Abstract
Background:
Neuropsychiatric adverse effects of interferon (IFN) alpha are well known. There is little clinically relevant research on animal models of depression with recombinant human IFN alpha 2b (rhIFN-α2b).
Aim:
To identify an appropriate dose and duration of administration of recombinant human interferon alpha-2b (rhIFN-α2b) to establish a convenient and clinically relevant murine model of chronic rhIFN-α2b-induced depression using the forced swim test (FST).
Materials and Methods:
Using a 4×3 factorial design, rhIFN-α2b was administered subcutaneously to mice (n=180) in the dose range of 400, 800, and 1600 IU/g/day for 5, 10, and 15 days; saline-treated mice formed the control groups. In each group, 1 day after the last dose, the mice were assessed for immobility in the FST. In another experiment, at these same doses and time points, the effect of rhIFN-α2b on murine motility was assessed in the small open field.
Results:
We found that rhIFN-α2b significantly increased immobility in the FST. The immobility was detectable by day 5 and did not increase with duration of IFN treatment. The immobility was apparent with the 400 IU/g/day dose and was not greater at higher IFN doses. At no dose or time point did rhIFN-α2b alter murine motility in the small open field.
Conclusion:
We conclude that rhIFN-α2b-induced behavioral despair, represented by immobility in the FST, is not due to reduced basal motility. The FST may therefore be used as a convenient Swiss albino mouse model of chronic rhIFN-α2b-induced depression with a 400–1600 IU/g/day dose administered subcutaneously for 5–15 days. The most economical model is 400 IU/g/day administered for 5 days
Keywords: Animal model, depression, forced swim test, interferon alpha 2b
INTRODUCTION
Interferons (IFNs) are a group of multifunctional cytokines that help protect the host against viral and parasitic infections and against certain tumors. IFNs are produced by infected cells. They prevent the multiplication of viruses and induce immune-mediated clearance of the viruses. There are three major categories of human IFNs: alpha, beta, and gamma.[1]
Recombinant human interferon alpha-2b (rhIFN-α2b) is a cytokine that is used in the treatment of tumors such as hairy cell leukemia, malignant melanoma, follicular lymphoma, and AIDS-related Kaposi's sarcoma. It is also used in infectious diseases such as chronic hepatitis B and condylomata acuminata. It is supplied in combination with ribavirin for use in chronic hepatitis C in specific circumstances.[2]
Despite their therapeutic benefits, the IFN alpha (IFN-α) cytokines are associated with a high burden of central nervous system adverse effects. These include mood symptoms, neurovegetative symptoms, and cognitive symptoms.[3] Depression is a common adverse effect of IFN-α and may occur in 21–58% of treated patients.[4,5] For example, in a randomized, double-blind, placebo-controlled study of 40 patients who had been prescribed high-dose IFN-α for malignant melanoma, Musselman et al.[6] found that 9 of 20 placebo-treated patients and 2 of 20 paroxetine-treated patients developed major depression during the first 12 weeks of IFN-α therapy. Severe depression necessitated the premature discontinuation of IFN-α in 7 of 20 placebo patients and 1 of 20 paroxetine patients. In a different report, Janssen et al.[7] described attempted and completed suicides in patients receiving IFN-α. Mechanisms underlying these IFN-α-induced adverse effects were reviewed by Capuron and Miller[3] and Miller.[8]
Animal models are helpful for the generation and testing of hypotheses relevant to clinical contexts. Animal models of IFN-α-induced depression are important because of the frequency and severity of IFN-α-induced depression. Regrettably, there has been little work on the subject. In a pathbreaking study, Makino et al.[9] demonstrated that the intravenous (i.v.) tail vein administration of different IFN-α preparations resulted in a dose-dependent increase in immobility in the forced swim test (FST), a standard animal model of depression in which an increased duration of immobility signifies behavioral despair.[10] The increased immobility in the FST peaked 15 min after dosing. Chronic administration of IFN-α for 7 days was similarly associated with a prolongation of immobility. In contrast, neither acute nor chronic dosing with IFN beta or gamma prolonged immobility.
In a separate series of experiments, Makino et al.[11] examined the effects of different IFNs in rats exposed to the FST. They found that a single 60 IU/g (but not 0.6 or 6.0 IU/g) i.v. dose of recombinant human IFN-α2b significantly prolonged immobility 15 min after administration to rats exposed to the test; human IFNs beta and gamma had no such effect. Repeated i.v. administration of IFN-α2b 6 IU/g for 7 days also prolonged immobility as assessed 15 min after the last dose; again, human IFNs beta and gamma had no such effect. In addition, acute dosing with recombinant rat IFNs alpha, beta, and gamma did not induce behavioral despair in the FST. As neither human nor rat IFNs influenced rodent locomotor activity, Makino et al. concluded that human IFN-α2b (but not rat or other human IFNs) has a specific depressogenic effect which is not an artifact of altered basal motility, and which is not mediated by IFN alpha/beta receptors. In a further series of elegantly conducted experiments using centrally acting and peripherally acting opioid probes in a murine model, Makino et al.[12] found that the depressogenic effect of human IFN-α is likely mediated by activation of central mu but not delta or kappa opioid receptors.
In contrast to the studies of Makino et al.[11,12] Loftis et al.[13] found that 3 weeks of i.p. administration of pegylated IFN-α2a or IFN-α2b (650 μg/week) failed to induce behavioral despair in Lewis rats exposed to the FST. Possible explanations are many: the sample size of 10 per group may not have been adequate; the IFN dose may not have been adequate; pegylated IFN may have less behavioral toxicity; Lewis rats may not be appropriate for the animal model; and the inclusion of female animals may have contaminated the range of behavioral responses.
The tail suspension test has also been studied as a murine model of IFN-α-induced depression. Yamano et al.[14] administered synthetic and natural IFN-α by the i.v. route to mice and assessed behavioral despair in the tail suspension test 15 min after dosing; both drugs increased immobility in a dose-dependent fashion. Repeated subcutaneous (s.c.) administration of both drugs across 7 days also dose-dependently prolonged immobility on this test with the assessments performed 30 min after the last dose.
Animal models are likely to have greater value the more closely they reflect clinical contexts. In patients who develop depression with IFN-α, symptoms of depression persist through the day to the next, when the next dose of medication falls due. Therefore, for an animal model to have validity, it is necessary to examine whether chronic dosing with IFN-α is associated with the persistence of behavioral despair through the day and into the next. However, even in their chronic dosing models, Makino et al.[9,11,12] and Yamano et al.[14] conducted the behavioral despair assessments just 15–30 min after the last dose of IFN-α; and the chronic dosing study of Loftis et al.[13] failed to elicit behavioral despair. Therefore, we still do not know whether the animals will exhibit behavioral despair a day after chronic dosing, as do humans who suffer IFN-α-induced depression.
We therefore conducted the present set of experiments to determine a suitable dose of IFN-α which, after repeated administration for a suitable period, would induce behavioral despair detectable in the FST 24 h after the last IFN-α dose. We also chose to study a s.c. route of administration of IFN-α because it is easier for the researcher and less traumatic to the animal than the i.v. route that was adopted by Makino et al.[9,11,12]
MATERIALS AND METHODS
Animals
The study was conducted on 2-month-old male Swiss albino mice obtained from the Central Animal Research Facility at the National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore. The mice were housed five per cage with free access to water and standard laboratory diet and were maintained and studied under daylight-driven light-dark cycle conditions, and under ambient temperature and humidity conditions, in a disturbance-free environment. The experimental procedures were approved by the animal ethics committee at NIMHANS.
Drugs
Commercial rhIFN-α2b (Intalfa; Intas Pharmaceutical Ltd, Ahmedabad, India) was purchased for the study. Stock solution was made up with distilled water into different aliquots containing 100,000 IU/ml. Prepared stock solutions were immediately stored at –20°C. Solutions for administration were prepared each day from these stock solutions, depending on the need for the day. The medication was administered subcutaneously into the nape of the neck. Control mice received 0.9% w/v physiological saline.
Three different doses of rhIFN-α2b were studied: 400, 800, and 1600 IU/g/day. Each dose was administered for each of three different time periods: 5, 10, and 15 days. The 1600 IU dose was chosen for study because it results in murine tissue levels of IFN that approximate those occurring during viral infection[15] (and this dose is associated with murine behavioral changes 10 and 24 h after a single injection).[16] The 400 and 800 IU doses were chosen because although they do not have the same magnitude of effect as the 1600 IU dose in single-dose studies,[16] in the interest of economy we wished to determine whether they suffice to induce murine behavioral changes in under repeated dosing conditions. In this context, the Makino et al.[11] showed that a far lower dose of IFN-α2b sufficed to induce behavioral despair under chronic dosing conditions when compared with single dosing conditions.
Forced swim test
This experiment comprised a 4×3 factorial design. There were four groups of mice (three IFN-α2b groups and saline controls; n=10 per group) studied for each of three different durations (5, 10, and 15 days) of IFN administration.
The FST for the assessment of behavioral despair essentially followed the standard procedure described elsewhere.[17,18] Two swim sessions were conducted: a baseline 10-min free exposure session, 1 day before the start of the study, to acclimatize the mice to the FST; and an end point 6-min session to assess behavioral despair, 24 h after the last IFN-α2b dose. The FST apparatus was a glass cylinder measuring 25 cm in height and 10 cm in internal diameter. The cylinder was filled to a height of 15 cm with fresh water at ambient tropical temperature. Mice were judged to be immobile when they floated in an upright position and made only small movements to keep the head above water. Mice were tested individually, and the time of immobility was recorded during the last 4 min of the 6-min period of exposure, leaving the first 2 min for habituation.
The entire end point session was recorded using a video camera. Recorded video clips were subsequently coded, scrambled, and scored by a trained rater who was blinded to the experimental group to which the mice belonged.
Small open field test
This experiment comprised four groups of mice, as in the FST, with 15 mice per group. Each group was studied after 5, 10, and 15 days of IFN-α2b administration.
The small open field test for motility monitoring followed that described by Van Ree and De Wied,[19] as adapted in our laboratory.[20–22] The apparatus comprised a glass cylinder measuring 45 cm in height and 19 cm in internal diameter. The floor of the cylinder was marked into four quadrants. A mouse placed in the cylinder was allowed 3 min for habituation to the new environment; afterward, the number of quadrants crossed by the mouse during a 3-min period of monitoring was recorded; at least three paws should have entered a quadrant for it to count. As with the FST, the motility assessment sessions were recorded using a video camera; the recorded video clips were coded, scrambled, and subsequently scored blind by a trained rater.
Statistical analysis
The data were analyzed using appropriate models of analysis of variance (ANOVA). In the FST analyses, drug and day of administration were the between-subject factors. In the small open field analyses, drug was the between-subject factor and day of administration was the within-subject factor. The Student-Newman-Keuls (SNK) post hoc test was used for multiple comparisons when the overall ANOVA was significant. Alpha for significance was set at P<0.05.
RESULTS
Eight mice died of unknown causes during the course of the FST experiment: one in the vehicle 15-day group; one in each of the three IFN-α2b 800-IU/g/day groups; one in the IFN-α2b 1600 IU/g/day for 5 days and one in the IFN-α2b 1600 IU/g/day for 10-day group; and two in the IFN-α2b 1600 IU/g/day for 15-day group. Five mice died in the motility experiment: two in the IFN-α2b 800 IU/g/day group and three in the IFN-α2b 1600 IU/g/day group.
Forced swim test
The FST data are presented in Table 1. A two-way ANOVA showed that there was a significant main effect for treatment groups (F=29.73, df=3,100, P<0.001). The main effect for duration of treatment was not significant (F=2.01, df=2,100, P=0.14). The treatment × duration interaction was also not significant (F=0.28, df=6,100, P=0.95).
Table 1.
Mean (SD) immobility scores in the FST in mice treated with saline or IFN-α2b (400, 800, or 1600 IU/g/day) for 5, 10, or 15 days*
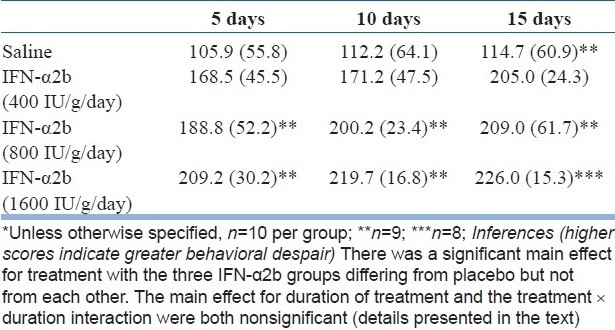
Because a longer duration of administration did not significantly increase behavioral despair, we chose to further examine only the day 5 data. In this analysis, a one-way ANOVA showed that there was a significant difference between groups (F=8.63, df=3,34, P<0.001) with the three IFN-α2b differing from the saline group but not from each other (SNK test).
Small open field test
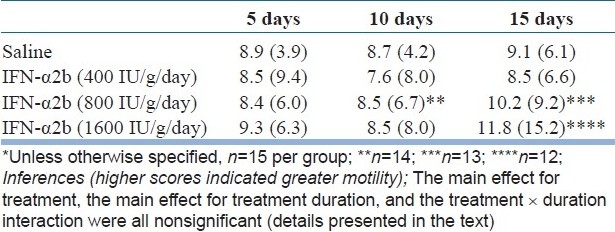
The small open field data are presented in Table 2. A two-way repeated measures ANOVA showed that there was no significant main effect for treatment groups (F=0.27, df=3,51, P=0.85) nor a significant main effect for treatment duration (Pillai's trace=0.02, F=0.63, df=2,50, P=0.53). The treatment × duration interaction was also not significant (Pillai's trace=0.01, F=0.10, df=6,102, P=1.00).
Table 2.
Mean (SD) motility scores in the small open field in mice treated with saline or IFN-α2b (400, 800, or 1600 IU/g/day) and assessed after 5, 10, and 15 days*
DISCUSSION
Many mechanisms have been proposed to explain the constellation of signs and symptoms that comprise the syndrome of IFN-α-induced depression. These mechanisms include alterations in serotonin mechanism, depletion in dopamine, changes in the hypothalamo-pituitary axis with increase in cortisol levels, and activation of neural circuits that subserve anxiety and alarm. These mechanisms were reviewed by Capuron and Miller[3] and Miller.[8]
We found that IFN-α2b increased immobility in the FST. This effect was apparent after 5 days of treatment itself and did not significantly increase in magnitude after 10 and 15 days of IFN administration. The increased immobility was apparent with the 400 IU/g/day dose and was no greater at higher IFN doses. At no dose did IFN-α2b alter animal motility in the small open field, as assessed after 5, 10, and 15 days of IFN administration. These findings suggest that 400 IU/g/day is the most economical dose of rhIFN-α2b for establishment of behavioral despair in the FST and that the behavioral despair is not an artifact of IFN-induced alteration in basal animal motility.
The use of this animal model could guide research which seeks to examine, for example, whether different treatments with potential antidepressant efficacy could treat or even prevent rhIFN-α2b-induced depression, much as was done in the clinical trial reported by Musselman et al.[6] The advantage of animal models is that they can answer questions that may pose ethical challenges in clinical contexts. We could find only one study that used an animal model to investigate the efficacy of antidepressant treatment in IFN-α-induced depression: this study, however, examined only a single dose of imipramine that was administered 30 min before dosing with synthetic or natural IFN-α; and in this study, the IFN-α was administered in a single i.v. dose just 15 min before exposure to the tail suspension test.[14] This study, therefore, is very far removed from clinical situations in which patients receive IFN-α in high doses for weeks or longer, and in whom depressive symptoms are chronic, as opposed to manifesting immediately after IFN dosing. Thus, we believe that our model is simple, practical, and clinically relevant.
Limitations
Lower doses of rhIFN-α2b may induce significant behavioral despair in the FST than the 400 IU/g/day that we recommend. However, doses that are too low may not sufficiently approximate those occurring in murine tissues during viral infection[15] and may therefore be less relevant to clinical contexts in which high doses are often used.[6] Briefer durations of administration than the 5 days that we recommend may also be associated with significant behavioral despair. However, such briefer durations would no longer qualify for a repeated dosing schedule and this, again, would no longer resemble clinical contexts. Our study findings allow researchers to choose the dose (400–1600 IU/g/day) and duration of administration (5–15 days) that are most relevant to their specific research question.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Hayden FG. Antimicrobial agents: Antiviral agents (nonretroviral) In: Hardman JG, Limbird LE, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill; 2001. pp. 1313–47. [Google Scholar]
- 2.Krensky AM, Strom TB, Bluestone JA. Immunomodulators: Immunosupressive agents, tolerogens, and immunostimulants. In: Hardman JG, Limbird LE, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill; 2001. pp. 1463–84. [Google Scholar]
- 3.Capuron L, Miller AH. Cytokines and psychopathology: Lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–24. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: Recognition and management. CNS Drugs. 2005;19:105–23. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asnis GM, De La Garza R., 2nd Interferon-induced depression in chronic hepatitis C: A review of its prevalence, risk factors, biology, and treatment approaches. J Clin Gastroenterol. 2006;40:322–35. doi: 10.1097/01.mcg.0000210099.36500.fe. [DOI] [PubMed] [Google Scholar]
- 6.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–96. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 7.Janssen HL, Brouwer JT, van der Mast RC, Schalm SW. Suicide associated with alfa-interferon therapy for chronic viral hepatitis. J Hepatol. 1994;21:241–3. doi: 10.1016/s0168-8278(05)80402-7. [DOI] [PubMed] [Google Scholar]
- 8.Miller AH. Mechanisms of cytokine-induced behavioral changes: Psychoneuroimunology at the translational interface. Brain Behav Immun. 2009;23:149–58. doi: 10.1016/j.bbi.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makino M, Kitano Y, Hirohashi M, Takasuna K. Enhancement of immobility in mouse forced swimming test by treatment with human interferon. Eur J Pharmacol. 1998;356:1–7. doi: 10.1016/s0014-2999(98)00474-9. [DOI] [PubMed] [Google Scholar]
- 10.Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245–55. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- 11.Makino M, Kitano Y, Komiyama C, Takasuna K. Human interferon-alpha increases immobility in the forced swimming test in rats. Psychopharmacology (Berl) 2000;148:106–10. doi: 10.1007/s002130050031. [DOI] [PubMed] [Google Scholar]
- 12.Makino M, Kitano Y, Komiyama C, Hirohashi M, Takasuna K. Involvement of central opioid systems in human interferon-alpha induced immobility in the mouse forced swimming test. Br J Pharmacol. 2000;130:1269–74. doi: 10.1038/sj.bjp.0703432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loftis JM, Wall JM, Pagel RL, Hauser P. Administration of pegylated interferon-alpha-2a or -2b does not induce sickness behavior in Lewis rats. Psychoneuroendocrinology. 2006;31:1289–94. doi: 10.1016/j.psyneuen.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Yamano M, Yuki H, Yasuda S, Miyata K. Corticotropin-releasing hormone receptors mediate consensus interferon-alpha YM643-induced depression-like behavior in mice. J Pharmacol Exp Ther. 2000;292:181–7. [PubMed] [Google Scholar]
- 15.Heremans H, Billiau A, De Somer P. Interferon in experimental viral infections in mice: Tissue interferon levels resulting from the virus infection and from exogenous interferon therapy. Infect Immun. 1980;30:513–22. doi: 10.1128/iai.30.2.513-522.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segall MA, Crnic LS. An animal model for the behavioral effects of interferon. Behav Neurosci. 1990;104:612–8. doi: 10.1037//0735-7044.104.4.612. [DOI] [PubMed] [Google Scholar]
- 17.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- 18.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 19.Van Ree, De Wied. Discussions in Neurosciences. 1. V. Geneva: Foundation for the Study for the Nervous System; 1988. Behavioural approaches to the study of the rat brain; p. 60. [Google Scholar]
- 20.Andrade C, Arunasmitha S, Pradhan N. Apomorphine-induced time-dependant potentiation of dopamine post-synaptic receptor response. NIMHANS J. 1990;8:53–5. [Google Scholar]
- 21.Andrade C, Pradhan N. Alpha-2 adrenergic effects of repeated electroconvulsive shocks. Indian J Psychiatry. 1991;33:140–2. [Google Scholar]
- 22.Andrade C. Electroconvulsive therapy: Methods and results of basic science research at NIMHANS. In: Khanna S, Channabasavanna SM, Keshavan MS, editors. Methods in Biological Psychiatry Research. New Delhi: Tata McGraw-Hill; 1995. pp. 114–38. [Google Scholar]


