Abstract
Background:
Exercise training as a part of cardiac rehabilitation aims to restore patient with heart disease to health. However, left ventricular ejection fraction (LVEF) is clinically used as a predictor of long-term prognosis in coronary artery disease (CAD) patients, there is a scarcity of data on the effectiveness of exercise-based cardiac rehabilitation on LVEF.
Objective:
To investigate the effectiveness of exercise-based cardiac rehabilitation on LVEF in early post-event CAD patients.
Patients and Methods:
In a single blinded, randomized controlled trial, post-coronary event CAD patients from the age group of 35-75 years, surgically (Coronary artery bypass graft or percutaneous coronary angioplasty) or conservatively treated, were recruited from Golsar Hospital, Iran. Exclusion criteria were high-risk group (AACVPR-99) patients and contraindications to exercise testing and training. Forty-two patients were randomized either into Study or Control. The study group underwent a 12-week structured individually tailored exercise program either in the form of Center-based (CExs) or Home-based (HExs) according to the ACSM-2005 guidelines. The control group only received the usual cardiac care without any exercise training. LVEF was measured before and after 12 weeks of exercise training for all three groups. Differences between and within groups were analyzed using the general linear model, two-way repeated measures at alfa=0.05.
Results:
Mean age of the subjects was 60.5 ± 8.9 years. There was a significant increase in LVEF in the study (46.9 ± 5.9 to 61.5 ± 5.3) group compared with the control (47.9 ± 7.0 to 47.6 ± 6.9) group (P=0.001). There was no significant difference in changes in LVEF between the HExs and CExs groups (P=1.0).
Conclusion:
A 12-week early (within 1 month post-discharge) structured individually tailored exercise training could significantly improve LVEF in post-event CAD patients.
Keywords: Cardiac rehabilitation, coronary artery disease, ejection fraction, exercise training
INTRODUCTION
Middle Eastern countries, including Iran, are joining the global obesity pandemic and its consequences such as coronary artery disease (CAD).[1] CAD is one of the most common causes of morbidity and mortality in different communities worldwide.[2–4] Despite the lack of accurate data, there is some evidence to indicate that CAD is increasing in magnitude in Iran,[2] accounting for about 50% of all deaths per year.[5] While age-adjusted mortality from CAD is gradually falling in developed countries,[3,6] the rate has increased by 20–45% in Iran.[7,8]
Left ventricular ejection fraction (LVEF) as a clinical index of myocardial contractility and its pumping action[9,10] is a well-established predictor of mortality and long-term prognosis in acute myocardial infarction.[10,11] However, exercise training is the core component of cardiac rehabilitation and secondary prevention of CAD, there is a less body of evidence regarding the effectiveness of exercise training on LVEF in CAD patients. Previous published studies mainly studied this outcome in heart failure patients or they used a heterogeneous subject group with respect to the time gap between coronary event and start of exercise training or total duration of the program. The purpose of this study was to determine the effect of early (within 1 month post-discharge) structured individually tailored exercise training on LVEF in post-event CAD patients. This is a part of a larger multicenter study and, to our knowledge, is the first of such in the country.
PATIENTS AND METHODS
Study design
The study procedure was designed in accordance with the Helsinki Declaration new revision 2000. This was a single blinded randomized (ratio 2:1) controlled trial in which the effectiveness of early structured individually tailored exercise training on LVEF was studied. Eligible patients who gave a written informed consent were allocated into study or control groups by means of block randomization (block size of 6) using the concealed envelope method. The assessor of main outcome, a cardiologist, was unaware of the allocation of patients and, due to the nature of the study, authors were not able to mask more arms. A flowchart of the study is summarized in Figure 1.
Figure 1.
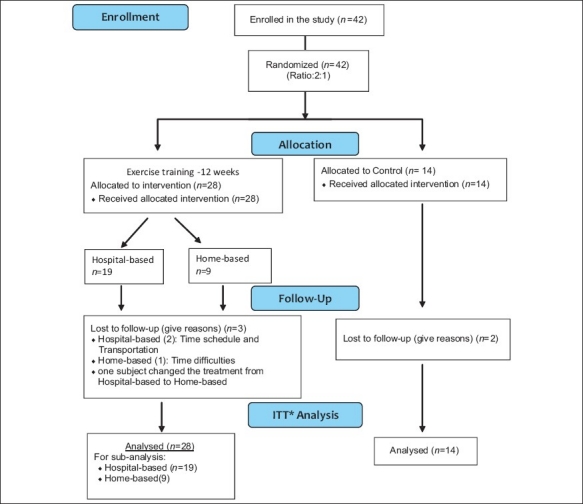
Flow diagram of the study
Subjects
Post-coronary event, patients who were treated surgically (CABG or PTCA) or conservatively were recruited between July and November 2009 at the Golsar Hospital, Rasht, Iran. The Golsar Hospital is a general hospital and offers cardiac care, including angioplasty and CABG as well as outpatient cardiac rehabilitation programs.
Inclusion criteria
Patients of both sexes were screened for eligibility criteria including age group of 35–75 years who were post-event (within 1 month post-discharge) CAD patients treated either surgically (CABG or PTCA) or conservatively.
Exclusion criteria
High-risk group patients (AACVPR-99)[12] or any systemic, orthopedic or neurological conditions that restrict participating in aerobic exercise and patients who were contraindicated for exercise testing and training were excluded from the study.
Procedure
The ethical committee of Golsar Hospital approved the study. All eligible patients were explained about the procedure and written informed consent was obtained from them before allocating them into different groups. Eligible and consenting patients were randomly (ratio 2:1) assigned to exercise-based cardiac rehabilitation or control group by means of block randomization (block size of 6) and concealed envelope method. Base line data included the LVEF measured by echocardiography, and demographic and clinical evaluation was taken. Patients in the study group underwent a 12-week structured individually tailored exercise training either in the form of a Center-based program (CExs) or a Home-based program (HExs). After 12 weeks of exercise training, subjects were reassessed clinically for the primary outcome and results were compared pre- and post-intervention with the control group. All patients underwent a graded exercise test (GXT) with Bruce protocol at baseline in order to risk stratify the patients (AACVPR-99), and the results of the test included MET and achieved HRPeak were used as baseline for exercise prescription according to the Karvonen formula.
Exercise training group
Authors used the ACSM-2005[13] guidelines as principle for exercise prescription for the study group. The intensity of the prescribed exercise was calculated based on heart rate reserve achieved during the graded exercise test (Bruce protocol) as well as the rating of perceived exertion (RPE). Target heart rate range (THRR) using the Karvonen formula was applied to prescribe exercise intensity. All recruited subjects were given orientation to the program. A session of informal health education about their condition was given by the physiotherapist to the patients and to their family members. Risk factor modifications advice according to the risk factors of each patient, life style modification and smoking cessation advice were given prior to the start of the rehabilitation program. Awareness about cardiac rehabilitation, exercise program, adherence to the program and its benefits, which they attend, was explained to the study group to increase the rate of attendance and compliance.
Patients in the study group allocated to either a CExs training program or a HExs training program according to the convenience and preference of patients but same guidelines (ACSM-2005),[13] were used for both subgroups for prescribing the intensity of the exercise.
Group IA. Center-based group
This group underwent a structured, supervised exercise training program for a period of 12 weeks. They attended a minimum of 3 days exercise-based cardiac rehabilitation in the hospital set-up. The exercise program consisted of 5–10 min warm up (breathing exercise, stretching exercise and walking on treadmill) followed by graded aerobic training and 5–10 min cool down. Graded aerobic training was mainly treadmill walk three to five times per week, with an intensity of 40–70% of HRR achieved in the exercise test applying the Karvonen formula, and RPE of 11–14 for a duration of 20–40 min (ACSM guidelines).[13]
Group IB. Home-based group
Exercise component of cardiac rehabilitation program for the home-based group was an individualized tailored program of aerobic exercises; preferably, brisk walking, as it is shown in the literature that brisk walking provides an activity intense enough to increase aerobic capacity in healthy sedentary as well as cardiac patients.[14] Initial sessions of exercise prescription and training were given in the department under physiotherapist supervision, and then the program protocol was given to the patient to do at home for 12 weeks. Intensity of exercise converted to a safe range of speed of walk that the patient achieved on a treadmill to use as a base for brisk walking.
Patients were also trained in palpating the pulse and calculating the heart rate, and to rate the RPE of 11–14. The exercise program consisted of 5–10 min warm up, including breathing exercise, stretching exercise and gentle active exercise, to larger muscle groups like the lower limb and trunk muscles followed by graded aerobic training and cool down. Graded aerobic training was mainly brisk walking for three to five times per week with an intensity of 40–70% of HRR achieved in the exercise test applying the Karvonen formula, converted to a speed of walk and RPE of 11–14 for a duration of 20–40 min (according to the ACSM guidelines).[13]
Patients in the HExs group were regularly contacted by phone every 2 weeks to find out their adherence to the program and advice or changes in program if necessary and to monitor the progress. The exercise log was reviewed every 15 days. Subjects were also advised to contact the physiotherapist if any advice or help was needed. A trained physiotherapist gave them detailed awareness of the signs and symptoms to be monitored while doing the exercise program, dos and don’ts and the criteria for the termination of exercise were well explained to them.
Exercise intensity progression
As the conditioning effect of exercise training, progression of the exercise intensity was done as needed. As the RPE falls with improving fitness, the intensity of exercise was increased at 5–10% of the maximum heart rate and by maintaining RPE of 11–14 throughout the 12 weeks duration. For the first 4 weeks, patients performed the exercise training for 15–20 mins, from the 5th to 8th week increased to 20–30 mins and the final 9th to 12th week duration was increased to 30–40 mins.
Monitoring
RPE
RPE provides a subjective means of monitoring exercise intensity. HR-VO2 relationship could be evaluated further in relation to individual RPE, which is helpful in monitoring the exercise intensity. This method is appropriate for setting exercise intensity in persons with low fitness, cardiac patients and those who are under medication that affect HR response to exercise, taking into account personal fitness level, environmental conditions and general fatigue level.[14] Light to moderate intensity (RPE of 11–14) is suitable for cardiac patients.
It is important to use standardized instruction to reduce problems of misinterpretation of RPE. The following instruction is recommended by the ACSM guidelines:[13]
“During the exercise we want you to pay close attention to how hard you feel the exercise work rate is. This feeling should reflect your total amount of exertion and fatigue, combining all sensations and feeling of physical stress, effort and fatigue. Do not concern yourself with any one factor such as leg pain, shortness of breath or exercise intensity, but try to concern on your total inner feeling of exertion. Try not to underestimate or overestimate your feeling of exertion. Be as accurate as you can.”
Other symptomatic complaints such as degree of chest pain, angina, burning sensation discomfort and dyspnea are collected from the patients routinely.[13]
Indications for termination of exercise
Detailed awareness of signs and symptoms to be monitored while doing the exercise program and subjective symptoms and criteria for the termination of exercise were well explained to the subjects.
Group II: Control group
In the control group, subjects underwent baseline assessment and these patients were instructed to follow medical treatment advised by their physician and only education program were given to them. They were not advised any extra formal exercise training program.
Reassessment
After 12 weeks, post-intervention re-evaluation was done by echocardiography in both the study as well as the control group.
Data analysis
Sample size was determined using a pilot study of 10 patients. Within-group improvement of 5% in LVEF was considered as clinically significant. At alfa=0.05 and power of 90%, authors determined a sample size of 42 subjects. Analyses were performed by using an intention to treat approach. Statistical software SPSS v17 was used to infer the data. The General Linear Model, including repeated measures, was used to analyze the results.
RESULTS
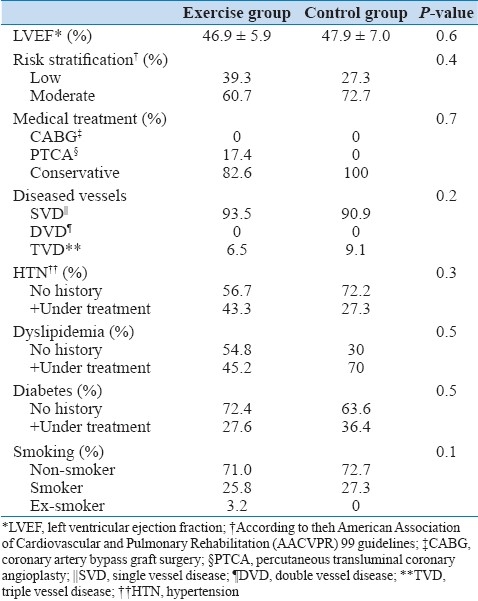
A total of 42 (32 male, 10 female) subjects with mean age of 60.5 ± 8.9 years enrolled in the study. All subjects completed their course of exercise training with a minimum of 70% attendance in the exercise sessions. Both groups had similar demographic and clinical characteristics at baseline with respect to the LVEF, risk stratification, number of diseased vessels, life style, educational level and diet habits, but there was a significant difference between the groups with respect to age [Tables 1 and 2].
Table 1.
Baseline demographic characteristics of patients assigned to the exercises and control groups
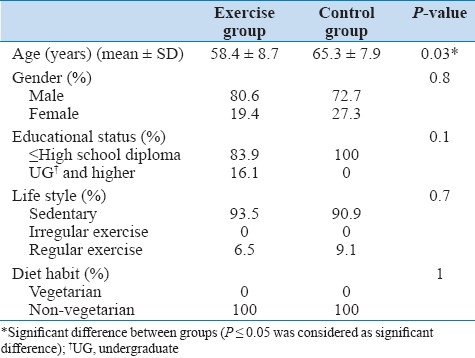
Table 2.
Baseline clinical characteristics of patients assigned to the exercises and control groups
Baseline LVEF in the study group was 46.9 ± 5.9 and in the control group was 47.9 ± 7.0. There was a significant improvement in LVEF after 12 weeks of exercise training in the study group (46.9 ± 5.9 to 61.5 ± 5.3) compared with the control (47.9 ± 7.0 to 47.6± 6.9) group (P=0.001) [Figure 2].
Figure 2.
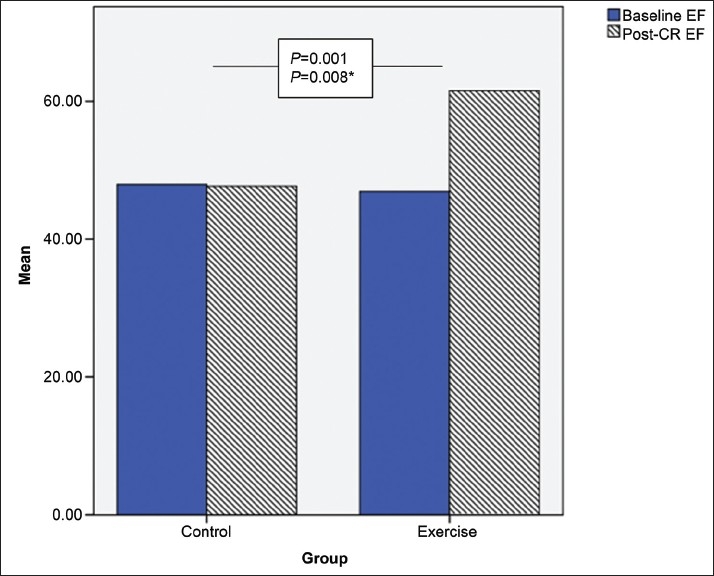
Changes in left ventricular ejection fraction (pre-post) exercise training
Because there was a significant difference between the study and control groups with respect to age, a second analysis after adjusting for the age variable still showed a significant improvement in LVEF in the study group compared with the control group (P=0.008) [Table 3].
Table 3.
Changes in LVEF in the study and control groups following 12 weeks exercise training

A subgroup analysis of the results between HExs and CExs showed that there was no significant difference in changes of LVEF between the two protocols [Table 3].
DISCUSSION
Although decreased left ventricular systolic function is a well-established independent predictor of mortality in CAD patients, little information is available regarding the effect of exercise training on LVEF.[11] The existing literature either focuses more on heart failure patients or lacks methodological uniformity regarding the type of patients, time gap between post-discharge to start of exercise training in post-event patients or the intensity and type of exercise given to the patients. Koch, Duard and Broustet (1992) in a randomized clinical trial studied the effect of graded physical exercise on EF and found no significant effect. But, their study was conducted on chronic heart failure patients.[15]
Adachi, Koiket and Obayshi (1996) reported improvement in cardiac function (such as stroke volume) both at rest and during exercise only with high-intensity exercise training.[16]
The present study demonstrated two important findings. First, an early (within 1 month post-discharge) 12 weeks structured exercise training program in post-event coronary artery disease patients could significantly improve the myocardial contractility in terms of LVEF. Second, a structured individually tailored HExs training could be as effective as center-based programs and safely used not only in low-risk but also in moderate-risk (AACVPR-99) CAD patients. These programs could be started as early as 2 weeks post-discharge in uncomplicated patients. These findings are in consistent with results from Haddadzadeh, Maiya et al. in their recent study in India, which found a similar effect in a RCT.[17] Giallauria et al. also found a favorable remodeling from 6 months exercise training program in patients with moderate left ventricular dysfunction.[18] As evidence shows, there are many difficulties and barriers to long term-center-based exercise training, and only 25–30% of the eligible patients attend exercise-based cardiac rehabilitation programs. Individually tailored HExs training programs could be an alternative method in improving myocardial contractility without affecting the efficacy of the programs.
To the best of our knowledge, this was the first RCT in Iran to investigate the effectiveness of structured exercise training program on LVEF. Applying the finding into practice amplifies the importance of secondary prevention and effectiveness of early exercise-based cardiac rehabilitation programs on the overall cardiac condition of the patients. Keeping in mind the increasing number of cardiovascular diseases in the Middle East, including Iran, forwarding the message to the policy makers, insurance companies for covering the cardiac rehabilitation expenses, hospital administrative for necessity of such programs and adjusting the need of the common people with the available resources in the form of home-based programs without losing the efficacy is a must.
Limitation of the study
One of the limitations of this study, like many other rehabilitation programs, was the inability to randomize the patients in center-based or home-based groups due to the universal barriers of cardiac rehabilitation, e.g. transportation, economical aspect and far distances of centers to rural areas and reaching the center three to five times per week. It can be an interest of consideration for future studies.
In conclusion, a 12-week early (within 1 month post-discharge) structured individually tailored exercise training could significantly improve the LVEF in post-event coronary artery disease patients. However, a structured individually tailored HExs program could be as effective as a center-based program in improving LVEF.
ACKNOWLEDGMENT
The authors would like to thank the reviewers in the International Conference on Multi-Disciplinary Approach in Healthy & Participatory Aging (MAHPA), Mumbai, in which a preliminary report of the pilot study was presented and comments were considered in the paper. The authors would also like to thank the primary funding agency, Armaghan Educational Institute, through the Ministry of Education of Iran, as a part of a larger multicenter study. Thanks are also due to the physiotherapists S. Alidoust and M. Monfaredi and the head nurse M. Pourgholi from the Department of Physiotherapy, for their minute to minute help during the study conduct. The authors would especially like to thank Prof. Anoush Barzigar, Head of Cardiology Department, Heshmat Heart Center, Dr. Azizollah-Zadeh, Chief Manager of Golsar Hospital, Rasht, Iran and Dr. A. Ershadi, Department of Angioplasty and Exercise Test Unit of Golsar Hospital for their complete support and cooperation.
Footnotes
Source of Support: Armaghan Educational Institute, Ministry of Education, Iran
Conflict of Interest: Authors agreed that there was no source of conflict of interest.
REFERENCES
- 1.Bahrami H, SadatSafavi M, Pourshams A, Kamangar F, Nouraei M, Semnani S, et al. Obesity and hypertension in an Iranian cohort study; Iranian women experience higher rates of obesity and hypertension than American women. BMC Public Health. 2006;6:158–66. doi: 10.1186/1471-2458-6-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hadaegh F, Harati H, Ghanbarian A, Azizi F. Prevalence of coronary heart disease among Tehran adults: Tehran Lipid and Glucose Study. East Mediterr Health J. 2009;15:157–66. [PubMed] [Google Scholar]
- 3.Castelli WP. Epidemiology of coronary heart disease: The Framingham study. Am J Med. 1984;76:4–12. doi: 10.1016/0002-9343(84)90952-5. [DOI] [PubMed] [Google Scholar]
- 4.Keil U. [The worldwide WHO MONICA Project: results and perspectives] Gesundheitswesen. 2005;67(Suppl 1):S38–45. doi: 10.1055/s-2005-858240. [DOI] [PubMed] [Google Scholar]
- 5.Hatmi ZN, Tahvildari S, Gafarzadeh Motlag A, Sabouri Kashani A. Prevalence of coronary artery disease risk factors in Iran: A population based survey. BMC Cardiovasc Disord. 2007;7:32. doi: 10.1186/1471-2261-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sytkowski PA, D’Agostino RB, Belanger A, Kannel WB. Sex and time trends in cardiovascular disease incidence and mortality: The Framingham heart study, 1950-1989. Am J Epidemiol. 1996;143:338–50. doi: 10.1093/oxfordjournals.aje.a008748. [DOI] [PubMed] [Google Scholar]
- 7.Prevention and control of cardiovascular disease. Alexandria, World Health Or-ganization Regional Office for the Eastern Mediterranean. 1995;24 [Google Scholar]
- 8.Zali M, Kazem M, Masjedi MR. [Health and disease in Iran]. Tehran, Islamic Republic of Iran, Deputy of Research, Ministry of Health. 1993 (Bulletin No 10) [Google Scholar]
- 9.Ratchford AM, Hamman RF, Regensteiner JG, Magid DJ, Gallagher SB, Merenich JA. Attendance and graduation patterns in a group model health maintenance organization.Alternative cardiac rehabilitation program. J Cardiopulm Rehabil. 2004;24:150–6. doi: 10.1097/00008483-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Johnson N, Fisher J, Nagle A, Inder K, Wiggers J. Factors associated with referral to outpatient cardiac rehabilitation services. J Cardiopulm Rehabil. 2004;24:165–70. doi: 10.1097/00008483-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Dutcher JR, Kahn J, Grines C, Franklin B. Comparison of left ventricular ejection fraction and exercise capacity as predictors of two and five-year mortality following acute myocardial infarction. Am J Cardiol. 2007;99:436–41. doi: 10.1016/j.amjcard.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 12.Guidelines for Cardiac Rehabilitation and Secondary Prevention Program. 3rd ed. Champaign, IL: Human kinetics; 1999. American Association of Cardiovascular and Pulmonary Rehabilitation. [Google Scholar]
- 13.7th ed. Philadelphia: Lippincott, Williams and Wilkines; 2006. American College Of Sports Medicine (ACSM) – Guidelines for exercise testing and prescription. [Google Scholar]
- 14.Machionni N, Fattirolli F, Fumagalli S, Oldridge N, Del Lungo F, Morosi L, et al. Improved exercise tolerance and quality of life with cardiac rehabilitation of older patients after myocardial infarction: results of a randomized controlled trial. Circulation. 2003;107:2201–6. doi: 10.1161/01.CIR.0000066322.21016.4A. [DOI] [PubMed] [Google Scholar]
- 15.Koch M, Douard H, Broustet JP. The benefit of graded physical exercise in chronic heart failure. Chest. 1992;101(5 Suppl):231S–235S. doi: 10.1378/chest.101.5_supplement.231s. [DOI] [PubMed] [Google Scholar]
- 16.Adachi H, Koike A, Obayashi T, Umezawa S, Aonuma K, Inada M, et al. Does appropriate endurance exercise training improve cardiac function in patients with prior myocardial infarction? Eur Heart J. 1996;17:1511–21. doi: 10.1093/oxfordjournals.eurheartj.a014715. [DOI] [PubMed] [Google Scholar]
- 17.Haddadzadeh MH, Maiya AG, Padmakumar R, Devasia T, Kansal N, Borkar S. Effectiveness of cardiac rehabilitation on myocardial contractility in post-event coronary artery disease patients: A randomized controlled trial. Physiotherapy. 2010;8:5–12. [Google Scholar]
- 18.Giallauria F, Cirillo P, Lucci R, Pacileo M, De Lorenzo A, D’Agostino M, et al. Left ventricular remodelling in patients with moderate systolic dysfunction after myocardial infarction: Favourable effects of exercise training and predictive role of N-terminal pro-brain natriuretic peptide. Eur J Cardiovasc Prev Rehabil. 2008;15:113–8. doi: 10.1097/HJR.0b013e3282f00990. [DOI] [PubMed] [Google Scholar]


