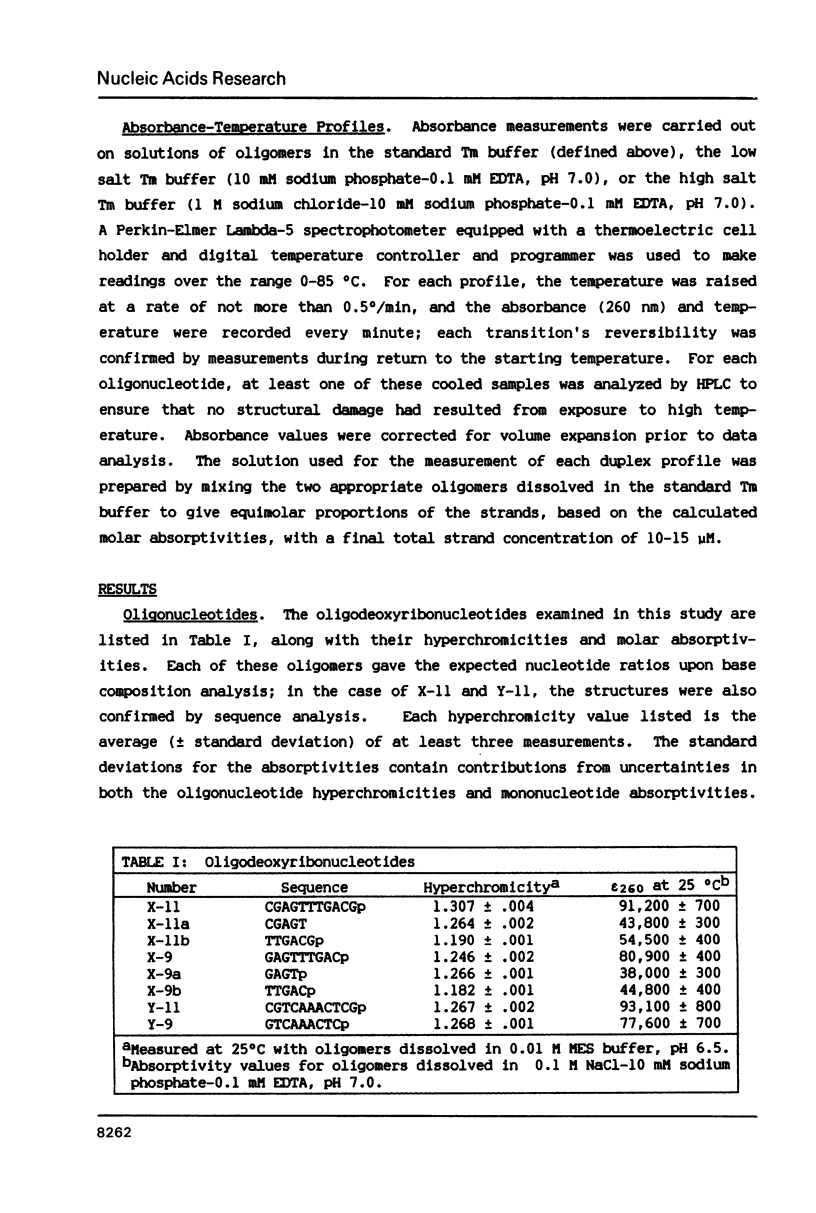
Abstract
An accurate method for deriving molar absorptivity-temperature profiles applied to a set of single-stranded oligodeoxyribonucleotides shows that the undecamer CGAGTTTGACGp exists in a hairpin conformation involving Watson-Crick base pairing between the two terminal CG dinucleotides. The hairpin, which has a transition midpoint of 40 degrees C in 0.115 M Na+, is unusually stable in comparison with previously reported hairpins. A non-linear least squares analysis of the undecamer's profile in terms of a two-state equilibrium model indicates that the hairpin-to-coil transition occurs with an enthalpy change about twice that expected if only combinations of Watson-Crick base-paired stacking interactions are considered. The analogous hairpin structure (containing an identical CG/CG stem) assignable to the complementary strand CGTCAAACTCGp does not form above 0 degrees C. Measurements on the two undecamers indicate that variation in non Watson-Crick interactions within the loops of two similar hairpins can produce a difference in stability of at least 2.2 kcal/mol (25 degrees C, 0.115 M Na+), roughly equal to the amount contributed to a double helix by a 5'-CG-3'/5'-CG-3' base-paired stacking interaction.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboul-ela F., Koh D., Tinoco I., Jr, Martin F. H. Base-base mismatches. Thermodynamics of double helix formation for dCA3XA3G + dCT3YT3G (X, Y = A,C,G,T). Nucleic Acids Res. 1985 Jul 11;13(13):4811–4824. doi: 10.1093/nar/13.13.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albergo D. D., Marky L. A., Breslauer K. J., Turner D. H. Thermodynamics of (dG--dC)3 double-helix formation in water and deuterium oxide. Biochemistry. 1981 Mar 17;20(6):1409–1413. doi: 10.1021/bi00509a001. [DOI] [PubMed] [Google Scholar]
- Appleby D. W., Kallenbach N. R. Theory of oligonucleotide stabilization. I. The effect of single-strand stacking. Biopolymers. 1973;12(9):2093–2120. doi: 10.1002/bip.1973.360120915. [DOI] [PubMed] [Google Scholar]
- Black D. M., Gilham P. T. A new method for sequence analysis of oligodeoxyribonucleotides. Nucleic Acids Res. 1985 Apr 11;13(7):2433–2442. doi: 10.1093/nar/13.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Cantor C. R., Tinoco I., Jr Absorption and optical rotatory dispersion of seven trinucleoside diphosphates. J Mol Biol. 1965 Aug;13(1):65–77. doi: 10.1016/s0022-2836(65)80080-8. [DOI] [PubMed] [Google Scholar]
- Elson E. L., Scheffler I. E., Baldwin R. L. Helix formation by d(TA) oligomers. 3. Electrostatic effects. J Mol Biol. 1970 Dec 28;54(3):401–415. doi: 10.1016/0022-2836(70)90118-x. [DOI] [PubMed] [Google Scholar]
- Freier S. M., Petersheim M., Hickey D. R., Turner D. H. Thermodynamic studies of RNA stability. J Biomol Struct Dyn. 1984 Mar;1(5):1229–1242. doi: 10.1080/07391102.1984.10507514. [DOI] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. II. Small hairpin loops. J Mol Biol. 1973 Feb 5;73(4):497–511. doi: 10.1016/0022-2836(73)90096-x. [DOI] [PubMed] [Google Scholar]
- Haasnoot C. A., de Bruin S. H., Berendsen R. G., Janssen H. G., Binnendijk T. J., Hilbers C. W., van der Marel G. A., van Boom J. H. Structure, kinetics and thermodynamics of DNA hairpin fragments in solution. J Biomol Struct Dyn. 1983 Oct;1(1):115–129. doi: 10.1080/07391102.1983.10507429. [DOI] [PubMed] [Google Scholar]
- Lawson T. G., Regnier F. E., Weith H. L. Separation of synthetic oligonucleotides on columns of microparticulate silica coated with crosslinked polyethylene imine. Anal Biochem. 1983 Aug;133(1):85–93. doi: 10.1016/0003-2697(83)90225-7. [DOI] [PubMed] [Google Scholar]
- Marky L. A., Blumenfeld K. S., Kozlowski S., Breslauer K. J. Salt-dependent conformational transitions in the self-complementary deoxydodecanucleotide d(CGCAATTCGCG): evidence for hairpin formation. Biopolymers. 1983 Apr;22(4):1247–1257. doi: 10.1002/bip.360220416. [DOI] [PubMed] [Google Scholar]
- Marky L. A., Breslauer K. J. Calorimetric determination of base-stacking enthalpies in double-helical DNA molecules. Biopolymers. 1982 Nov;21(11):2185–2194. doi: 10.1002/bip.360211107. [DOI] [PubMed] [Google Scholar]
- Martin F. H., Uhlenbeck O. C., Doty P. Self-complementary oligoribonucleotides: adenylic acid-uridylic acid block copolymers. J Mol Biol. 1971 Apr 28;57(2):201–215. doi: 10.1016/0022-2836(71)90341-x. [DOI] [PubMed] [Google Scholar]
- Nadeau J. G., Singleton C. K., Kelly G. B., Weith H. L., Gough G. R. Use of ribonucleosides as protecting groups in synthesis of polynucleotides with phosphorylated terminals. Biochemistry. 1984 Dec 4;23(25):6153–6159. doi: 10.1021/bi00320a040. [DOI] [PubMed] [Google Scholar]
- Petersheim M., Turner D. H. Base-stacking and base-pairing contributions to helix stability: thermodynamics of double-helix formation with CCGG, CCGGp, CCGGAp, ACCGGp, CCGGUp, and ACCGGUp. Biochemistry. 1983 Jan 18;22(2):256–263. doi: 10.1021/bi00271a004. [DOI] [PubMed] [Google Scholar]
- Scheffler I. E., Elson E. L., Baldwin R. L. Helix formation by dAT oligomers. I. Hairpin and straight-chain helices. J Mol Biol. 1968 Sep 28;36(3):291–304. doi: 10.1016/0022-2836(68)90156-3. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Borer P. N., Dengler B., Tinoco I., Jr Stability of RNA hairpin loops: A 6 -C m -U 6 . J Mol Biol. 1973 Feb 5;73(4):483–496. doi: 10.1016/0022-2836(73)90095-8. [DOI] [PubMed] [Google Scholar]
- Wickstrom E., Tinoco I., Jr The stability of RNA hairpin loops containing A-U-G: An-U-G-Um. Biopolymers. 1974 Nov;13(11):2367–2383. doi: 10.1002/bip.1974.360131116. [DOI] [PubMed] [Google Scholar]
- van Boom J. H., van der Marel G. A., Westerink H., van Boeckel C. A., Mellema J. R., Altona C., Hilbers C. W., Haasnoot C. A., de Bruin S. H., Berendsen R. G. Synthesis and conformational analysis of synthetic DNA fragments. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):403–409. doi: 10.1101/sqb.1983.047.01.047. [DOI] [PubMed] [Google Scholar]
- van Knippenberg P. H., Heus H. A. The conformation of a conserved stem-loop structure in ribosomal RNA. J Biomol Struct Dyn. 1983 Oct;1(2):371–381. doi: 10.1080/07391102.1983.10507448. [DOI] [PubMed] [Google Scholar]



