Abstract
Background:
Seborrheic dermatitis (SD) is an inflammatory skin disorder in which colonies of Malassezia furfur have been found in affected areas.
Aim:
The aim of this study was to evaluate the efficacy of itraconazole in the treatment of severe SD.
Materials and Methods:
Itraconazole was given to 30 patients of SD in a dose of 100 mg twice daily for 1 week followed by 200 mg/day for first 2 days of the following 2 months. The response was noted on day 15, 30, 60, and 90. The clinical response was graded as markedly effective, effective, or ineffective.
Results:
Clinical improvement (evaluated as markedly effective or effective) was observed in 83.3% cases.
Conclusion:
The anti-inflammatory activity of oral itraconazole suggests that it should be the first-line therapy in severe SD.
Keywords: Seborrheic dermatitis, itraconazole, pityriosporum, intermittent therapy
Introduction
Seborrheic dermatitis (SD) is a common chronic erythematous scaly eruption usually seen in areas rich in sebaceous glands. Two main factors are the presence of a generous amount of epidermal lipids and colonization by Malassezia species (lipophilic, usually non pathogenic yeast forms, a part of normal human cutaneous flora; however, under the influence of certain predisposing factors, they become pathogenic). Clinical features show varied morphology. Erythematous reddish yellow, poorly circumscribed patches with fine scale, which are mildly pruritic, are seen. Common sites are scalp (dandruff), eye brows, perinasal areas, ears, retroauricular areas, neck, and anterior and posterior trunk (annular or petaloid forms are seen).
There are several topical and systemic therapies. Itraconazole is a highly keratinophilic and lipophilic triazole. Secretion in sebum is a major route by which the drug reaches the stratum corneum. The aim of the study was to determine the efficacy and safety of oral itraconazole capsule 100 mg given twice daily for 7 days and consecutive usage in the treatment of the severe SD.
Materials and Methods
The study was an open noncomparative clinical trial in which 30 patients were evaluated. Written consent was obtained from each patient before entering the study. The patients were clinically evaluated according to the following items: Itching, burning erythema, scaling, and seborrhea. This study was carried out in the winter and early spring seasons, in which SD is normally exacerbated. The clinical efficacy parameter was the proportion of patients who achieved complete or nearly complete disappearance of clinical features. Patients who had used systemic antibiotics, systemic antifungals, topical steroids, topical antifungals, selenium sulfide or zinc pyrithione within 15 days prior to the study were excluded. The patients included belonged to the age group of 18 years or above. In all the patients, complete blood count, liver function test (LFT), serum urea, serum creatinine, and mycological examination were done before starting the therapy. In the treatment protocol, itraconazole capsule 100 mg twice a day was given for 1 week; then after a 3-week interval 100 mg capsule was given twice a day for 2 days of following months for two consecutive months. The evaluation of results was performed on day 15, day 30, day 60, and day 90. The clinical response was graded as markedly effective, effective, or ineffective. The mycological examination was done after treatment to evaluate further.
Results
Thirty patients were enrolled in the study. Out of these 30 patients, 21 were male and 9 were female. In all of these patients, response to conventional therapy was very unsatisfactory. The evaluation of clinical improvement showed at the end of 90 days was that a markedly effective result was seen in 18 patients (60%). A moderate response was seen in 7 patients (23.3%) and in 5 patients (16.6%) no improvement was seen. None of the patients showed any adverse effect with itraconazole [Figure 1].
Figure 1.
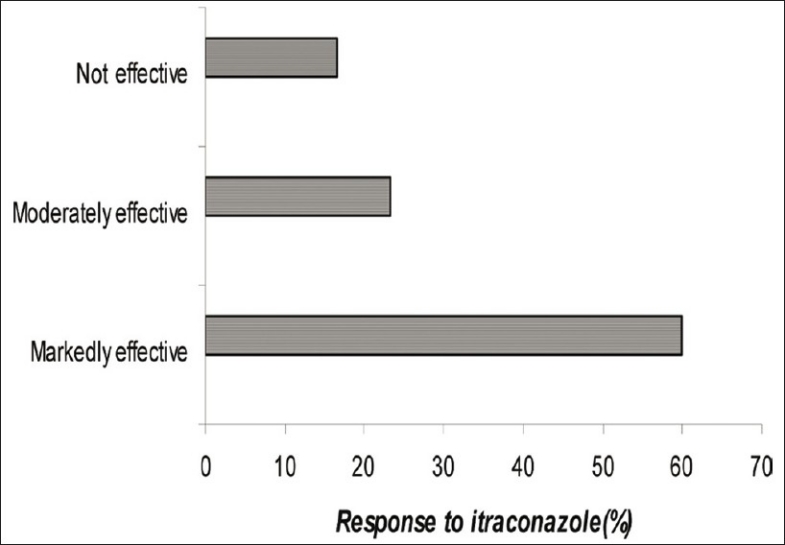
Percentage of the response of itraconazole therapy in seborrhoeic dermatitis
Discussion
SD is an inflammatory disorder of the skin characterized by erythema and scaling over the areas rich in sebaceous glands, the scalp, face, chest, back, and flexural areas. The enigma of SD is yet unsolved.[1] Probably the organism Pityrosporum ovale, Pityrosporum lipase activity, skin surface lipids, and lastly immune dysfunction may play a role. It has been suggested that SD is an inflammatory response to these organisms.[2] Malassezia restricta/globosa, which plays an important role in the pathogenesis of SD,[3] stimulates cytokine production by keratinocytes. Many studies showed that antimycotics are effective in clearing lesions with a reduction in the number of Malassezia yeasts.
The efficacy of azole and imidazole antimycotics may be due to their anti-inflammatory action. They inhibit the cell wall lipid synthesis of the organisms (antifungal activity of the azoles is via inhibition of lanosterol 14α demethylase) and may have some effects on the skin surface lipids.[4] Itraconazole is anti-inflammatory primarily because of its inhibitory effect on the synthesis of 5-hypoxygenase metabolites, which are involved in several inflammatory diseases such as SD. In one study, Mastaro treated 30 patients with SD of head and face, who used 150 or 200 mg once per week for 2-3 months. A total of 67% of patients showed marked improvement.[5] Faergemann evaluated 10 patients with sebopsoriasis of the scalp and face. Patients were started on itraconazole 50 mg per day for 2 weeks. If this dosage was well tolerated, the dose was increased to 100 mg per day for another 4 weeks. Five patients were cured.[6]
In our study, patients were given itraconazole 200 mg per day for 7 days and then 200 mg per day for 2 days in the following 2 months. By the end of 90 days, the treatment was found to be markedly effective in 60% cases, moderately effective in 23.3%, and no improvement was noticed in 16.6%. The use of oral itraconazole capsule clearly diminished the symptoms (especially erythema and desquamation) of the disease. Patients also noticed the reduction of burning and itching sensation.
The anti-inflammatory activity of oral itraconazole and its efficacy on Malassezia yeast suggest that itraconazole may be one of the safe and effective treatments of SD although it is evident that future research is needed about the efficacy and safety of this drug in a comparative or placebo-controlled clinical trial.[3]
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Holden CA, Jones Berth J. Rooks Textbook of Drematology. In: Burns Tony, Bradthnach Stephens, Cox Neil, Griffiths Ctristopher., editors. 7th ed. New York: Blackwell Science; 2004. pp. 10–15. [Google Scholar]
- 2.Valia RG, Valia Ameet R. Valia R. G., editor. Eczema IADVL text book of Dermatology. (2nd ed) 2001;1:430. [Google Scholar]
- 3.Crespo Erchiga Vicente, Eveline Gucho. Superficial diseases caused by malassezia species; Topley and Wilson's Text book of Medical Mycology. In: Merz Willium G., Hay Roderick J., Hoddu Arnold., editors. 10th ed. 2005. pp. 202–219. [Google Scholar]
- 4.Gupta AK, Kohli Y, Li A, Faergemann J. In vitro susceptibility of the seven-malassezia species to ketoconazole, variconazole.itraconazole and terbinafine. Br. J. Dermatol. 2000;14:758–65. doi: 10.1046/j.1365-2133.2000.03294.x. [DOI] [PubMed] [Google Scholar]
- 5.Mastaro H. Treatment of seborrhoeic dermatitis with antifungal drugs. J. Clin Exp Med. 1995;173:1026–7. [Google Scholar]
- 6.Faergemann J. Pityrosporum species as a cause of allergy and infection. Allergy. 1999;54:413–9. doi: 10.1034/j.1398-9995.1999.00089.x. [DOI] [PubMed] [Google Scholar]


