Abstract
Background:
Even though herpes zoster is a common condition its incidence and pattern of occurrence in the era of HIV disease is significant.
Aim:
To analyze the incidence, pattern of occurrence and evolution of herpes zoster with special attention to provocative factors if any.
Materials and Method:
This was an analytical study conducted for 2 years based on a preformed proforma containing preliminary information, a detailed clinical evaluation regarding the segment of involvement, morphology, pattern of lesions, complications, disseminations etc. and investigations to establish provocative factors if any.
Results:
Incidence of herpes zoster was mainly in the fourth and third decades of life. A definite history of chicken pox was present in only 63.4% cases. In the majority (70%) herpes zoster occurred spontaneously. In 30% cases, immunosuppression due to chemotherapy, malignancy, HIV infection, diabetes mellitus were observed. The commonest segment affected was thoracic (42.4%) followed by cranial (28.2%) and cervical (12.1%). Majority resolved in 7–14 days except immunosuppressed. 34.6% of the patients had complications such as secondary bacterial infection, post herpetic neuralgia, and motor weakness. Ten patients had HIV infection as a provocative factor.
Conclusion:
The results of incidence and clinical pattern of herpes zoster is almost parallel to the previous studies. Any factors of immunosuppression should be checked, especially HIV, particularly in disseminated and long-lasting cases.
Keywords: Clinical study, herpes zoster, immunosuppression
Introduction
Herpes zoster caused by the neurodermotropic virus called “varicella zoster virus” is distributed worldwide. This benign localized viral disease has been recognized as a distinct entity since ancient times. It manifests as a result of reactivation of the virus laid dormant in the sensory ganglion[1–3] following a clinical or sub clinical varicella (chicken pox) infection early in life or occasionally in utero.
The reactivation of the virus may be due to immunosuppression (inherited, acquired or iatrogenic) or spontaneous. In HIV disease it presents in diverse manner such as multidermatomal involvement, crusted, nodular or vesiculopustular, ulcerative, and ecthymatous[4–6] lesions that may be widely disseminated[4,7–9] or localized.[10]
Although in most cases the manifestations are classical, there are occasions, particularly of visceral involvement that can challenge the knowledge of even an experienced physician.
Another important and most troublesome complication is post herpetic neuralgia (PHN), which is very tiring to the patient as well as to the physician[11] and to date there is no single effective drug to cure this distressing problem.
Materials and Methods
Two hundred five patients, in the age group 3–85 years with herpes zoster attending the various departments of the Calicut Medical College, Kerala, India, were included in the study for a period of 2 years. Permission was obtained from the Calicut Medical College Ethical Committee before performing the study. Informed consent was obtained from patients.
Preliminary information such as age, sex, address, and occupation were noted. A detailed history regarding the prodromal and presenting symptoms, day of occurrence of skin lesions after prodrome, nature of pain, its intensity and duration, and other symptoms if any, were elicited and recorded. Also, history of chicken pox and previous attack of herpes zoster were elicited. History suggestive of provocative factors such as drugs, recent trauma, surgery, irradiation, immunosuppressive and cytotoxic chemotherapy, diabetes mellitus, pulmonary TB, HIV infections were inquired. A thorough dermatological examination regarding the segment of involvement, morphology, and pattern of the lesions, regional lymph node enlargement, motor complications, dissemination of the lesions in other areas of the body etc. were noted. Whenever necessary, opinion from other specialists such as the ophthalmologist, chest physician, diabetologist, and the general physician were sought.
At first reporting, the following investigations were carried out. Tzanck smear (in Leishman's stain) examination for ballooned epithelial cells and multinucleated giant cells, complete haemogram, blood sugar, urine routine exam, and enzyme-linked immunosorbent assay test for HIV antibodies were conducted. All the patients were reviewed weekly for 1 month and monthly for two more months.
Results
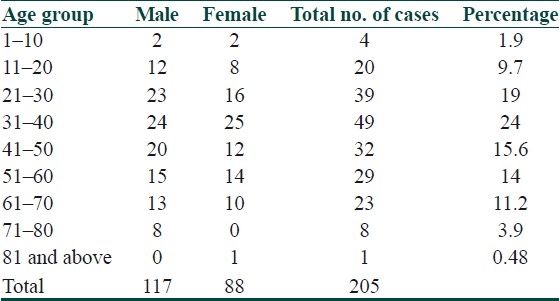
Out of 205 cases, 117 were males and 88 were females. The age ranged from 3–85 years. One hundred nine were below 40 years and 96 were above 40 years. The age and sex distribution is given in Table 1.The maximum incidence was in the age group of 31–40 years (24%), which is followed by that of 21–30 years (19%), and 41–50 years (15.6%). Minimum incidence was observed in the age group 1–10 years and 71–80 years (1.9% and 3.9%). Out of 205 cases, 130 (63.4%) had definite history of chicken pox, most of them within 20 years of age. The remaining 75 cases (36.6%) were neither aware of nor had chicken pox at all.
Table 1.
Age and sex distribution of zoster
Sixty-two patients had one or more suspected provoking factors. Twenty patients were on steroids for ailments such as bronchial asthma, contact dermatitis, exfoliative dermatitis, pemphigus, lepra reactions, systemic lupus erythematosis, idiopathic thrombocytopenic purpura, and erythema multiforme. Nineteen patients had various types of malignancies and were on chemotherapy/radiotherapy or on both, 11 patients were diabetic, ten patients had HIV infection [Figures 1 and 2], and two patients had pulmonary tuberculosis.
Figure 1.
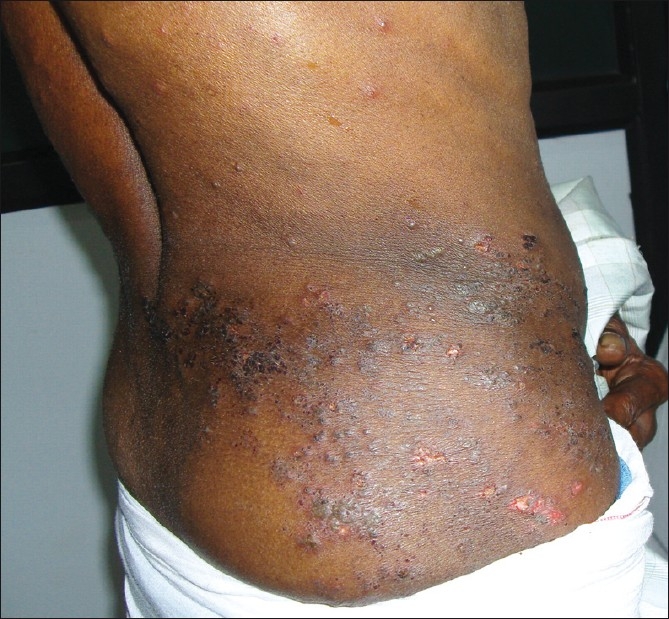
Extensive Herpes zoster in an HIV patient
Figure 2.
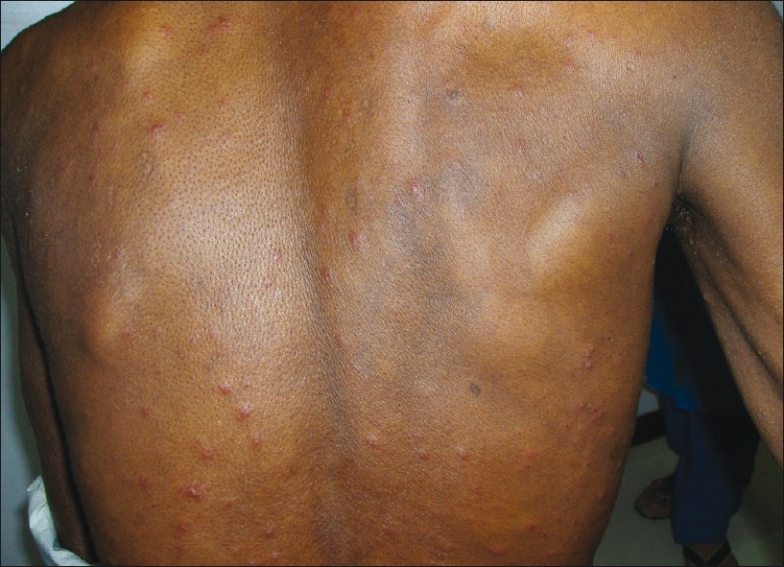
Disseminated herpes zoster in an HIV patient
Twenty-two cases had significant constitutional symptoms such as fever, headache and arthralgia, 2-3 days prior to or concurrently with the onset of vesicles.
94.6% cases had segmental neuralgia some times during the course of the disease varying in intensity from mild to very severe producing insomnia. Pain preceded the vesicles in 123 (60%) cases. Concurrently started with the vesicles in 65 and followed by 2–3 days in six patients. Five patients had preherpetic itching started 2–3 days prior.
The segment wise distribution of zoster is given in Table 2 Thoracic dermatome was commonly (87) affected [Figure 3] and among thoracic, T4 segment was common, followed by the Trigeminal nerve (56) [ophthalmic branch (33), maxillary (15), and mandibular (8)]. Two cases of facial nerve involvement with Ramsay hunt syndrome were present. Twenty-five patients had cervical, 16 lumbar, and 10 had sacral nerve involvement. Nine patients had more than one dermatome involvement. Twenty-seven cases had aberrant vesicles ranging from 2–16 in distant areas.
Table 2.
Segmental distribution of zoster
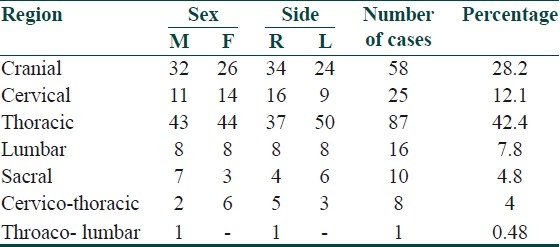
Figure 3.
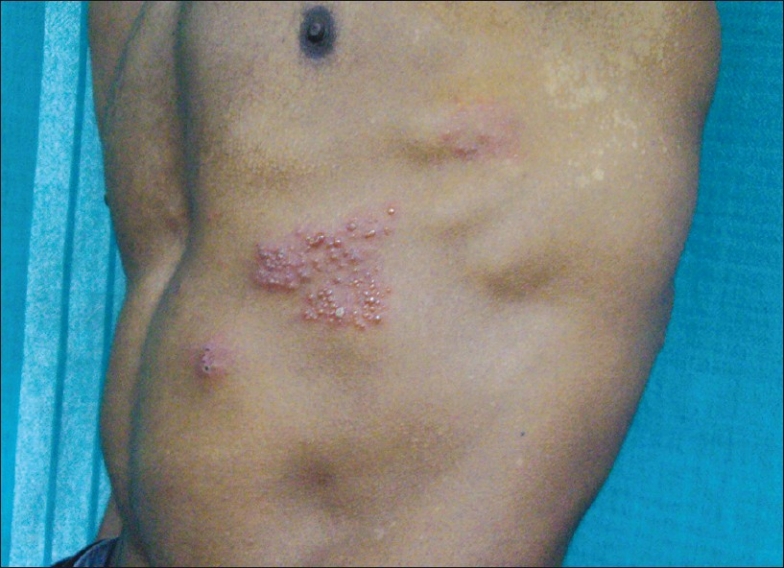
Herpes zoster of the thoracic segment
One hundred five had lesions on the right side and 100 cases on the left side. Sixty percent had tender regional adenopathy at the time of presentation, in the rest, 25% developed lymphadenopathy by 1 week. Fifteen percent never developed lymphadenopathy. The period of resolution ranged from 7–18 days with an average of 9–12 days.
Out of 205 patients 71 (34.6%) developed complications. The complications observed were secondary bacterial infection (28 cases, 13.6%), severe ulceration (four cases, 1.95%), scarring (eight cases, 3.9%), PHN (21 cases, 10.2%), motor weakness (three cases – in a 25-year old female right ulnar weakness, 71-year old man and 42- year old woman – Ramsay-hunt syndrome with facial weakness), depigmentation (five cases, 2.4%) and post herpetic itching (two cases).
PHN occurred in 21 patients of whom 12 were males and nine were females. It was a continuous deep nagging pain in seven and intermittent lancinating or radicular pain in the rest. It was common in thoracic segments followed by trigeminal branches.
Discussion
The study of 205 patients of herpes zoster revealed that the majority of the patients affected were adults, (55%) below the age of 40 years and (45%) above 40 years. This was similar to the study by Pavithran and Sehgal et al.[1,12] but in contrast to other reports in the literature.[13–15] Males outnumbered females in the ratio of 1.75:1.3 in this study which is similar to a study from south India[14] but in contrast to the Western studies[15,16] where both males and females were equally affected. Trauma and stress as a result of their occupation and outdoor activity may be the predisposing factor for the male preponderance in Indian setup.
In 70% of the patients, no provocative factors could be elicited. Among the rest (30%) the commonest provocative factor was steroid intake for various ailments (20 cases) followed by malignancies.[16] with or without irradiation (19 cases) diabetes mellitus (11 cases) and HIV infection (10 cases).[17–24] Depressed CMI associated with the above conditions[18,25–27] could be the possible factor responsible for the development of zoster and for its extensiveness.
The prevalence of prodromal or concurrent constitutional symptoms were found to be lower in this study (10.7%), particularly in older patients comparing to older Indian studies.[1,28]
In accordance to the previous literature reports,[29–31] in most of the patients, the presenting symptom was pain (94.6%) then vesiculations (94%) and pain coinciding with the onset of the eruptions of vesicles were in more than two thirds of the cases. 87.5% of the patients below the age of 20 years had neuralgic pain at the onset of the lesions contrary to the previous studies. 85% of the patients had strict dermatomal distribution, but 15% had vesicles beyond the dermatome. Even with this slightly altered morphology in the latter group, the course of the illness was not different from the former excepting for the slightly prolonged resolution time.
The pattern of dermatomal involvement was slightly different from earlier Indian studies.[1,32] Thoracic segment was common,[25] then cranial nerve involvement, unlike the previous studies where thoracic segment was followed by cervical and lumbar segments. Even though the reports claim[29,31] that the clinical picture as well as the course of the disease is severe in fifth decade and above, in this study, severe rash with hemorrhagic and extensive grouped vesicles were encountered only in less than 15% of the older population. PHN was observed mainly in older age group (above 60) and was more in thoracic segments in contrast to reports in ophthalmic zoster.[13,33] Since more than 70% of the cases were of less than 50 years of age, the incidence of PHN is less in this study.
Out of ten HIV patients, nine had localized lesions as in normal patients, but one had dissemination, bulla formation, and necrosis. Out of ten HIV cases, Zoster was the presenting symptom in three patients.[18,31,34–36] But unusual morphologies as reported in some studies[4,5,10,37] were not encountered in this study except in one.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Pavithran K. A clinical study of five hundred cases of herpes zoster. Antiseptic. 1986;83:682–5. [Google Scholar]
- 2.Peeneys N. Diseases caused by viruses. In: Elder D, editor. Lever's Histopathology of the skin. 8th ed. Philadelphia: Lippincott – Raven; 1997. pp. 569–89. [Google Scholar]
- 3.Whitley RJ. Varicella zoster virus. In: Mandell GL, Bennet JE, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York: Churchill Livingstone; 1995. pp. 1345–51. [Google Scholar]
- 4.Schacker T, Corey L. Herpes virus infections in HIV infected person. In: Devita VT, Hailman Samuel, Lisenberg SA, editors. Textbook of AIDS. 4th ed. Philadelphia: Lippincott – Raven; 1997. pp. 267–80. [Google Scholar]
- 5.Smith KJ, Skelton HG, Yeager J. Cutaneous findings in HIV - 1 positive patients: A 42 - months’ prospective study. J Am Acad Dermatol. 1994;3:746–54. doi: 10.1016/s0190-9622(94)70236-5. [DOI] [PubMed] [Google Scholar]
- 6.Watson PN, Evens RJ. Post herpetic neuralgia: A review. Arch Neural. 1986;43:836–40. doi: 10.1001/archneur.1986.00520080074027. [DOI] [PubMed] [Google Scholar]
- 7.Happenjans WB, Bibler MR, Orme RL. Prolonged cutaneous herpes zoster in acquired immunodeficiency syndrome. Arch Dermatol. 1988;126:1048. [PubMed] [Google Scholar]
- 8.Reusser P. Herpesvirus resistance to antiviral drugs: A review of the mechanisms, clinical importance and therapeutic options. J Hosp Infect. 1996;33:235–48. doi: 10.1016/s0195-6701(96)90010-9. [DOI] [PubMed] [Google Scholar]
- 9.Bernhard P, Obel N. Chronic ulcerating acyclovir resistant varicella zoster lesions in an AIDS patient. Scand J Infect Dis. 1996;27:623–5. doi: 10.3109/00365549509047078. [DOI] [PubMed] [Google Scholar]
- 10.Buchbinder SP, Katz MI, Hessol NA, Liu JY, O’Malley PM, Underwood R, et al. Herpes zoster and human immunodeficiency virus infection. J Infect Dis. 1992;166:1153–6. doi: 10.1093/infdis/166.5.1153. [DOI] [PubMed] [Google Scholar]
- 11.Thiers BH, Sahn EE. Varicella zoster virus infections. In: Samuel L, Moschella, Harry J, Hurley, editors. 3rd ed. Philadelphia: W.B. Saunders Company; 1992. pp. 797–806. [Google Scholar]
- 12.Sehgal VN, Rege VL, Kharangate VN. The natural history of Herpes Zoster. Indian J Dermatol Venereol Leprol. 1976;42:86–89. [PubMed] [Google Scholar]
- 13.Ananthanarayan R, Panicker J. Human Immuno-deficiency virus infection- AIDS. In: Ananthanarayan R, Panicker J, editors. Textbook of Microbiology. 5th ed. Hyderabad: Orient Longman Ltd; 1996. pp. 538–52. [Google Scholar]
- 14.Whitley RJ. Varicella Zoster Virus Infections. In: Isselbacher KJ, Wilson J, editors. Harrison's Principles of Internal Medicine. 14th ed. New York: McGraw Hill Inc; 1998. pp. 1086–8. [Google Scholar]
- 15.Liesegang TJ, Rochester MN. The varicella zoster virus: Systemic and ocular features. J Am Acad Dermatol. 1984;11:165–91. doi: 10.1016/s0190-9622(84)70148-4. [DOI] [PubMed] [Google Scholar]
- 16.Fueyo MA, Lookingbill DP. Herpes zoster and occult malignancy. J Am Acad Dermatol. 1984;11:480–2. doi: 10.1016/s0190-9622(84)70195-2. [DOI] [PubMed] [Google Scholar]
- 17.Kien FA, Lafleur FL, Gendler E. Herpes zoster: A possible early clinical sign for development of acquired immunodeficiency syndrome in high-risk individuals. J Am Acad Dermatol. 1986;14:1023–8. doi: 10.1016/s0190-9622(86)70127-8. [DOI] [PubMed] [Google Scholar]
- 18.Rajashekar TS, Singh G, Shivakumar V, Okade R. Recurrent herpes zoster duplex symmetricus in HIV infection. Indian J Dermatol. 2008;53:33–4. doi: 10.4103/0019-5154.39741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulick RM. Varicella zoster virus disease in patients with human immunodeficiency virus infection. Arch Dermatol. 1990;126:1086–8. [PubMed] [Google Scholar]
- 20.Das AL, Sayal SK, Gupta CM. Herpes zoster in patients with HIV infection. Indian J Dermatol Venereol Leprol. 1997;63:101–14. [PubMed] [Google Scholar]
- 21.Kar HK, Gautam RK, Jain RK. Disseminated cutaneous herpes zoster: A clinical predictor of human immunodeficiency virus infection. Indian J Dermatol Venereol Leprol. 1995;61:40–1. [PubMed] [Google Scholar]
- 22.Gilson IH, Barnett JH, Conant MA. Disseminated ecthymatous herpes varicella zoster virus infection in patients with acquired immunodeficiency syndrome. J Am Acad Dermatol. 1989;20:637–42. doi: 10.1016/s0190-9622(89)70076-1. [DOI] [PubMed] [Google Scholar]
- 23.Varsha D, Subhash H, Chetan O. Natural history of herpes zoster in the era of AIDS. Indian J Dermatol Venereol Leprol. 1998;64:169–72. [PubMed] [Google Scholar]
- 24.Das AL. Herpes zoster in patients with HIV infection. Indian J Dermatol Venereol Leprol. 1997;63:101–4. [PubMed] [Google Scholar]
- 25.Pranesh N, Tandon VK, Kumar R. Herpes zoster: A clinical study. Indian J Dermatol Venereol Leprol. 1972;38:152–5. [PubMed] [Google Scholar]
- 26.Jain C. Recurrent herpes zoster in a child with systemic lupus erythemtous. Indian J Dermatol Venereol Leprol. 1995;61:38–9. [PubMed] [Google Scholar]
- 27.Kar PK, Ramasastry CV. HIV prevalence in patients with Herpes Zoster. Indian J Dermatol Venereol Leprol. 2003;69:116–9. [PubMed] [Google Scholar]
- 28.Chaudhary SD, Pahwa DA. A clinico-epidemiologic profile of herpes zoster in North India. Int J Dermatol Venereol Leprol. 1987;53:213–6. [PubMed] [Google Scholar]
- 29.Highet AS, Kurtz J. Viral infections. In: Champion RH, Burton JL, Ebling FJ, editors. Textbook of Dermatology. 5th ed. Oxford: Blackwell Scientific Publications; 1992. pp. 867–952. [Google Scholar]
- 30.Oxman MN, Alani R, Varicella, Zoster Herpes. Dermatology in General Medicine. In: Fitzpatrick TB, Elsen ZA, Wolff K, editors. 4th ed. New York: McGraw-Hill Inc; 1993. pp. 2543–72. [Google Scholar]
- 31.Dubey AK, Jaisankar TJ, Thappa DM. Clinical and morphological characteristics of herpes zoster in south India. Indian J Dermatol. 2005;50:203–7. [Google Scholar]
- 32.Mandal BK. Herpes Zoster in the immunocompromised populations. Indian J Dermatol Venereol Leprol. 2006;5:235–43. [Google Scholar]
- 33.Kost Rg, Strauss SE. Post herpetic neuralgia: Pathogenesis, treatment and prevention. N Engl J Med. 1996;335:32–41. doi: 10.1056/NEJM199607043350107. [DOI] [PubMed] [Google Scholar]
- 34.Colebunders R. Herpes Zoster in African patients. A clinical predictor of human immunodeficiency virus infection. J Infect Dis. 1988;157:314–8. doi: 10.1093/infdis/157.2.314. [DOI] [PubMed] [Google Scholar]
- 35.Mandal BK. Herpes zoster in the immunocompromized populations. Indian J Dermatol. 2006;51:235–43. [Google Scholar]
- 36.Linnemao CC. Emergence of acyclovir resistant varicella zoster virus in an AIDS patient on prolonged acyclovir therapy. AIDS. 1990;4:577. doi: 10.1097/00002030-199006000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Wood MJ, Johnson RW, Mckendrick MW. A randomized trial of Acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med. 1994;330:896–900. doi: 10.1056/NEJM199403313301304. [DOI] [PubMed] [Google Scholar]


