Abstract
Coronary perforation is a rare complication of percutaneous coronary intervention. We present two different types of coronary intervention, but both ending with coronary perforation. However, these perforations were tackled successfully by covered stents. This article reviews the incidence, causes, presentation, and management of coronary perforation in the present era of aggressive interventional cardiology. Coronary perforations are classified as type I (extraluminal crater), II (myocardial or pericardial blushing), and III (contrast streaming or cavity spilling). Types I and II coronary perforations are caused by stiff or hydrophilic guidewires. Type I has a benign prognosis, whereas type II coronary perforations have the potential to progress to tamponade. Type III coronary perforations are caused by balloons, stents, or other intracoronary devices and commonly lead to cardiac tamponade necessitating pericardial drainage. However, type III perforations can be managed with covered stents without need for surgical intervention.
Keywords: Cardiac tamponade, coronary perforation, covered stent
INTRODUCTION
Since the introduction of percutaneous coronary intervention (PCI) in 1977 along with availability of advanced coronary hardware and newer adjunctive pharmacotherapy, PCI is increasingly used not only in simple coronary lesions, but also in complex coronary anatomies. Thus, following complex coronary interventions there is relatively higher rate of complications, such as coronary dissection, coronary perforation, acute coronary syndrome, and arrhythmias. Hence, an interventional cardiologist has to anticipate these complications, try to avoid these complications and know how to tackle them to prevent mortality and morbidity.
Coronary perforation is a rare PCI complication leading to pericardial effusion with or without tamponade and if left undiagnosed or untreated it is life-threatening. We present two different types of coronary interventions, but both ending with coronary perforation. However, the coronary perforations were tackled successfully by covered stents. This review summarizes the incidence, causes, presentation, and management of coronary perforation in the present era of aggressive interventional cardiology.
Case 1
A 48-year-old man was referred for PCI with history of exertional angina and a positive treadmill test. He had undergone coronary angiography six months earlier in another institute, which had revealed 100% occlusion of mid left anterior descending artery (LAD) after a large second diagonal artery (D2) with thrombolysis in myocardial infarction (TIMI) grade 0 flow and retrograde filling of LAD from right system. There was 100% occlusion of proximal left circumflex artery with bridging collaterals filling a large obtuse marginal artery. The right coronary artery was dominant and normal. He was advised coronary artery bypass surgery, but he went to another institute abroad and underwent an attempted PCI to the totally occluded mid LAD. A drug eluting stent was deployed in LAD-D2. No details of the procedure were available. He was taking aspirin 75 mg and clopidogrel 75 mg once daily. His routine blood tests including renal parameter were normal. After 6 Fr sheath insertion into right femoral artery and vein, he received intravenous unfractionated heparin 2000 Units.
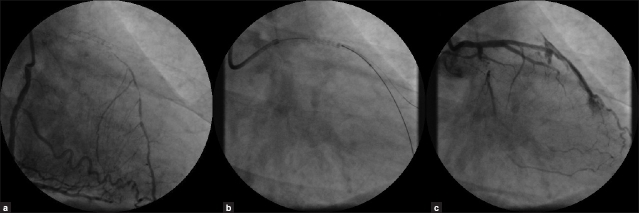
Coronary angiogram showed patent stent in LAD-D2 with TIMI 3 flow and a totally occluded mid-LAD after D2. There was retrograde filling of LAD from right system [Figure 1a]. After discussing with the patient who was still refusing surgical intervention, we proceeded with PCI of chronic totally occluded LAD. Using 6 Fr XB guiding catheter a PT graphix standard wire was tried unsuccessfully to cross the LAD through the stent struts. This wire was placed in D2. Miracle Bros 12 wire was used to cross the totally occluded LAD smoothly with good torque response [Figure 1b]. Further, 5000 units heparin was administered. Using 1.5×20 mm Fire Star RX balloon through the stent, an attempt was made to dilate the LAD at 12 atmospheres (atms). The first image taken after dilatation showed a large perforation of LAD through the stent with free extravasation of contrast into the pericardium indicating Type III perforation (Ellis-classification) [Figure 1c]. There was no rupture of the balloon.
Figure 1.
(a) Right coronary angiogram showing retrograde filling of left anterior descending artery. (b) Fluoroscopy showing placement of a guide wires in diagonal artery and left anterior descending artery (through the stent). (c) Left coronary angiogram post-dilatation showing Type III perforation of left anterior descending artery across the stent with cavity spilling of dye
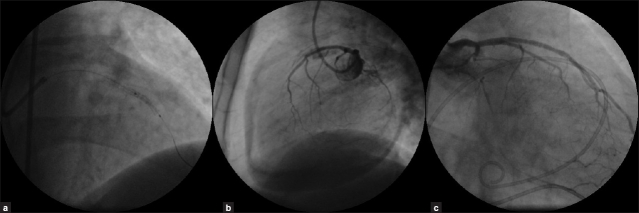
Initially, patient remained hemodynamically stable. There were no ischemic changes on monitor. Immediately, the area of perforation was sealed with the use of a rapid exchange covered stent (JOSTENT GraftMaster [Abbot vascular Inc.] 3 × 16 mm at 14 atms) [Figure 2a] deployed within the previous stent, with rapid cessation of contrast extravasation and a TIMI 3 Flow in D2. Subsequent left coronary injection showed residual Type II perforation, but there was large pericardial collection [Figure 2b] from the previous perforation. Patient developed hypotension (blood pressure 80/60 mmHg) and pulsus paradoxus of 30 mmHg. Immediate percutaneous pericardiocentesis was performed using 6F sheath, 350 ml of fresh blood was drained with rapid hemodynamic improvement. A pigtail catheter was introduced into the pericardium. Intravenous protamine 30mg was administered to reverse heparin effect. Activated clotting time was 144 seconds. Following this he was shifted to CCU on inotropic support. Further, 550 ml of hemorrhagic pericardial fluid was drained over 24 hours. There was no significant drop in hemoglobin. Check angiogram done next day showed complete sealing of the LAD perforation [Figure 2c]. He remained well and was discharged two days later after serial echocardiography showed no pericardial effusion. He was advised to undergo coronary artery bypass surgery at a later date. He was continued on aspirin and clopidogrel along with antianginal medications.
Figure 2.
(a) Fluoroscopy showing deployment of a covered stent within a previous stent. (b) Left coronary angiogram showing residual perforation with a large pericardial effusion. (c) Left coronary angiogram 24-hours post-deployment of a covered stent showing complete sealing of left anterior descending artery perforations.
Case 2
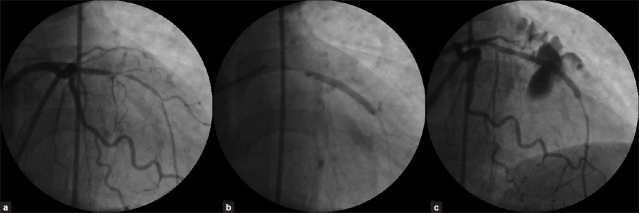
A 57-year-old female with a history of long standing hypertension, hyperlipidemia presented with exertional anginal class II and a positive treadmill exercise test. Her routine blood investigations were normal including renal parameters. At cardiac catheterization, after 6 Fr sheath insertion into right femoral artery, she was given 2000 units of unfractionated heparin. The coronary angiogram showed normal non dominant right coronary and dominant left circumflex artery with LAD showing mid long 95% stenosis [Figure 3a]. It was decided to predilate the lesion followed by implantation of a drug-eluting stent. Further, 6000 units of intra-arterial heparin was given. After cannulating left coronary ostium, with 6F JL 3.5 guide catheter and crossing the lesion with BMW 0.014” wire, the lesion was pre-dilated using 1.5 × 15 mm Crescendo balloon at 12 atms pressure. Following this, a drug-eluting stent was deployed in the mid LAD at 14 atms pressure [Figure 3b].
Figure 3.
(a) Left coronary angiogram showing tight long mid left anterior descending artery stenosis. (b) Left coronary angiogram showing deployment of a long drug-eluting stent in mid left anterior descending artery. (c) Left coronary angiogram post-stent deployment showing Type III perforation of left anterior descending artery with extensive myocardial and pericardial blushing
Post stenting angiogram showed large dye extravasations with extensive myocardial and pericardial blushing along with contrast streaming into the pericardium indicating a type III coronary perforation [Figure 3c]. Immediately, the area of perforation at the mid part of the stent was sealed with the use of a rapid exchange covered stent (JOSTENT GraftMaster 3 × 16 mm at 10 atms) [Figure 4a] deployed within the previous stent, with rapid cessation of contrast extravasation.
Figure 4.
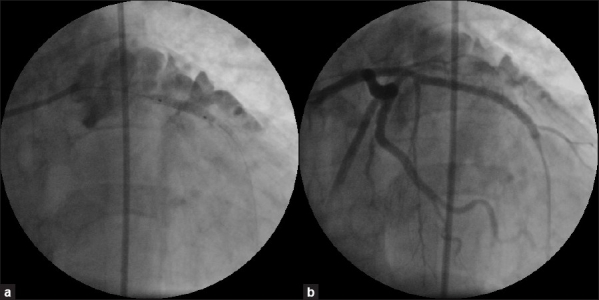
(a) Fluoroscopy showing deployment of a covered stent within a previous stent at the perforation site. (b) Left coronary angiogram post- deployment of a covered stent showing complete sealing of left anterior descending artery perforation
Post-covered stent angiogram showed complete sealing of the perforation [Figure 4b]. Echocardiogram showed minimal pericardial effusion, which had disappeared next day. Rest of her stay was uneventful.
DISCUSSION
Incidence and predictors of coronary perforation
Coronary artery perforation is a rare but potentially fatal complication of PCI that can result in life threatening cardiac tamponade. In the present era, the incidence of coronary perforation ranges from 0.19% to 0.59%.[1–8] Coronary perforation occurs when a dissection or intimal tear completely penetrates the arterial wall leading to either vessel puncture with minimal dye staining or vessel rupture with brisk extravasations of blood and dye into the pericardial space.[9] Among the possible factors predicting its appearance are:
Clinical factors: Advanced age,[10] female sex,[1,10] renal impairment,[4,7] non-ST-elevation myocardial infarction patients[8]
Angiographic factors: Chronic total occlusion,[2,8] coronary artery calcification,[2,3,8] type-C lesions,[2,3] tortuous vessels, target lesions in the circumflex and right coronary arteries, long target lesions (>10 mm), and eccentric lesions.[2,3]
Technique-associated factors: Use of hydrophilic/ extra stiff wires,[3,6] atherectomy devices,[1,2] increased balloon to artery ratio,[9,10] intravascular ultrasound guided PCI optimization and high-pressure stent post-dilatation,[5] and cutting balloons.[11]
Common causes of coronary perforation
During PCI, perforation occurs as a consequence of guide wire advancement, balloon/stent advancement, balloon/stent inflation, over sizing or ruptured balloon/stent, or from subintimal passage of the balloon/stent into a vessel with severe dissection.[12] It is reported that perforation after stenting is mainly caused by excessive overdilatation or an oversized stent implantation.[12] In the past, Witzke et al.[2] noted that coronary perforation occurred more frequently with debulking techniques (directional atherectomy/rotablation) than with non-debulking (balloon angioplasty/stent) techniques (1% versus 0.26%). They also noted that, about fifty percent of the coronary perforations were guide wire related.[2]
Coronary perforation occurs commonly due to distal migration of guide wire with or without wire fracture or while crossing the lesion with guide wire or inadvertently positioned outside the arterial bed. Hydrophilic-coated guide wires (guide wires with a polymer coated tip, used in tortuous severely stenotic coronary lesions) may confer an increased risk of perforation due to their low coefficient of friction and ease of distal migration.[9] In a recent analysis,[6] coronary perforation occurred as a complication of wire manipulation in 66% (with 89% of this group being hydrophilic wires), of coronary stenting in 16%, of angioplasty alone in 8%, and of rotational atherectomy in 11%. In another study,[8] vessels were perforated by wires (52%), balloons (26%), and stents (21%).
In a study involving guide wire coronary perforation, in the multivariate analysis, the use of hydrophilic guidewires (OR=2.33; 95% CI, 1.34–4.05) and treatment of chronic occlusions (OR=3.31; 95% CI, 1.05–10.46) were independently related to guide wire perforation with cardiac tamponade (sub acutely, up to 3 days following the procedure) seen in 46% of the patients.[13] Teis et al. observed that in these guide wire perforations, the passage of blood to the pericardium took place very slowly because of the small perforation produced by the guide wire.[13] Hence, coronary perforation secondary to guide wires may be silent initially, but patients may develop hypotension or chest pain later progressing to tamponade. An urgent transthoracic echocardiogram can diagnose tamponade early in these patients.
Classification and presentation of coronary perforation
Ellis et al.[10] classified coronary perforation into three types: Type I, extraluminal crater without extravasation; Type II, pericardial or myocardial blushing; Type III, perforation ≥1-mm diameter with contrast streaming; and cavity spilling. Often, coronary perforation is associated with significant morbidity and mortality.[1,6,8] Mortality is high and is reported to range from 5.9% to 7%.[1,6,8]
Major adverse clinical outcomes including cardiac tamponade occurs more frequently in patients with Type III perforations.[2,3] In recent studies, multivariate analysis identified chronic total occlusion, coronary calcium and non-ST elevation myocardial infarction as independent predictors of coronary perforation[8] and the perforation grade and chronic renal insufficiency were the only predictors of mortality.[4] Patients can develop acute myocardial infarction.[1,3,6] Pericardial effusion occurs in nearly 40% of the cases[2] and around 10–20% of the cases can have tamponade.[2,8] Tamponade may not be immediately evident and can present later especially with post-PCI hypotension. Epicardial and intramyocardial hematomas compressing left atrium have been reported.[14] In a study, 30-day mortality and major adverse cardiac events were reported to be significantly more in patients with coronary perforation.[7]
Management of coronary perforation
Conventional management to treat coronary perforation includes prolonged balloon inflation (proximal to or at the level of perforation to prevent hemo-pericardium)[14] and reversal of the anticoagulation with protamine to achieve an activated clotting time of less than 150 seconds.[5] As previously reported, the use of protamine in patients with coronary perforation seems to be safe without any increase in vessel/stent thrombosis (if a stent has already been deployed in the parent vessel),[2] but should be avoided in diabetic patients with a history of protamine-insulin use. Platelet transfusion is useful in patients treated with abciximab. In the presence of normal renal function, infusions of eptifibatide and tirofiban may be stopped with prompt reversal given their short half-lives.[9] Pericardial tamponade and its outcome are not affected with the use of glycoprotein (GP) IIb/IIIa antagonists.[2,3] Continuation of antiplatelet therapy resulted in no overt rebleeding.[5] In a study, analyzing three randomized trials, there was no significant difference in composite end points including complicated coronary perforation between bivalirudin monotherapy and use of unfractionated heparin plus GP IIb/IIIa inhibitors.[7] Pericardiocentesis is done if cardiac tamponade is present.
Prolonged balloon inflation
A balloon (with a balloon to artery ratio ~1.0) should initially be positioned over the site of contrast extravasation and inflated for at least 10 minutes depending on occurrence of ischemia.[9] Repeat inflation of the balloon may be performed every 5–10 minutes till perforation closes or ischemia occurs. Prolonged balloon inflation with a perfusion balloon catheter is ideal at low pressure to seal the perforation maintaining myocardial perfusion. In the passive auto-perfusion balloon catheter with dual lumen shaft, blood enters the lumen of the balloon catheter proximal to the balloon through side holes, travels along the balloon lumen then exits distally.
Type I and II perforations are predominately caused by stiff or hydrophilic guide wires and tend to heal spontaneously and require no more than observation.[3,8] However, type II coronary perforations have the potential to progress to tamponade. Furthermore, perforation with guide wire may cause delayed pericardial effusions or late pseudoaneurysm formation.[15] It should be borne in mind that in few cases of perforation caused by intracoronary guide wires, it has been noted that condition could progress to cardiac tamponade even though there was no contrast extravasations during the procedure.[2,12,15] In type I and II perforations, if there is significant extravasations, prolonged balloon inflation is useful in these patients. If this fails or there is large Type III perforation immediate covered stents have to be deployed to seal the perforation and prevent hemo-pericardium.[16,17,18]
Covered stents for proximal to mid coronary perforations
Covered stents have revolutionized the management of coronary perforation specifically, those perforations occurring in large epicardial arteries involving proximal and mid segments.[16,17,18] Covered stents, though used extensively in occluding coronary aneurysms, have been used in coronary perforations for long time. Expanded polytetrafluoroethylene [PTFE] covered stents for coronary and saphenous vein graft use have been developed by Boston Scientific (the Symbiot stent, consisting of a double layer of PTFE surrounding a modified self-expanding RADIUS-like nitinol stent), Abbott (the Jostent Coronary Stent Graft, consisting of a single PTFE layer sandwiched between two coaxial 316L stainless steel, slotted-tube, balloon-expandable stents), and Cardiovasc (the Nuvasc Stent Graft, a stainless steel stent surrounded by PTFE coated with the synthetic peptide P-15 a cell adhesion protein to promote endothelialization).
Recently, highly deliverable pericardial covered stents have been used in coronary perforation.[19] Venous covered stent usage has also been reported for venous graft perforation.[15,18,20] Autologous vein-covered stents have been used before,[18] but isolating the graft (typically a cephalic vein) by cut down and mounting and suturing it onto a metallic stent is practically impossible in an emergency situation.
Covered stents effectively seal coronary perforations, especially involving proximal or mid-segments of the artery where delivery of these devices is relatively easy. Briguori et al.[16] reported a 91% successful closure rate of Type I and II perforations with PTFE-covered stents and a significantly lower incidence of cardiac tamponade or need for emergency surgery. However, for covered stents to be used the target vessel must be of appropriate size, accessibility, free of significant side branches and the area of perforation must be clearly delineated. However, in tortuous and calcified vessels, it can be difficult to advance a covered stent through the vessel's lumen due to bulkiness and lack of flexibility of the stent. This technique is effective in larger epicardial vessels, but not in distal coronary perforations. If covered stents fail, patients require urgent surgical intervention, which carries high morbidity and mortality.[9] If covered stents are unavailable, bare metal stents with narrow struts can be attempted across the perforation to seal it.[21]
In a study using PTFE-covered stents in various clinical settings, subacute stent thrombosis and angiographic restenosis occurred in 5.7% and 31.6% of the patients, respectively.[22] In the RECOVERS trial, PTFE covered stents implanted in saphenous vein grafts showed a higher incidence of 30-day subacute myocardial infarction than standard stents (10.3% vs 3.4%, respectively).[23] This incidence of subacute thrombosis and restenosis in the PTFE-covered stent is relatively higher than in standard stents, which may be related to delayed endothelialization and increased susceptibility to thrombus formation in these stents.[24] Hence, long-term dual antiplatelet therapy at least for a year is advisable.
Surgery
Operative repair of coronary perforation includes either ligation or suturing of the vessel for hemostasis and bypass grafting to the distal vessel. In addition, pericardial patch/ Teflon felt wrapping repair of the perforation with or without coronary bypass grafting is advocated especially when multiple stents with coronary perforation and subepicardial hematoma are present.[25]
Other non-surgical alternatives for distal coronary perforations
Non-surgical alternatives for distal perforations are the use of metal coils or Gel foam to embolize the vessel.[26,27] Other embolization materials described in literature are coagulated blood from the patient, thrombin, two component fibrin-glue, collagen, transcatheter subcutaneous tissue delivery, cyanoacrylate liquid glue, denatured alcohol, tris-acryl gelatin microsphere, or polyvinyl alcohol particles and use of a local drug delivery catheter.[26–36] The predominant hindrance in the use of embolic materials is the availability of an interventional cardiologist who has experience in using these materials which involve complex hardware and maneuvers. Other important disadvantage is that the use of embolic materials results in permanent loss of the vessel lumen beyond the site of deployment and subsequent infarction. In addition, foreign body reaction to absorbable gelatin sponge has been noted leading to granulomatous arteritis and coronary occlusion. This was stronger with gelatin sponge when compared to microcoils.[37] Overall, non-surgical management is successful in most cases of coronary perforation. Emergency coronary artery bypass surgery is required in 2.9 % to 5.2 % of the cases.[2,6]
Real-world experience
In the first case report, we describe an unusual mechanism of PCI related coronary perforation. In this patient, there was a true balloon-induced LAD rupture across the stent, possibly associated with a fractured stent strut. When available, intravascular ultrasound would describe the stent pathology clearly. Perforations associated with stents usually occur from the edges or the overlapped portions. Isolated strut rupture is extremely rare. Since the perforation was large and across a stent, treatment with prolonged balloon inflation would not have sealed the tear. Hence, a covered stent was directly deployed within the previous stent and sealing off the perforation. This illustrates that a free rupture mandates the use of a covered stent. If a covered stent cannot reach the rupture site either due to its bulkiness or involvement of a small branch, it can be placed into the main vessel across the ruptured branch and sacrifice the smaller branch. In the second patient, there was a large perforation secondary to over-sized stent and stent over inflation. Type III perforations are more often associated with balloon/stent/device use and majority of these perforations can be managed by percutaneous methods using covered stents as seen in these two patients. Lansky et al.[17] reported in a large number of coronary perforation patients, the use of PTFE covered JOSTENT GraftMaster (available diameter 2.75 to 5 mm and length 12, 16, 19 and 26 mm) with overall procedure success rate of 96%. Of the 41 patients, more than third experienced life-threatening complications before covered stent implantation, including pericardial tamponade (12%), cardiogenic shock (10%), and cardiac arrest (2.4%). A total of 52 covered stents were used to treat the 41 perforations (mean 1.3 per lesion). They noted that these stents are compatible with guides ≥7 Fr. Kalusky et al.[38] reported that GraftMasters of ≤5 mm can be easily deployed via 6 Fr guiding catheters as demonstrated in these two patients. In a recent study, treating a coronary artery perforation with two guide catheters through dual access enabled a rapid delivery of covered stent or coils to the vessel, without losing control of the perforation site.[39] This needs to be evaluated in a large number of patients.
In a recent study of grade III perforation,[40] over 90% were complex lesions of type B2 and C, 28% were chronic total occlusions, and within a small vessel (≤2.5 mm) in 32%. The device causing perforation was balloon (50%), guide wire (18%), rotablation, and directional atherectomy (3.6% each). Following perforation, success rate of prolonged balloon inflation was 55%, covered stent was 85%, coronary artery bypass graft surgery and surgical repair was 44% and coil embolization was 100%, although it was used in only 1.8% of the cases. Major in-hospital and long-term (38 months) adverse cardiac event rate was 55% and 42%, respectively.
CONCLUSIONS
In conclusion, management of coronary perforation requires early detection and angiographic classification. Caution is needed while advancing guide wires and dilating the coronary lesion either pre-stent, during or post-stent implantation. Immediate sealing of the ruptured coronary vessel (if proximal) using covered stents along with reversal of heparin anticoagulation and relief of hemodynamic compromise can salvage a potentially life-threatening complication. Use of covered stents in proximal large perforations does not need any additional skill for an experienced interventionalist. However, distal coronary artery perforations need use of various embolic materials, which an interventional cardiologist should be aware and have experience in using them.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Fasseas P, Orford JL, Panetta CJ, Bell MR, Denktas AE, Lennon RJ, et al. Incidence, correlates, management, and clinical outcome of coronary perforation: Analysis of 16,298 procedures. Am Heart J. 2004;147:140–5. doi: 10.1016/s0002-8703(03)00505-2. [DOI] [PubMed] [Google Scholar]
- 2.Witzke CF, Martin-Herrero F, Clarke SC, Pomerantzev E, Palacios IF. The changing pattern of coronary perforation during percutaneous coronary intervention in the new device era. J Invasive Cardiol. 2004;16:257–301. [PubMed] [Google Scholar]
- 3.Ramana RK, Arab D, Joyal D, Steen L, Chol L, Lewis B, et al. Coronary artery perforation during percutaneous coronary intervention: incidence and outcomes in the new interventional era. J Invasive Cardiol. 2005;17:603–5. [PubMed] [Google Scholar]
- 4.Javaid A, Buch AN, Satler LF, Kent KM, Suddath WO, Lindsay J, et al. Management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol. 2006;98:911–4. doi: 10.1016/j.amjcard.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Shirakabe A, Takano H, Nakamura S, Kikuchi A, Sasaki A, Yamamoto E, et al. Coronary perforation during percutaneous coronary intervention. Int Heart J. 2007;48:1–9. doi: 10.1536/ihj.48.1. [DOI] [PubMed] [Google Scholar]
- 6.Kiernan TJ, Yan BP, Ruggiero N, Eisenberg JD, Bernal J, Cubeddu RJ, et al. Coronary artery perforations in the contemporary interventional era. J Interv Cardiol. 2009;22:350–3. doi: 10.1111/j.1540-8183.2009.00469.x. [DOI] [PubMed] [Google Scholar]
- 7.Doll JA, Nikolsky E, Stone GW, Mehran R, Lincoff AM, Caixeta A, et al. Outcomes of patients with coronary artery perforation complicating percutaneous coronary intervention and correlations with the type of adjunctive antithrombotic therapy: Pooled analysis from REPLACE-2, ACUITY, and HORIZONS-AMI trials. J Interv Cardiol. 2009;22:453–9. doi: 10.1111/j.1540-8183.2009.00494.x. [DOI] [PubMed] [Google Scholar]
- 8.Shimony A, Zahger D, Van Straten M, Shalev A, Gilutz H, llia R, et al. Incidence, risk factors, management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol. 2009;104:1674–7. doi: 10.1016/j.amjcard.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 9.Rogers JH, Lasala JM. Coronary artery dissection and perforation complicating percutaneous coronary intervention. J Invasive Cardiol. 2004;16:493–9. [PubMed] [Google Scholar]
- 10.Ellis SG, Ajluni S, Arnold AZ, Popma JJ, Bittl JA, Eigler NL, et al. Increased coronary perforation in the new device era.Incidence, classification, management and outcome. Circulation. 1994;90:2725–30. doi: 10.1161/01.cir.90.6.2725. [DOI] [PubMed] [Google Scholar]
- 11.Maruo T, Yasuda S, Miyazaki S. Delayed appearance of coronary artery perforation following cutting balloon angioplasty. Catheter Cardiovasc Interv. 2002;57:529–31. doi: 10.1002/ccd.10335. [DOI] [PubMed] [Google Scholar]
- 12.Nair P, Roguin A. Coronary perforations. Eurointervention. 2006;2:363–70. [PubMed] [Google Scholar]
- 13.Teis A, Fernández-Nofrerías E, Rodríguez-Leor O, Tizón H, Salvatella N, Valle V, et al. Coronary artery perforation by intracoronary guide wires: Risk factors and clinical outcomes. Rev Esp Cardiol. 2010;63:730–4. doi: 10.1016/s1885-5857(10)70148-1. [DOI] [PubMed] [Google Scholar]
- 14.Krabatsch T, Becher D, Schweiger M, Hetzer R. Severe left atrium compression after percutaneous coronary intervention with perforation of a circumflex branch of the left coronary artery. Interact Cardiovasc Thorac Surg. 2010;11:811–3. doi: 10.1510/icvts.2010.246017. [DOI] [PubMed] [Google Scholar]
- 15.Fukutomi T, Suzuki T, Popma JJ, Hosokawa H, Yokoya K, Inada T, et al. Early and late clinical outcomes following coronary perforation in patients undergoing percutaneous coronary intervention. Circ J. 2002;66:349–56. doi: 10.1253/circj.66.349. [DOI] [PubMed] [Google Scholar]
- 16.Briguori C, Nishida T, Anzuini A, Di Mario C, Grube E, Colombo A. Emergency polytetrafluoethylene-covered stent implantation to treat coronary ruptures. Circulation. 2000;102:3028–31. doi: 10.1161/01.cir.102.25.3028. [DOI] [PubMed] [Google Scholar]
- 17.Lansky AJ, Yang YM, Khan Y, Costa RA, Pietras C, Tsuchiya Y, et al. Treatment of coronary artery perforations complicating percutaneous coronary intervention with a polytetrafluoroethylene-covered stent graft. Am J Cardiol. 2006;98:370–4. doi: 10.1016/j.amjcard.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 18.Jamshidi P, Mahmoody K, Erne P. Covered stents: A review. Int J Cardiol. 2008;130:310–8. doi: 10.1016/j.ijcard.2008.04.083. [DOI] [PubMed] [Google Scholar]
- 19.Jokhi PP, McKenzie DB, O’Kane P. Use of a novel pericardial covered stent to seal an iatrogenic coronary perforation. J Invasive Cardiol. 2009;21:E187–90. [PubMed] [Google Scholar]
- 20.Baruah DK. Covered stent to treat saphenous venous graft perforation--a case report. Catheter Cardiovasc Interv. 2010;76:844–6. doi: 10.1002/ccd.22401. [DOI] [PubMed] [Google Scholar]
- 21.Karabulut A, Topçu K. Coronary perforation due to sirolimus-eluting stent's strut rupture with post-dilatation. Kardiol Pol. 2011;69:183–7. [PubMed] [Google Scholar]
- 22.Gercken U, Lansky AJ, Buellesfeld L, Desai K, Badereldin M, Muller R, et al. Results of the Jostent coronary stent graft implantation in various clinical settings: Procedural and follow-up results. Catheter Cardiovasc Interv. 2002;56:353–60. doi: 10.1002/ccd.10223. [DOI] [PubMed] [Google Scholar]
- 23.Stankovic G, Colombo A, Presbitero P, van den Branden F, Inglese L, Niccoli L, et al. Randomized evaluation of polytetrafluoroethylenecovered stent in saphenous vein grafts (RECOVERS trial) Circulation. 2003;108:37–42. doi: 10.1161/01.CIR.0000079106.71097.1C. [DOI] [PubMed] [Google Scholar]
- 24.Takano M, Yamamoto M, Inami S, Xie Y, Murakami D, Okamatsu K, et al. Delayed endothelialization after polytetrafluoroethylene-covered stent implantation for coronary aneurysm. Circ J. 2009;73:190–3. doi: 10.1253/circj.cj-07-0924. [DOI] [PubMed] [Google Scholar]
- 25.Inoue Y, Ueda T, Taguchi S, Kashima I, Koizumi K, Noma S. Teflon felt wrapping repair for coronary perforation after failed angioplasty. Ann Thorac Surg. 2006;82:2312–4. doi: 10.1016/j.athoracsur.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Yeo KK, Rogers JH, Laird JR. Use of stent grafts and coils in vessel rupture and perforation. J Interv Cardiol. 2008;21:86–99. doi: 10.1111/j.1540-8183.2007.00302.x. [DOI] [PubMed] [Google Scholar]
- 27.Pershad A, Yarkoni A, Biglari D. Management of distal coronary perforations. J Invasive Cardiol. 2008;20:E187–91. [PubMed] [Google Scholar]
- 28.Störger H, Ruef J. Closure of guide wire-induced coronary artery perforation with a two-component fibrin glue. Catheter Cardiovasc Interv. 2007;70:237–40. doi: 10.1002/ccd.21115. [DOI] [PubMed] [Google Scholar]
- 29.Goel PK. Delayed and repeated cardiac tamponade following microleak in RCA successfully treated with intra arterial sterile glue injection. Catheter Cardiovasc Interv. 2009;73:797–800. doi: 10.1002/ccd.21924. [DOI] [PubMed] [Google Scholar]
- 30.Aleong G, Jimenez-Quevedo P, Alfonso F. Collagen embolization for the successful treatment of a distal coronary artery perforation. Catheter Cardiovasc Interv. 2009;73:332–5. doi: 10.1002/ccd.21823. [DOI] [PubMed] [Google Scholar]
- 31.Canton T, Pajín F, Lanciego C, Moreu J. An alternative treatment for iatrogenic coronary perforation. Rev Esp Cardiol. 2009;62:328–9. doi: 10.1016/s1885-5857(09)71566-x. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka S, Nishigaki K, Ojio S, Yasuda S, Okubo M, Yamaki T, et al. Transcatheter embolization by autologous blood clot is useful management for small side branch perforation due to percutaneous coronary intervention guide wire. J Cardiol. 2008;52:285–9. doi: 10.1016/j.jjcc.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Trehan VK, Nigam A. Cyanoacrylate glue for type iii lad perforation. Indian Heart J. 2008;60:612–4. [PubMed] [Google Scholar]
- 34.To AC, El-Jack SS, Webster MW, Stewart JT. Coronary artery perforation successfully treated with tris-acryl gelatin microsphere embolisation. Heart Lung Circ. 2008;17:423–6. doi: 10.1016/j.hlc.2007.06.521. [DOI] [PubMed] [Google Scholar]
- 35.Jamali AH, Lee MS, Makkar RR. Coronary perforation after percutaneous coronary intervention successfully treated with local thrombin injection. J Invasive Cardiol. 2006;18:E143–5. [PubMed] [Google Scholar]
- 36.Habara M, Kinoshita Y, Suzuki T. Novel use of a local drug delivery catheter for coronary perforation. J Invasive Cardiol. 2011;23:E236–9. [PubMed] [Google Scholar]
- 37.Kawano H, Arakawa S, Satoh O, Matsumoto Y, Hayano M, Miyabara S. Foreign body granulomatous change from absorbable gelatin sponge and microcoil embolization after a guide wire-induced perforation in the distal coronary artery. Intern Med. 2010;49:1871–4. doi: 10.2169/internalmedicine.49.3750. [DOI] [PubMed] [Google Scholar]
- 38.Kaluski E, Gerula C, Randhawa P, Haider B, Klapholz M. Massive coronary perforation and shock: From appropriate labeling to appropriate calls. Acute Card Care. 2009;11:181–6. doi: 10.1080/17482940903003000. [DOI] [PubMed] [Google Scholar]
- 39.Ben-Gal Y, Weisz G, Collins MB, Genereux P, Dangas GD, Teirstein PS, et al. Dual catheter technique for the treatment of severe coronary artery perforations. Catheter Cardiovasc Interv. 2010;75:708–12. doi: 10.1002/ccd.22331. [DOI] [PubMed] [Google Scholar]
- 40.Al-Lamee R, Ielasi A, Latib A, Godino C, Ferraro M, Mussardo M, et al. Incidence, predictors, management, immediate and long-term outcomes following grade III coronary perforation. JACC Cardiovasc Interv. 2011;4:87–95. doi: 10.1016/j.jcin.2010.08.026. [DOI] [PubMed] [Google Scholar]