Abstract
Obsessive–compulsive disorder (OCD) is a psychiatric disease characterized by anxiety-provoking thoughts (obsessions) leading to repeated, time-consuming behaviors (compulsions) that may or may not provide temporary relief. With an approximate prevalence of 2–3% of the general population and 0.6% in the Indian population, OCD is a debilitating disorder that can significantly affect nearly every aspect of a patient's life, and in some cases, lead to suicide.
Keywords: Anterior capsulotomy, obsessive–compulsive disorder surgery, psychosurgery, refractory obsessive–compulsive disorder
INTRODUCTION
Surgery for obsessive–compulsive disorder (OCD) is reserved for patients with the most severe cases of the disease, when pharmacological and psychotherapeutic alternatives have been exhausted. The main inclusion criteria for surgery include a minimum trial by three drugs, 20 sessions of cognitive and behavioral therapy (CBT) (or failed CBT) and Yale-Brown obsessive compulsive scale (YBOCS) of 24 and above.
Anterior capsulotomy, a lesion in the anterior limb of internal capsule, can be effective in ameliorating OCD symptoms. We describe the first case report of successful capsulotomy as per the recommendations of the core group of psychiatric disorders surgery.
OCD has an approximate prevalence rate of 2–3% in the general population and 0.6% in the Indian population. OCD is a debilitating disease that can significantly affect almost all aspects of patient's life, and in some cases, lead to suicide.[1] Surgery for OCD is reserved for patients with the most severe cases of the disease. Literature estimates have put 10-40% of the patients as treatment refractory.[2–4] These patients can be eligible for surgical intervention.
The four different targets currently being used are anterior capsule (AC), cingulate gyrus (CG), subcaudate tractotomy and limbic leucotomy. Nucleus accumbens is another promising target for this surgery. Two methods of surgery are employed for altering these targets. One involves performing lesion and the other involves stimulation of these targets using deep brain stimulation (DBS). In a lesion, a radiofrequency unit is used to produce (destroy) a thermal lesion of calculated volume. This is permanent and irreversible. In DBS, an electrode is implanted at the site of the target and current is delivered through a pacemaker to alter the signals emanating from the target. The pacemaker is implanted in the infraclavicular region and is connected by extension wires, tunneled subcutaneously to the electrodes that are implanted in the brain. The amount of current and thereby the stimulation/inhibition of the target site can be controlled by an external programmer. DBS offers an exceptional benefit of reversibility and titrability. Recently, US Food and Drug Administration (FDA) under humanitarian device exemption category, approved the use of DBS for OCD. Both these procedures are performed using stereotactic techniques, which offer a high degree of accuracy (within 1–2 mm).
Last year in May, a group of eminent psychiatrists from across India met to review the literature on psychiatric disorder surgery. They accepted OCD as the only indication for treatment. The choice of target and the type of therapy (lesion/DBS) was left to the surgeon to decide. The selection criteria and the guidelines for OCD surgery included:
DBS/ablations may be considered a viable treatment option for treatment resistant patients of OCD at the present time.
A protocol drawn by the Multicenter Study group for OCD surgery[5–6] for selection of patients for DBS will be implemented for selecting patients of OCD for neurosurgical intervention.
Any center desirous of undertaking a surgical program should form a review committee of one or more psychiatrists, a neurologist and a neurosurgeon not involved directly in the treatment for reviewing suitability for surgery. This is recommended to ensure that patients are adequately assessed. This committee will not play the role of IRB. The role of such a committee will be to ensure that all measures recommended by the International OCD DBS group are fulfilled.
We recently performed bilateral anterior capsulotomy for intractable OCD.
CASE REPORT
Mr. V, a 62-year-old engineer from Karnataka, had anxious, determined, short-tempered, reserved and dominating personality. He developed depression after the demise of his father in 1990. He was treated for the same in Mysore. One year later, his symptom progressed to anxiety, dominated by obsessive and compulsive symptoms, which gradually increased in severity. He had taken voluntary retirement and had not been working since past 15 years. He was also unable to withdraw his pension, as he could not sign for himself. His obsessive symptoms included insisting on repeated checking and verifying documents, checks and money. His compulsions included repeated washing of the hands (about 80–100 times at the time of admission), spending long time in the toilet (about 3–4 hours), repeatedly asking the same questions and verifying the answers multiple times. There were no ideas of reference on persecution. There were no hallucinations. Since the last 2 years, he had been confined to home due to these symptoms. His disease had significantly affected his and his caregiver's quality of life. He had been on selective serotonin reuptake inhibitors (SSRIs), including Fluoxetine, Fluvoxamine, Sertraline, Clomipramine, Escitalopram and many others, in maximum doses. He had also undergone behavioral therapy during these 15 years (more than 20 out-patient sessions) under the care of a psychiatrist, without any significant change in his symptoms. At the time of admission, he was on a daily dose of Risperidone 2 mg, Quetiapine 75 mg, Alprzolam 0.5 mg, Escitalopram 10 mg and fluoxetine 100 mg. Both the referring psychiatrist and the psychiatrist who independently assessed him for surgery were satisfied with the duration of the treatment (medical and behavioral therapy) and had found him to be adequate to establish intractability.
On examination, he was intelligent, anxious, well oriented and had normal memory. He had preserved insight, was co-operative and repetitive. His thought process had occasional tangentiality but could be brought back to rational thinking. He had no other focal neurological deficits.
He was evaluated by two psychiatrists independently, who established the diagnosis of OCD as per DSM IV criteria. The neurosurgeon (author) also concurred with them and decided to offer surgical option. The patient underwent neuropsychological testing that included Minnesota Multiphasic Personality Inventory (MMPI) and Rorschach. The MMPI revealed “Fake Bad” protocol/psychoses. The F% was 66.67%, implying weakening ties with realty. It was reported as obsessive–compulsive personality disorder and psychoses. However, it was also noted that the patient was very defensive in taking the tests (and was also tired). His YBOCS was 38/40 and Hamilton depression score was 24. His Beck's Anxiety Inventory reflected a score of 26, revealing moderate degree of anxiety. The Mini-Mental State Examination score was 30, revealing a normal cognitive profile. Based on the clinical history, patient interview and results of various assessments, the team of pschiatrists concluded that this was a case of intractable OCD and qualified for surgical intervention. There were two options availabe for surgery: DBS or ablative surgery (lesion). The patient and his wife came from a city that was 4 hours drive from Banglore (the closest town to have flight connections with Mumbai). They had not traveled independently for many years and felt that it would be difficult for them to make frequent visits to Mumbai for postoperative programming. Hence, they decided to opt for lesion.
The case was than referred to the psychiatric surgery review board, which comprised a neurosurgeon, neurologist and psychiatrist not involved in surgery or patient care. They found that the surgical treatment was a logical option for improving the patients’ condition.
As the patient could not sign (because of OCD), an informed consent was obtained from his sister and wife. Patients’ consent for surgery was recorded on video.
A preoperative planning magnetic resonance imaging (MRI) was performed 1 day prior to surgery. IR and T2-weighted coronal images were used to identify the internal capsule. A surgical target, 3 mm anterior to the posterior border of anterior commisure and 2 mm inferior to the AC–PC plane, was selected. This was the bottom of the target. On the day of surgery, a stereotactic computed tomography (CT) scan was performed and fused with the preoperative MRI. The target was approached through a precoronal burr hole under local anesthesia. Neurophysiological response was noted starting from 20 mm above the target to 3 mm below the target on the right side and from 15 mm above till 3 mm below the target on the left side. The response in the form of decrease in anxiety, more calmness and pseudosmile was noted nearer the targets. Radiofrequency lesioning was done bilaterally at 75°C for 60 seconds.
Post-op CT scan confirmed the appropriate target [Figure 1]. He had good improvement in his OCD symptoms but had severe confusion and disorientation in the initial three postoperative days. He was also having high-grade fever and hyponatremia, for which there was no identifiable apparent cause. Reduction in the dosage of psychotropic medications was done on consultation with the psychiatrist. At the time of discharge, he had very good relief of his OCD symptoms. The YBOCS at the time of discharge (1 week postoperatively) was 9 and the depression score was 6. He had no anxiety. After 3 months of follow-up, the family reported that his obsession was well controlled, and, he had lesser anxiety and depression. The apathy had reduced. His progress and medical management is being done by the referring psychiatrist who has been keenly interested in the surgical option.
Figure 1.
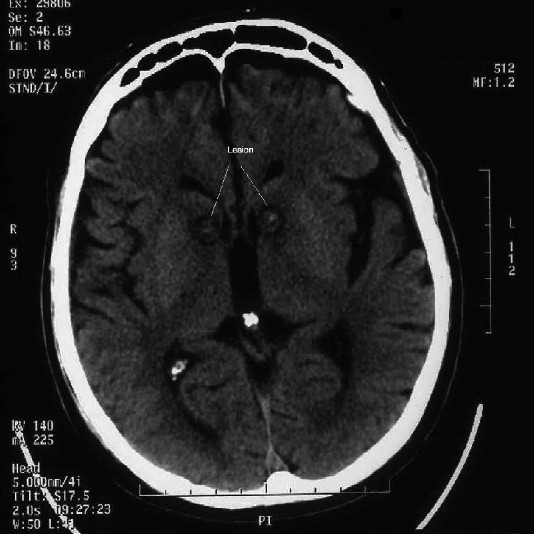
Axial CT scan showing bilateral anterior capsule lesions
DISCUSSION
There is a convergence of evidence implicating the corticostriatothalamocortical (CSTC) loop involving orbitofrontal cortex (OFC), anterior cingulate cortex (ACC) and basal ganglia as central to the pathophysiology of OCD.[7–9] Positron emission tomography (PET) studies on patients with OCD confirm that elevated glucose metabolism occurs in the bilateral thalamus, caudate, and OFC regions.[9] These observations have reinforced the relevance of the surgical targets (CG, AC and subcaudate tractotomy) for OCD treatment. Anterior capsulotomy has been practiced for the treatment of intractable OCD since the early 1950s.[10] Lars Leksell in Sweden popularized this target aimed at interrupting the fronto-thalamic fibers. From 1952 to 1957, Leksell operated on 117 patients. On long-term follow-up, it was noted that 78% of patients obtained good or fair outcome.[10] Several other authors[11–17] have compared the effects of capsulotomy and cingulotomy and concluded that capsulotomy was more effective. In another review, it was found that the correct placement of right AC lesion correlated with a better outcome than those placed in the left AC.[13]
In 1999, Nuttin showed that electrical stimulation of the anterior limbs of the internal capsules induced beneficial effects in a patient with intractable OCD.[18] Later, a multicenter trial reported an improvement of YBOCS from a mean of 34 to 20 in 27 patients.[5] The average follow-up was 31 months. They observed that as their experience improved, the number of patients responding increased from 35 to 75%. They also observed that the more ventral contacts (contacts in the vicinity of nucleus accumbens) were more effective. At the last follow-up, work, school or homemaking functioning was described as fair or good in 21 of 25 patients. Capacity for independent living was considered fair or good in 20 of 25 patients. Patients required high voltage for stimulation and the average pacemaker life was over 12–18 months. This observation calls for caution in using DBS for OCD as the cost of the therapy can increase substantially.
Intraoperative confirmation of target localization can be obtained by stimulation-evoked responses. In 20% of the patients, intraoperative stimulation produces reduction of anxiety and tension.[10,17] This was seen in the most ventral part of the target, which prompted Laitinen to extend the lesion below the intercommisural plane, a target close to present day target of nucleus accumbens. We also had a similar observation when we were 4–6 mm above the target.
Surgery for OCD, though rewarding, is highly dependent on appropriate patient selection. It is important that the patient be under a care of psychiatrist, who would be agreeable to manage him postoperatively as the patient would need proper psychiatric support.
Capsulotomy is a relatively safe procedure. Early postoperative side effects include short-lasting confusion with occasional loss of bladder control, which was also seen in our patient. Lack of initiative or apathy is also a known complication and would last for several weeks after surgery.[12] Another side effect is fatigue that can also last for a few weeks.[10] Besides these, there are no significantly noticeable complications.
CONCLUSION
Surgery for OCD has been practiced for now more than five decades. Intractable OCD is a big drain on the patient and his/her family. Recent understanding of OCD symptomatology and pathophysiology has contributed in target refinement and reducing complications associated with surgery. Presently, this surgical option is highly underutilized. However, we should take a lesson from the past and ensure that this renaissance of surgery continues and does not die an unnatural death.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Stein DJ. Obsessive-compulsive disorder. Lancet. 2002;360:397–405. doi: 10.1016/S0140-6736(02)09620-4. [DOI] [PubMed] [Google Scholar]
- 2.Ferrao YA, Shavitt RG, Bedin NR, de Mathis ME, Carlos A, Fontenelle LF, et al. Clinical features associated to refractory obsessive-compulsive disorder. J Affect Disord. 2006;94:199–209. doi: 10.1016/j.jad.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Lopes AC, de Mathis ME, Canteras MM, Salvajoli JV, Del Porto JA, Miguel EC. [Update on neurosurgical treatment for obsessive compulsive disorder] Rev Bras Psiquiatr. 2004;26:62–6. doi: 10.1590/s1516-44462004000100015. [DOI] [PubMed] [Google Scholar]
- 4.Schruers K, Koning K, Luermans J, Haack MJ, Griez E. Obsessive-compulsive disorder: A critical review of therapeutic perspectives. Acta Psychiatr Scand. 2005;111:261–71. doi: 10.1111/j.1600-0447.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg BD, Gabriels LA, Malone DA, Jr, Rezai AR, Friehs GM, Okun MS, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15:64–79. doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doshi PK. Surgical treatment of obsessive compulsive disorders: Current status. Indian J Psychiatry. 2009;51:216–21. doi: 10.4103/0019-5545.55095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 8.Rauch SL, Jenike MA. Neurobiological models of obsessive-compulsive disorder. Psychosomatics. 1993;34:20–32. doi: 10.1016/S0033-3182(93)71924-6. [DOI] [PubMed] [Google Scholar]
- 9.Saxena S, Brody AL, Schwartz JM, Baxter LR. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry Suppl. 1998;35:26–37. [PubMed] [Google Scholar]
- 10.Christensen DD, Laitinen LV, Schmidt LJ, Hariz MI. Anterior capsulotomy for treatment of refractory obsessive-compulsive disorder: Results in a young and an old patient. Stereotact Funct Neurosurg. 2002;79:234–44. doi: 10.1159/000070837. [DOI] [PubMed] [Google Scholar]
- 11.Fodstad H, Strandman E, Karlsson B, West KA. Treatment of chronic obsessive compulsive states with stereotactic anterior capsulotomy or cingulotomy. Acta Neurochir (Wien) 1982;62:1–23. doi: 10.1007/BF01402207. [DOI] [PubMed] [Google Scholar]
- 12.Laitinen LV. Psychosurgery today. Acta Neurochir Suppl (Wien) 1988;44:158–62. doi: 10.1007/978-3-7091-9005-0_30. [DOI] [PubMed] [Google Scholar]
- 13.Lippitz BE, Mindus P, Meyerson BA, Kihlstrom L, Lindquist C. Lesion topography and outcome after thermocapsulotomy or gamma knife capsulotomy for obsessive-compulsive disorder: Relevance of the right hemisphere. Neurosurgery. 1999;44:452–8. doi: 10.1097/00006123-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Mindus P. Present-day indications for capsulotomy. Acta Neurochir Suppl (Wien) 1993;58:29–33. doi: 10.1007/978-3-7091-9297-9_6. [DOI] [PubMed] [Google Scholar]
- 15.Mindus P, Bergstrom K, Levander SE, Noren G, Hindmarsh T, Thuomas KA. Magnetic resonance images related to clinical outcome after psychosurgical intervention in severe anxiety disorder. J Neurol Neurosurg Psychiatry. 1987;50:1288–93. doi: 10.1136/jnnp.50.10.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyman H, Mindus P. Neuropsychological correlates of intractable anxiety disorder before and after capsulotomy. Acta Psychiatr Scand. 1995;91:23–31. doi: 10.1111/j.1600-0447.1995.tb09737.x. [DOI] [PubMed] [Google Scholar]
- 17.Laitinen LV, Singounas E. Intraoperative electrical stimulation of the brain in patients with obsessive-compulsive neurosis. Appl Neurophysiol. 1988;51:317–23. doi: 10.1159/000099976. [DOI] [PubMed] [Google Scholar]
- 18.Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999;354:1526. doi: 10.1016/S0140-6736(99)02376-4. [DOI] [PubMed] [Google Scholar]


