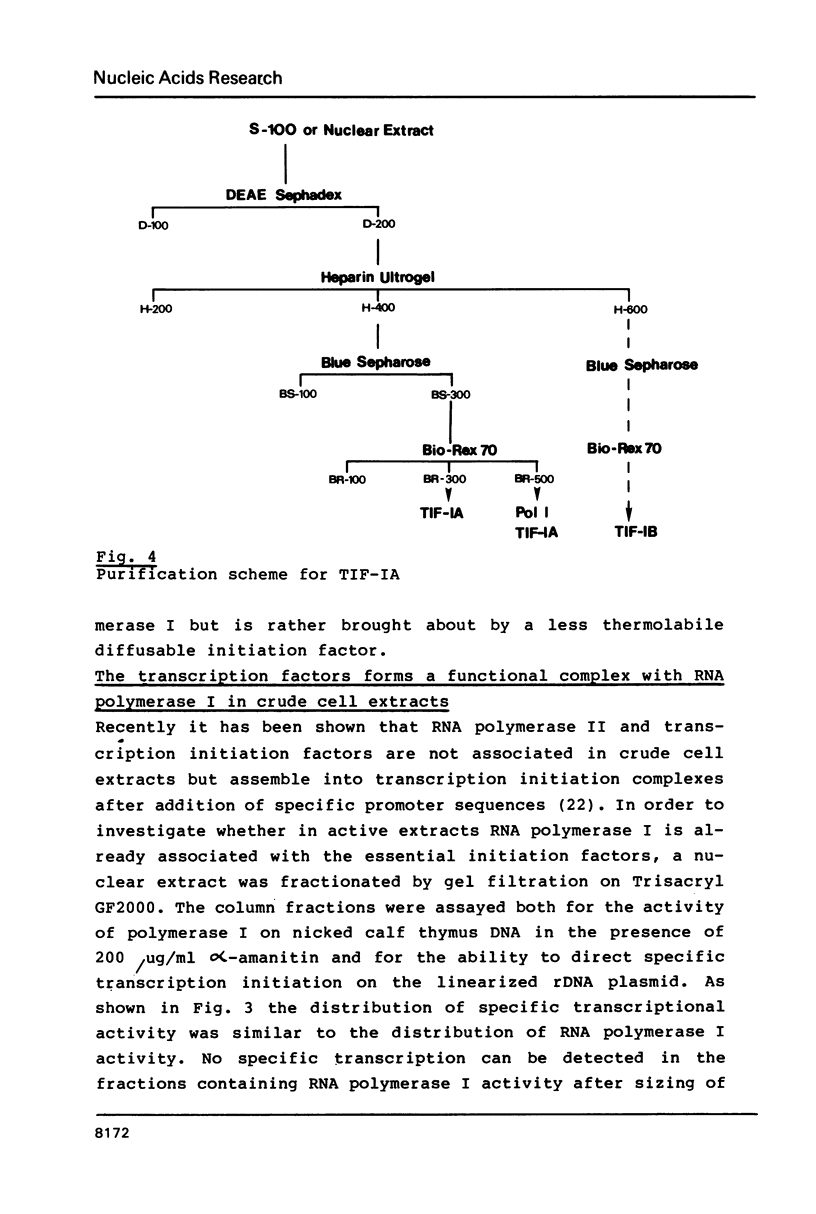
Abstract
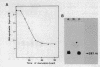
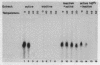
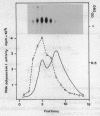
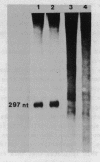
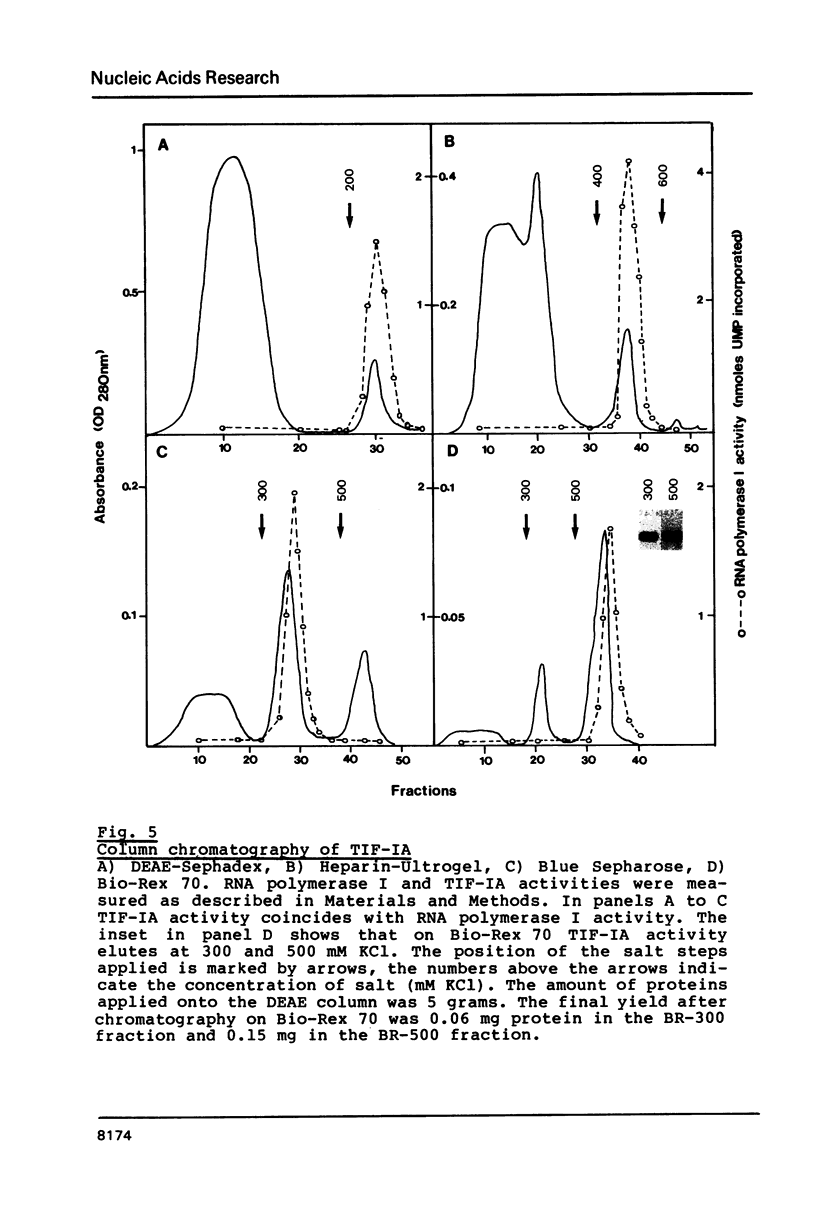
Mouse RNA polymerase I requires at least two chromatographically distinct transcription factors (designated TIF-IA and TIF-IB) to initiate transcription accurately and efficiently in vitro. In this paper we describe the partial purification of TIF-IA by a four-step fractionation procedure. The amount or activity of TIF-IA fluctuates in response to the physiological state of the cells. Extracts from quiescent cells are incapable of specific transcription and do not contain detectable levels of TIF-IA. Transcriptionally inactive extracts can be restored by the addition of TIF-IA preparations that have been highly purified from exponentially growing cells. During the fractionating procedure TIF-IA co-purifies with RNA polymerase I, suggesting that it is functionally associated with the transcribing enzyme. We suggest that only those enzyme molecules that are associated with TIF-IA are capable to interact with TIF-IB and to initiate transcription.
Full text
PDF




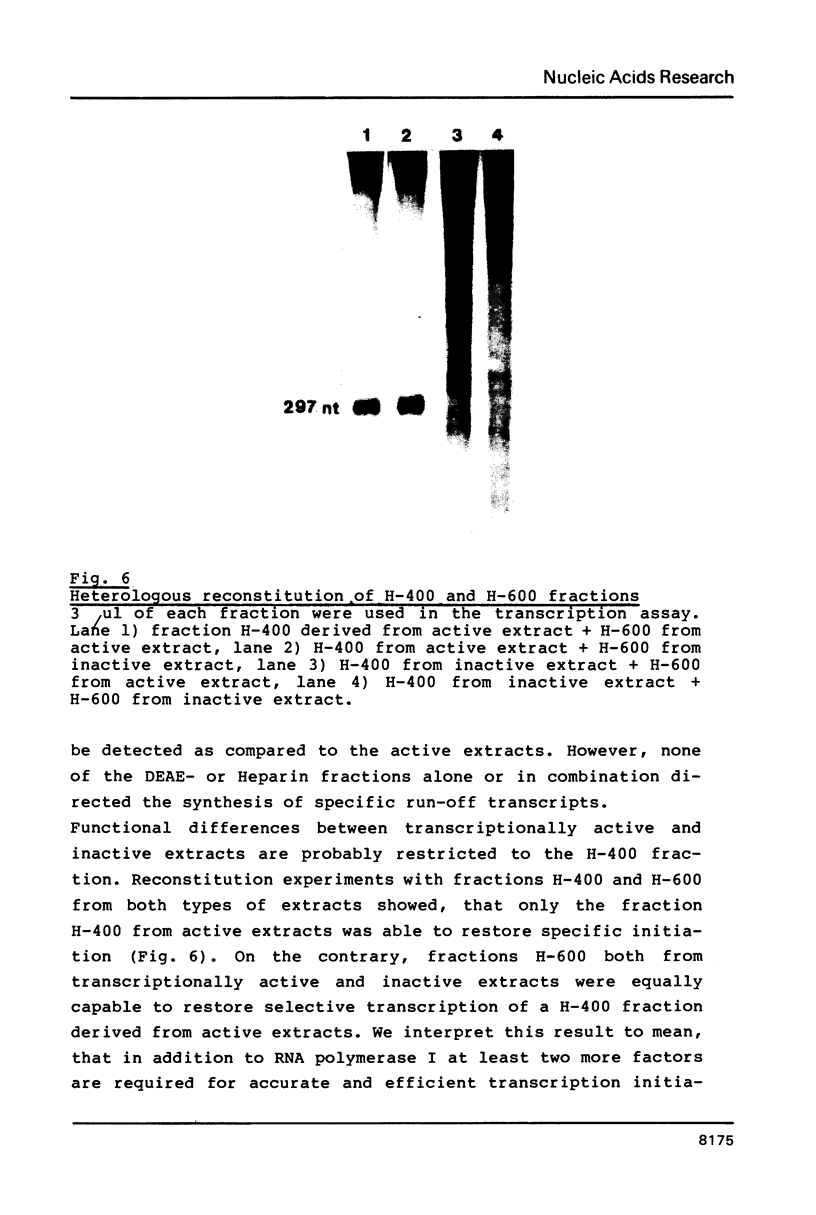





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry J., Gorski J. Uterine ribonucleic acid polymerase. Effect of estrogen on nucleotide incorporation into 3' chain termini. Biochemistry. 1971 Jun 8;10(12):2384–2390. doi: 10.1021/bi00788a032. [DOI] [PubMed] [Google Scholar]
- Cavanaugh A. H., Gokal P. K., Lawther R. P., Thompson E. A., Jr Glucocorticoid inhibition of initiation of transcription of the DNA encoding rRNA (rDNA) in lymphosarcoma P1798 cells. Proc Natl Acad Sci U S A. 1984 Feb;81(3):718–721. doi: 10.1073/pnas.81.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh A. H., Thompson E. A., Jr Hormonal regulation of transcription of rDNA: glucocorticoid effects upon initiation and elongation in vitro. Nucleic Acids Res. 1985 May 10;13(9):3357–3369. doi: 10.1093/nar/13.9.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt I., Grummt F. Control of nucleolar RNA synthesis by the intracellular pool sizes of ATP and GTP. Cell. 1976 Mar;7(3):447–453. doi: 10.1016/0092-8674(76)90175-6. [DOI] [PubMed] [Google Scholar]
- Grummt I. Nucleotide sequence requirements for specific initiation of transcription by RNA polymerase I. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6908–6911. doi: 10.1073/pnas.79.22.6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt I., Smith V. A., Grummt F. Amino acid starvation affects the initiation frequency of nucleolar RNA polymerase. Cell. 1976 Mar;7(3):439–445. doi: 10.1016/0092-8674(76)90174-4. [DOI] [PubMed] [Google Scholar]
- Grummt I. Specific transcription of mouse ribosomal DNA in a cell-free system that mimics control in vivo. Proc Natl Acad Sci U S A. 1981 Feb;78(2):727–731. doi: 10.1073/pnas.78.2.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida C. T., Kownin P., Paule M. R. Ribosomal RNA transcription: proteins and DNA sequences involved in preinitiation complex formation. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1668–1672. doi: 10.1073/pnas.82.6.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn B. D., Rae P. M. A component of Drosophila RNA polymerase I promoter lies within the rRNA transcription unit. Nature. 1983 Jul 14;304(5922):179–181. doi: 10.1038/304179a0. [DOI] [PubMed] [Google Scholar]
- Kurl R. N., Rothblum L. I., Jacob S. T. A purified fraction containing RNA polymerase I that can accurately transcribe rat ribosomal RNA gene. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6672–6675. doi: 10.1073/pnas.81.21.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learned R. M., Smale S. T., Haltiner M. M., Tjian R. Regulation of human ribosomal RNA transcription. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3558–3562. doi: 10.1073/pnas.80.12.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Sollner-Webb B. Transcription of mouse rRNA genes by RNA polymerase I: in vitro and in vivo initiation and processing sites. Cell. 1981 Nov;27(1 Pt 2):165–174. doi: 10.1016/0092-8674(81)90370-6. [DOI] [PubMed] [Google Scholar]
- Miller K. G., Tower J., Sollner-Webb B. A complex control region of the mouse rRNA gene directs accurate initiation by RNA polymerase I. Mol Cell Biol. 1985 Mar;5(3):554–562. doi: 10.1128/mcb.5.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y., Financsek I., Kominami R., Muramatsu M. Fractionation and reconstitution of factors required for accurate transcription of mammalian ribosomal RNA genes: identification of a species-dependent initiation factor. Nucleic Acids Res. 1982 Nov 11;10(21):6659–6670. doi: 10.1093/nar/10.21.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M., Shimada N., Higashinakagawa T. Effect of cycloheximide on the nucleolar RNA synthesis in rat liver. J Mol Biol. 1970 Oct 14;53(1):91–106. doi: 10.1016/0022-2836(70)90047-1. [DOI] [PubMed] [Google Scholar]
- NOWELL P. C. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960 May;20:462–466. [PubMed] [Google Scholar]
- Paule M. R., Iida C. T., Perna P. J., Harris G. H., Knoll D. A., D'Alessio J. M. In vitro evidence that eukaryotic ribosomal RNA transcription is regulated by modification of RNA polymerase I. Nucleic Acids Res. 1984 Nov 12;12(21):8161–8180. doi: 10.1093/nar/12.21.8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskas H. J., Thomas D. C., Green M. Biochemical studies on adenovirus multiplication. XVII. Ribosome synthesis in uninfected and infected KB cells. Virology. 1970 Apr;40(4):893–902. doi: 10.1016/0042-6822(70)90135-2. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Wilkinson J. A., Roan J., Reeder R. H. Nested control regions promote Xenopus ribosomal RNA synthesis by RNA polymerase I. Cell. 1983 Nov;35(1):199–206. doi: 10.1016/0092-8674(83)90222-2. [DOI] [PubMed] [Google Scholar]
- Soprano K. J., Dev V. G., Croce C. M., Baserga R. Reactivation of silent rRNA genes by simian virus 40 in human-mouse hybrid cells. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3885–3889. doi: 10.1073/pnas.76.8.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolunay H. E., Yang L., Anderson W. F., Safer B. Isolation of an active transcription initiation complex from HeLa cell-free extract. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5916–5920. doi: 10.1073/pnas.81.19.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandelt C., Grummt I. Formation of stable preinitiation complexes is a prerequisite for ribosomal DNA transcription in vitro. Nucleic Acids Res. 1983 Jun 11;11(11):3795–3809. doi: 10.1093/nar/11.11.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil P. A., Segall J., Harris B., Ng S. Y., Roeder R. G. Faithful transcription of eukaryotic genes by RNA polymerase III in systems reconstituted with purified DNA templates. J Biol Chem. 1979 Jul 10;254(13):6163–6173. [PubMed] [Google Scholar]
- Yamamoto O., Takakusa N., Mishima Y., Kominami R., Muramatsu M. Determination of the promoter region of mouse ribosomal RNA gene by an in vitro transcription system. Proc Natl Acad Sci U S A. 1984 Jan;81(2):299–303. doi: 10.1073/pnas.81.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F. L., Feigelson P. The rapid turnover of RNA polymerase of rat liver nucleolus, and of its messenger RNA. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2833–2837. doi: 10.1073/pnas.69.10.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]