Abstract
Background
The debate about returning research results has revealed different perspectives among researchers, participants and advisory groups with participants generally interested in obtaining their results. Given this preference, policies regarding return of individual research results may affect whether a potential subject chooses to participate in a study. Public attitudes, particularly those of African-Americans, toward this issue have been understudied.
Methods
In 2008–2009, we convened 10 focus groups in Durham, N.C. to explore attitudes about returning research results and how different policies might influence their likelihood to participate in genetic/genomic studies. Transcripts were complimented by a short anonymous survey. Of 100 participants, 73% were female and 76% African-American with a median age of 40–49 years.
Results
Although there was general interest in obtaining genetics research results, particularly individual results, discussants recognized many potential complexities. The option to obtain research results (individual or summary) was clearly valued and lack thereof was potentially a deterrent for genetic/genomic research enrollment.
Conclusions
Providing the option to learn research results may help strengthen relationships between investigators and participants and thereby serve as a positive influencing factor for minority communities. Consideration of the broader implications of returning research results is warranted. Engaging diverse publics is essential to gain a balance between the interests and burdens of participants and investigators.
Key Words: Public attitudes, Focus group, Research results
Introduction
The issue of returning research results has been the subject of much debate in recent years [1,2,3,4,5,6]. In general, investigators do not appear to provide access to research results to participants [2,7,8], despite reported interest in doing so by both researchers [7,9,10] and participants [11,12,13,14,15,16]. The additional time and resources required to return individual research results are likely to deter many researchers from doing so [8,9,12,17].
If returned, research results are typically disclosed as individual or summary reports. If samples are collected anonymously or pooled without the possibility of reidentification, a summary report would be the only option to provide information about study outcomes. However, summary reports may leave participants with uncertainty as to the significance of the findings for themselves (e.g. whether they have the genotype of interest) [18]. Beyond simply fulfilling participants’ preference for individual results [19], providing an individual report may also help build trust between the participants and the researchers, particularly among individuals and groups who may be concerned about the use of their genetic information [20].
Given the expanding attention to this issue, investigators have begun to study the general public's views in regards to returning research results [21,22,23,24,25] and, to a lesser extent, about the potential impact of returning research results (individual or summary) or not on recruitment in genetics and genomics studies [26]. Genetic and genomic studies are increasingly dependent on recruitment of large numbers of individuals both with and without disease. In this article, we describe the results of a study assessing public attitudes, predominantly of African-Americans, towards the return of genomics research results and the potential impact of these policies on research participation rates. These data provide additional insight into preferences, concerns and expectations regarding participation in genomic research.
Methods
Study Population
Participants were recruited from community locations across Durham, N.C., USA, through advertisements in community newspapers, flyers posted in public areas and word-of-mouth. Recruitment efforts were targeted toward African-Americans as this population group is frequently underrepresented in genetics/genomic research. These efforts included recruitment through predominantly African-American churches, flyers posted in community centers and libraries in predominantly African-American neighborhoods and advertising in newsletters and radio stations targeted toward the African-American community. A meal and USD 25 were provided as compensation for their participation in the focus group. Individuals were eligible to participate if (1) they were 18 years of age or older and (2) did not currently, or ever, hold a genetics-related job (i.e. scientist, technician and healthcare provider). The study was approved by the Duke University Health System Institutional Review Board.
Focus Group Design
A 3-part questioning route guide (a sequence of questions designed for the moderator in conversationalist sentences) was designed based on a review of the literature and the investigators’ experience engaging the public around genetic topics [27]. Employing a ‘funneling’ approach [27], initial questions were intentionally broad, encouraging discussants in free expression with subsequent questions becoming more targeted and issue-specific. Part I of the focus group introduced the topic of genetic and genomic research. Part II served to guide the discussion toward our specific study aims through presentation of 3 hypothetical research vignettes, each highlighting different options for returning genetic/genomic research results as well as data-sharing practices amongst other researchers (table 1). Vignette 1 (Bob)described a large-scale, case-control study in which research participants would not learn individual research results but would instead be mailed a summary report of the study's findings at its completion. Vignette 2 (Betty) described a large-scale study of the prevalence of an allele in African-Americans. Participants would not learn of individual research results, but could access the researcher's webpage that would provide regular updates about the study outcomes. Vignette 3 (Sam) described a large-scale, multi-site genomic study of individuals with a family history of a heart disease in which participants have the option to receive individual research results in-person from a genetic counselor and/or a summary report. Part III of the focus group continued the issue-based discussion encouraging personal reflection and opinion formation. This article will focus on public perspectives toward returning genetic/genomic research results. The focus groups were moderated by one of the authors (alternating between focus groups).
Table 1.
Brief description of each vignette used in focus group discussion (note that genetic/genomic concepts and terms were described in general terms using nontechnical language)
| Vignette | Description |
|---|---|
| 1 | A man (Bob) with type II diabetes was approached at his doctor's office to take part in a large-scale, case-control study that required a DNA sample and some health information. At the completion of the study, research participants would not learn of individual research results, but would instead be mailed a summary report of the study's findings at its completion. The research findings would be disseminated through publications and presentations at professional meetings. The researchers will also share the data with other scientists studying diabetes upon request. |
| 2 | A healthy woman (Betty) was approached to take part in a large-scale study of the prevalence of a candidate functional variant in African-Americans in which she would be required to provide only a DNA sample by mail. Participants would not learn individual research results, but would have access to the researchers' web-page that would provide regular updates about the study outcomes. The research findings would be disseminated through publications and presentations at professional meetings. In addition, the data will be submitted to an open government Internet database accessible to any researcher. |
| 3 | A man (Sam) at risk for heart disease (based on family history) was approached to take part in a large-scale, multi-site genomic study, which required a DNA sample and some health information. Participants would have the option to receive a summary report by mail and/or their individual research result via genetic counselor. The research findings would be disseminated through publications and presentations at professional meetings. All of the participant survey data and genetic results will be stored in a centralized Internet database accessible only to researchers involved in the study. |
Pilot Focus Groups
We held 2 pilot focus groups to obtain feedback on the understandability of the content and questions as well as meeting logistics (e.g. food, time and location). A total of 23 individuals participated (17 female/18 African-Americans). Eight had a high-school degree/GED or less and 11 had a Bachelor's or higher degree. The median age group was 30–39 years. The materials and moderator guide were revised accordingly, and a short survey was added to enable collection of perspectives from all discussants regardless of whether they were verbalized during the focus group.
Focus Groups
Ten focus groups were held between February 2008 and February 2009 at 7 locations within the Durham, N.C. community including neighborhood centers, churches, public libraries, and a Duke University campus building. Consent was obtained from discussants upon arrival. Discussants also completed a socio-demographic questionnaire at the beginning of the session and a short survey at the conclusion of the focus group, which enabled collection of perspectives from all discussants. Each focus group discussion was audio-recorded and transcribed.
Data Analysis
The focus groups yielded 3 datasets: (1) socio-demographic data, (2) audio-recorded and transcribed text from each focus group session, and (3) anonymous responses to a short survey. Socio-demographic information was not linked to the individual responses in the transcript or the short survey. Analysis of the transcripts was facilitated using the software package NVivo (QSR International), an established program for use in qualitative data analysis. Utilizing the moderator questioning route, the unabridged transcripts were first divided into sampling units based on the topic-focused discussion questions. Initial coding categories for the content of the interview responses were developed by the 3 members of the study team; areas of intercoder disagreement were resolved by consensus before proceeding. We obtained an average Cohen's Kappa statistic of 0.74 based on coding at least 20% of the data in each sampling unit by the authors and a research assistant. The semantic content analysis involved: (1) coding of themes, (2) inter-group and aggregate evaluation of redundancy and/or discrepancy of themes, and (3) potential temporal changes and/or associations between inter-group and aggregate participant responses.
For the survey, summary statistics were generated for each question; descriptive data were expressed as a percentage or mean. Paired t tests were conducted to assess differences in likelihood to participate in a study given a particular policy on returning research results. The analog scale responses were collapsed into binary responses (very/somewhat vs. not very/not at all). As the responses to the short survey were anonymous and not linked to demographic data, the data could not be analyzed for any associations between participant characteristics. Analyses were performed using the STATA statistics package.
Results
Focus Group Discussants
One hundred individuals participated in 10 focus groups. Discussants were mostly female (73%) and African-American (76%), between the ages of 40–49 years (36%; see table 2). By chance, 2 of the focus groups were comprised only of individuals who identified themselves as African-American and 1 group only of individuals who identified themselves as White.
Table 2.
Demographic characteristics of focus group participants (n = 100)
| Gender | |
| Female | 73 |
| Race∗ | |
| White (FG 4 all White) | 22 |
| African-American (FG 1, FG 5 all African-American) | 76 |
| American-Indian/Alaskan Native | 4 |
| Asian | 1 |
| Ethnicity | |
| Hispanic | 4 |
| Non-Hispanic | 69 |
| Unsure | 8 |
| No response | 19 |
| Highest level of education | |
| <High school | 3 |
| High school diploma/GED | 17 |
| Some college, but no degree | 26 |
| Associate degree | 10 |
| Bachelors degree | 23 |
| Masters/doctorial degree | 17 |
| No response | 4 |
| Age | |
| 18-29 | 16 |
| 30-39 | 12 |
| 40-49 | 36 |
| 50-59 | 13 |
| 60-69 | 12 |
| ≥70 | 6 |
| No response | 5 |
Totals more than 100 as participants could indicate more than one race.
Responses to Research Study Vignettes
To illustrate some of the different options for returning research results to the focus group discussants and to serve as the basis for discussion, 3 hypothetical vignettes were read. Following each vignette, discussants were asked to consider the scenario, whether it seemed like a fair study with respect to the issue of returning results and whether they would participate in a similar type of study if approached. In general, both positive and negative issues were raised about each vignette when discussing ‘fairness’ and ‘potential participation’ (table 3). When considering whether they would choose to participate in a similar study, reported factors fell across 3 general categories: motivations (e.g. personal benefit and altruism), balance of burden/harm with compensation (compensation being material or informational) and trustworthiness of the research study and the researchers.
Table 3.
Concerns raised by discussants about each vignette regarding fairness and issue of returning research results
| Vignette 1 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Lack of compensation | x | x | x | x | ||||||
| Concern about ability to understand | x | |||||||||
| Concern with study design | x | |||||||||
| Concern about misuse of sample | x | x | ||||||||
| Privacy/confidentiality concerns | x | x | x | x | ||||||
| No return of personal result | x | x | x | x | x | x | ||||
| Lack of options for return of result | x | x |
| Vignette 2 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Focus on a particular population | x | x | x | x | ||||||
| Lack of compensation | x | x | x | |||||||
| Uncertainty of a research result | ||||||||||
| Concern about ability to understand | x | |||||||||
| Mistrust of research in general | x | x | ||||||||
| Concern with study design | x | x | x | x | x | |||||
| Concern about misuse of sample | x | |||||||||
| Mistrust of government database | x | x | x | |||||||
| Privacy/confidentiality concerns | x | x | x | x | ||||||
| No return of personal result | x | x | x | x | x | x | ||||
| Lack of options for return of results | x | x |
| Vignette 3 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Lack of compensation | x | x | ||||||||
| Uncertainty of a research result | x | x | x | x | x | |||||
| Worry after learning personal result | x | x | ||||||||
| Mistrust of research in general | x | x | ||||||||
| Concern about misuse of sample | x | |||||||||
| Privacy/confidentiality concerns | x | x | x |
In response to Vignette 1, discussants identified the following positive attributes: being able to help medical research and other affected individuals (altruism) (mentioned by 8 groups), the potential personal or family benefit (6 groups), the low potential for harm due to already being affected (4 groups), and that the subject was fully informed about the study and what he would/would not receive with respect to individual or summary research results (2 groups). The biggest drawback of the study appeared to be the lack of availability of an individual research result (5 groups) and general concerns about privacy and confidentiality (4 groups). In most groups, the majority of discussants reported they would likely participate in this type of study. The fact that the prospective research participant already had the disease was a significant factor both with respect to whether the focus group discussants would participate and whether they would wish to learn of their individual research results.
‘… If I enter a study personally, I mean, I know people that have different opinions, but I would want to know the results personally. I mean that would be one of the motivations besides contributing to the study.’ (FG 4, male)
‘[I would want to know] … because it would be beneficial to me and also to my children.’ (FG 4, female)
‘Well, I think, as a participant, very little is asked of him. So, I mean, if it's a cost factor, I would be okay not getting results.’ (FG 2, female)
‘… It will be okay. You told me straight up … I wouldn't expect [a personal result].’ (FG 10, female)
‘I'd like options … of how the results are going to be, you know. What is the final conclusion? I want to know what I am a part of.’ (FG 3, female)
‘I don't think I would participate because I wouldn't get anything back … I would have too many unanswered questions.’ (FG 5, female)
More than twice as many issues were raised in response to Vignette 2 as compared to the other vignettes. Positive features of the study included the low burden to participate (5 groups) and the potential to contribute to research that could benefit one's population group (5 groups). As with the first vignette, the most frequently raised problem with the study was the lack of availability of individual research results (6 groups). Other negative issues included mistrust of the study design due to lack of contact with researchers (5 groups) and discomfort with the focus on African-Americans (4 groups). In addition, some discussants were uncomfortable that data would be stored in a database maintained by the government, in reference to databases operated by the National Institutes of Health or other federal agencies. Notwithstanding the concerns, the majority of discussants again reported they would likely participate in this type of study.
‘It's just something about that word, “government”, because there were other studies in the past on the African-American population. And I probably would not [participate].’ (FG 2, female)
‘… It's just too impersonal because you have got to send all this in. It can be lost.’ (FG 6, female)
‘I like the fact that it's completely anonymous, and there's no way to link [the sample] back to the person.’ (FG 10, female)
‘I would [participate], I mean, if I had an interest in just helping mankind … it's like donating blood. I go to donate blood – I'm not personally wanting to know where it's going. I just know that it's going to help somebody.’ (FG 3, female)
‘I would participate in it because it would be for the African-American people.’ (FG 5, female)
For Vignette 3, although the return of individual research results was considered a significantly positive aspect (5 groups), it was the availability of options for individual or summary results that was raised most frequently as an attractive feature of the study (7 groups). In addition, given the at-risk status of the prospective research subject, the potential personal and/or familial benefit was also repeatedly mentioned as an advantage to the participant (7 groups). Discussants also, however, expressed concern about the uncertainty of the research findings (5 groups), potential worry related to learning personal results (3 groups) and concerns about privacy and confidentiality (3 groups).
‘For some people [learning individual results] could be a very positive thing. For others it could be a very negative thing … I think it would be good for individuals to have the option of saying, yes, I want to know or no, I don't want to know.’ (FG 4, female)
‘In my case, if the researcher says that they have no idea, and they're looking for something, I don't want to know because if they don't know how to fix it, what – just the burden is on me to suffer and with no help from them.’ (FG 4, female)
‘It would be good to know if you have those things, but I don't think I would want my name in that information – connected – to be out.’ (FG 4, female)
‘I think I would want to know because whatever they gained, whatever they learned, you could always take it to your primary physician and if they – they would be able to explain things more … his primary physician should be able to take it from there.’ (FG 5, female)
In addition to the issues highlighted above, the apparent lack of compensation (e.g. food and money) was mentioned by 4 different groups in response to 1 or more vignettes. Although this was not specifically described in any vignettes, its absence was noted and described as an important factor to at least some of our discussants.
Perspectives on Learning Research Results
Building upon the vignette discussion, we sought to understand what factors would influence discussants’ interest in learning of their individual research results, whether they believed they were entitled to their individual results, and the perceived obligation of researchers to make individual research results available. Many discussants stated they would be interested in learning of their individual research result, regardless of the certainty of the results, type or severity of disease and availability of treatment. Some discussants noted that whether or not the research participant was affected with the disease being studied would influence the perceived value of learning of their research results. In addition, given the nature of genetic information, several discussants recognized that the research result may become significant in the future as more research is done.
‘That's your ending point, but that could be someone else's [starting point] – when something closes, another something else opens.’ (FG 3, female)
‘… I mean, there's not a cure now. Next week, there might be one.’ (FG 3, female)
‘… even if there's no treatment or cure, you can at least prepare … make sure that [your family] is set before your disease starts to really affect you, I guess. So yeah, I'd want to know.’ (FG 10, female)
When asked to consider if there were any circumstances in which they may not want to receive an individual result, several responded they would always want to receive them. Some discussants, however, indicated that they would be less interested in learning of their results if it would cause worry or be too traumatic to know (echoing earlier comments), while others indicated they would only want to learn their results if a treatment or intervention were available. A few also commented that they may not be able to understand the results.
‘Yeah, what's your DNA really gonna tell you? Somebody else with some of your traits, the T and A's might be – you know, what are they gonna tell you?’
‘They could tell you about potential predictions.’
‘But which would cause the stress level to increase. Why would you want to know about it?’ (conversation between 2 female discussants) (FG 7)
‘… sometimes getting information back from research that hasn't proven yet, can really set off a chain reaction in a person that could make things worse for them.’ (FG 10, female)
The balance of burden and benefits was repeatedly considered in both quantitative (e.g. time and discomfort vs. monetary compensation) and qualitative terms (e.g. risks of misuse of genetic information vs. personal relevance of results information). As highlighted below, loss of confidentiality and discrimination were also raised as a potential concern.
‘The only thing is would it prevent you from getting insurance or employment, or something like that?’ (female)
‘Or getting into a college, even?’ (male) (FG 4, conversation)
If it was raised spontaneously, we informed discussants of state and newly enacted federal protections to prevent health insurance and employment discrimination based on genetic information. Regardless, the moderator often mentioned these protections at the end of discussion. We did not probe whether existence of legal protections diminished fears about discrimination.
Discussants were divided as to whether they believed they were entitled to their results and no consensus emerged, as exemplified by the comments below. Some discussants thought that they should get something back since they were personally involved (e.g. providing a DNA sample) or because results may be personally relevant or useful (for themselves or family members). Others stated that if it was clearly disclosed in the informed consent, a person should not expect results. Several discussants qualified their opinions by saying they should have ‘the option’ to receive results.
‘If they tell you at the onset, you are not going to get the results back, then it's up to you to make that decision. You don't participate if you are expecting to get some results back because they done told you, you not.’ (FG 6, female)
‘If it's not for [commercial] profit and just for the good of the people, then I don't feel any entitlement.’ (FG 9, female)
‘Well, as long as it's not mandatory that a person receives back their individual results, I think, like I personally would have no problem being in a study.’ (FG 9, female)
In response to a question about whether researchers are ever justified in not returning individual results, some discussants agreed that researchers may be justified if they believed it may cause harm to the participants or were inconclusive. They also understood that the additional costs and effort required to recontact participants may affect the study design (e.g. smaller sample size).
‘[Justified] in not sharing what the information would mean, no, I don't think [so].’ (FG 5, female)
‘… if it could harm the person in some way, to make them do something different that may or may not help them.’ (FG 2, female)
‘If for some reason they find the study is flawed.’ (FG 4, male)
‘I think “too expensive” and “time consuming” would be 2 good factors. To be honest, whether I get the results back or not would fall low in the scale of me deciding whether to do a study or not.’ (FG 2, female)
Others felt that the lack of clinical relevance, clinical utility or even the potential for harm were not justifiable reasons for researchers not to return individual results. In addition, apart from the perceived value or harm of returning results, some simply felt that researchers should not decide on behalf of their participants regarding the clinical relevance or utility of results. Since the information may be important for their personal health and well-being, some believed that researchers did not have the authority or the medical expertise to make those types of decisions.
‘It's not their place because that “MD” would be behind their name if [it] was. That's not their call to make.’ (FG 3, female)
‘I think the person who's doing – that's participating – should have that option, and I don't think it should be the researcher.’ (FG 8, female)
‘I don't think that the researchers should make that decision, selection of choice for the participant. That is something that the participant should have say-so in.’ (FG 5, female)
In the survey, we asked discussants to consider the importance of 4 factors that may influence their interest to learn their individual research result. Three of the factors (accuracy of results, personal relevance and clinical utility) were equally important considerations (78–80% indicated them as very important), while potential harms for discrimination was a significantly less important factor (65% indicated it as very important; p < 0.001; data not shown).
Communication of Research Results
If individual research results were to be returned, the majority of groups preferred to receive their results in-person along with a written record. Some discussants indicated they might be willing to pay a small fee in order to receive their individual research result though most were not. Similar to the focus group dialogue, the survey results showed that most discussants preferred to receive their individual research results in-person, choosing to learn them from their physician (42%), followed in preference by a post letter from the researcher (32%) and a call from the researcher (16%).
Potential Impact on Participation in Research
Discussants were divided about whether they would participate in a genetics/genomics study that would provide a summary report only or no information at all. Some readily indicated that they would participate in such studies, while others stated that their decision to participate in a study that did not return individual results would depend on the disease and/or how detailed the summary report would be.
Discussants acknowledged that their personal reasons for participating in a particular study would likely affect their desire and/or expectation to receive individual research results (e.g. altruism vs. vested interest (affected participant or family member)). It was repeatedly commented that they may be willing to receive little or no benefit with respect to availability of individual research results if very little effort or time was required, it involved minimal discomfiture and if the results were not considered highly important (e.g. genetics of baldness). Lastly, the provision of research results, whether individual or summary results, was perceived as providing a layer of ‘transparency’ between the participant and the research as well as the researchers. This was especially important in Vignette 2 in which there was minimal contact with the research team.
‘Yeah, I'd feel like they're creating relative transparency. They're trying to share all this information. If I was really curious, which I assume I would be, then I could go and get as much information as they could give me … I'd want as much as I could have.’ (FG 9, female)
‘I think the attitude is “are you doing this for the good of your fellow man,” and that is your payback, or “do you need a personal payback?”’ (FG 8, male)
‘If I give a sample – [the] researcher, that's the person that I'm in contact with … I'm just not going to give you blood and you go [away] with it, and I don't know nothing.’ (FG 6, male)
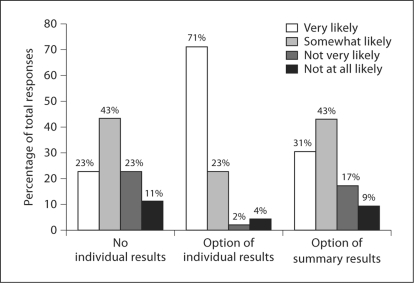
The survey data showed that although the majority of discussants indicated they would be very or somewhat likely to participate in a genetics research study regardless of whether results were returned, significant differences were noted based on whether individual or summary results were to be returned. Significantly more discussants (94%) indicated they would be very/somewhat likely to participate in a study that returned individual research results compared to one that offered to return a summary report (74%; p < 0.00001) versus one that did not return any results (66%; p < 0.00001) (fig. 1).
Fig. 1.
Likelihood of participating in a genetics research study given 3 hypothetical policies on returning results.
Discussion
The issue of returning research results continues to be a subject of intense interest by research participants, researchers and members of institutional review boards. The debate has revealed conflicting preferences and attitudes among researchers, research participants and advisory groups [28]. Our study provides an additional perspective of the general public (potential future research participants), particularly African-Americans, whose views have not been deeply considered in previous studies. Similar to previous findings and despite the differences in study populations (predominantly White vs. African-American), our focus group discussants overwhelmingly desired to receive individual research results [11,12,13,14,15,16,23,26]. More importantly, however, they desired the option to decide for themselves what type of information they might want to learn, similar to findings by Murphy et al. [23].
In general, discussants recognized several potential benefits and risks to learning individual genetic research results including personal benefits to themselves (and more often, to family members such as children), uncertainty of results and psychosocial or clinical harms, some of which have been experienced by other research participants [29,30]. Their perception of fairness with respect to the 3 vignettes appeared to be based on a balance of the burdens of study participation to the potential benefits of participation as well as consideration of the study participant's health situation (affected, unaffected and at-risk based on family history). Regardless of their personal preferences, some discussants admitted that it would be fair if told of the study's policy regarding the return of research results prior to enrollment. However, most discussants favored access to research results as (part of) the compensation for participation and viewed it as both a recognition of a participant's contribution to the research as suggested by others [31] and an opportunity to potentially receive direct benefit. In addition, discussants noted that physical or psychological harm could be incurred from inappropriate medical actions based on uncertain research results.
Although several groups have recommended that individual research results should be of clinical benefit if they are to be returned [4,31,32,33,34], our discussants disagreed with limiting the definition of benefit to clinical benefit. Making the distinction between clinically useful and nonuseful information is not required to determine researchers’ obligations but is important to consider when communicating results to participants [2]. Due to the unique situation of each participant highlighted in the vignettes, most discussants believed that researchers should not make these decisions on behalf of participants and some argued that researchers may not even be qualified to make a ‘clinical’ call. Indeed, this decision was considered a personal or medical decision which involved the individual and potentially his/her physician. Discussants were both distrustful and resentful of the paternalistic approach of researchers with respect to this issue, a characteristic that has been recognized in the field of research ethics [35,36].
Partridge and Winer [37] speculated that failure to provide research results may account for lower enrollment rates in clinical studies. Approximately one-third of discussants indicated that they would be ‘not very or not at all likely to’ consider participating in a genetics/genomics study that did not provide individual results or a summary. Although posed differently, another study also demonstrated a trend toward less positive views about participation, with up to 75% of prospective participants indicating they would be less likely to participate without access to results [26]. But given the challenges to recruiting African-Americans to participate in research [38,39,40], the sizable proportion of individuals who would decline to participate is noteworthy and warrants further investigation.
Issues of mistrust were voiced by several discussants, particularly due to past research abuses of minority populations. As suggested by our study and others [23], providing the option to learn of individual research results may serve as a significant, positive influencing factor in decisions about participation by enhancing trust between participants and researchers [41,42] and showing respect for participants [18,43]. Specifically, enhancing trust between researchers and participants may be particularly important for genetics/genomics research as this type of research may raise additional personal and community concerns, potentially affecting overall participation rates of minorities [44,45,46,47,48,49,50]. In addition, concerns regarding the perceived lack of benefit, to the individual or to the community [51,52], may be ameliorated through the provision of options to access individual or summary results.
Some assert that while the option to obtain research results should be provided, it should not necessarily be encouraged given concerns about the potential harms of unvalidated or inconclusive data, particularly for individual results [18]. Discussants felt strongly that research participants should be allowed to consider these potential harms and decide for themselves. Many of our discussants preferred to learn of the research results in-person. The delivery of these results could mimic a genetic counseling session including an overview of genetics as well as the details of the study and limitations for interpretation of the research result. While being the most comprehensive, this would also, of course, be the most costly and time-consuming approach. Other discussants indicated they would be comfortable receiving their research results by mail or accessing them through a secure online database. While less costly, these methods leave open the potential for misinterpretation and therefore, contact information should be provided to allow participants to follow up with someone familiar with the study to assist in the interpretation of the results. In addition, providing study information to participants at intervals throughout a project, perhaps through updates posted online, would create transparency enabling participants to stay informed of the research progress and engaged. The Coriell Personalized Medicine Collaborative is utilizing this latter approach, along with providing participants the option to select which results they wish to view and the opportunity to meet with a genetic counselor before and/or after viewing a result (http://cpmc.coriell.org/sections/about/faqs.aspx?pgid=13).
Our study has some limitations that should be noted. The attitudes of our focus group discussants may not be representative of the general public given the small sample size and recruitment from one region. In addition, responses to hypothetical scenarios are often positively biased [53], and, therefore, further studies are needed to test the impact of differing policies on returning research results, particularly in underrepresented populations. Although African-American opinions are well represented in the study, the opinions are not solely those of African-Americans.
Overall, our findings suggest general interest in having access to individual genetic/genomic research results, but that participants would still likely participate in studies even if they did not return individual or summary results. Although this might suggest that current policies of not returning results have little influence on decisions to participate in research, personal results disclosure was clearly valued by our discussants and lack thereof appeared to be a potential deterrent for genetic/genomic research enrollment. Our data also suggest that the benefits of returning results extend beyond the individual and can help strengthen the relationship between researcher and participant. Balancing the interests and burdens of participants and investigators, respectively, will continue to be a challenge in developing policies regarding the return of individual research results. As genetics and genomics research continues to expand, engaging the public is critical in the development of new policies to ensure respect, safety and interest of future research participants.
Acknowledgements
We would like to thank our research assistant, Ms. Genevieve Tindall, for her kind assistance on this project and the manuscript. This study was supported by NIH grant #1R03-HG-004312.
References
- 1.Renegar G, Webster CJ, Stuerzebecher S, Harty L, Ide SE, Balkite B, Rogalski-Salter TA, Cohen N, Spear BB, Barnes DM, Brazell C. Returning genetic research results to individuals: points-to-consider. Bioethics. 2006;20:24–36. doi: 10.1111/j.1467-8519.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- 2.Shalowitz DI, Miller FG. Communicating the results of clinical research to participants: attitudes, practices, and future directions. PLoS Med. 2008;5:e91. doi: 10.1371/journal.pmed.0050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knoppers BM, Joly Y, Simard J, Durocher F. The emergence of an ethical duty to disclose genetic research results: international perspectives. Eur J Hum Genet. 2006;14:1170–1178. doi: 10.1038/sj.ejhg.5201690. [DOI] [PubMed] [Google Scholar]
- 4.Bookman EB, Langehorne AA, Eckfeldt JH, Glass KC, Jarvik GP, Klag M, Koski G, Motulsky A, Wilfond B, Manolio TA, Fabsitz RR, Luepker RV, NHLBI Working Group Reporting genetic results in research studies: summary and recommendations of an NHLBI working group. Am J Med Genet A. 2006;140:1033–1040. doi: 10.1002/ajmg.a.31195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGuire AL, Caulfield T, Cho MK. Research ethics and the challenge of whole-genome sequencing. Nat Rev Genet. 2008;9:152–156. doi: 10.1038/nrg2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravitsky V, Wilfond BS. Disclosing individual genetic results to research participants. Am J Bioeth. 2006;6:8–17. doi: 10.1080/15265160600934772. [DOI] [PubMed] [Google Scholar]
- 7.Partridge AH, Hackett N, Blood E, Gelman R, Joffe S, Bauer-Wu S, Knudsen K, Emmons K, Collyar D, Schilsky RL, Winer EP. Oncology physician and nurse practices and attitudes regarding offering clinical trial results to study participants. J Natl Cancer Inst. 2004;96:629–632. doi: 10.1093/jnci/djh096. [DOI] [PubMed] [Google Scholar]
- 8.Rigby H, Fernandez CV. Providing research results to study participants: support versus practice of researchers presenting at the American Society of Hematology annual meeting. Blood. 2005;106:1199–1202. doi: 10.1182/blood-2005-02-0556. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez CV, Kodish E, Shurin S, Weijer C. Offering to return results to research participants: attitudes and needs of principal investigators in the children's oncology group. J Pediatr Hematol Oncol. 2003;25:704–708. doi: 10.1097/00043426-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Garcia J. Sharing research results with patients: the views of care-givers involved in a randomized controlled trial. J Reprod Infant Psychol. 1987;5:9–13. doi: 10.1080/02646838708403469. [DOI] [PubMed] [Google Scholar]
- 11.Snowdon C, Garcia J, Elbourne D. Reactions of participants to the results of a randomised controlled trial: exploratory study. BMJ. 1998;317:21–26. doi: 10.1136/bmj.317.7150.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Partridge AH, Burstein HJ, Gelman RS, Marcom PK, Winer EP. Do patients participating in clinical trials want to know study results? J Natl Cancer Inst. 2003;95:491–492. doi: 10.1093/jnci/95.6.491. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez CV, Santor D, Weijer C, Strahlendorf C, Moghrabi A, Pentz R, Gao J, Kodish E. The return of research results to participants: pilot questionnaire of adolescents and parents of children with cancer. Pediatr Blood Cancer. 2007;48:441–446. doi: 10.1002/pbc.20766. [DOI] [PubMed] [Google Scholar]
- 14.Richards MP, Ponder M, Pharoah P, Everest S, Mackay J. Issues of consent and feedback in a genetic epidemiological study of women with breast cancer. J Med Ethics. 2003;29:93–96. doi: 10.1136/jme.29.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moutel G, Duchange N, Raffi F, Sharara LI, Theodorou I, Noel V, de Montgolfier S, Callies I, Bricaire F, Herve C, Leport C. Communication of pharmacogenetic research results to HIV-infected treated patients: standpoints of professionals and patients. Eur J Hum Genet. 2005;13:1055–1062. doi: 10.1038/sj.ejhg.5201450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fong M, Braun KL, Chang RM. Native Hawaiian preferences for informed consent and disclosure of results from research using stored biological specimens. Pac Health Dialog. 2004;11:154–159. [PubMed] [Google Scholar]
- 17.Fernandez CV, Skedgel C, Weijer C. Considerations and costs of disclosing study findings to research participants. CMAJ. 2004;170:1417–1419. doi: 10.1503/cmaj.1031668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shalowitz DI, Miller FG. Disclosing individual results of clinical research: implications of respect for participants. JAMA. 2005;294:737–740. doi: 10.1001/jama.294.6.737. [DOI] [PubMed] [Google Scholar]
- 19.Dixon-Woods M, Jackson C, Windridge KC, Kenyon S. Receiving a summary of the results of a trial: qualitative study of participants' views. BMJ. 2006;332:206–210. doi: 10.1136/bmj.38675.677963.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz A, Caldwell C, Foster S. ‘What are they going to do with the information?’ Latino/Latina and African American perspectives on the Human Genome Project. Health Educ Behav. 2003;30:151–169. doi: 10.1177/1090198102251026. [DOI] [PubMed] [Google Scholar]
- 21.Godard B, Marshall J, Laberge C. Community engagement in genetic research: results of the first public consultation for the Quebec CARTaGENE project. Community Genet. 2007;10:147–158. doi: 10.1159/000101756. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman DJ, Murphy-Bollinger J, Scott J, Hudson KL. Public opinion about the importance of privacy in biobank research. Am J Hum Genet. 2009;85:643–654. doi: 10.1016/j.ajhg.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy J, Scott J, Kaufman D, Geller G, LeRoy L, Hudson K. Public expectations for return of results from large-cohort genetic research. Am J Bioeth. 2008;8:36–43. doi: 10.1080/15265160802513093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoeyer K, Olofsson BO, Mjorndal T, Lynoe N. Informed consent and biobanks: a population-based study of attitudes towards tissue donation for genetic research. Scand J Public Health. 2004;32:224–229. doi: 10.1080/14034940310019506. [DOI] [PubMed] [Google Scholar]
- 25.Wendler D, Emanuel E. The debate over research on stored biological samples: what do sources think? Arch Intern Med. 2002;162:1457–1462. doi: 10.1001/archinte.162.13.1457. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman D, Murphy J, Scott J, Hudson K. Subjects matter: a survey of public opinions about a large genetic cohort study. Genet Med. 2008;10:831–839. doi: 10.1097/GIM.0b013e31818bb3ab. [DOI] [PubMed] [Google Scholar]
- 27.Krueger RA, Casey MA. Focus Groups: A Practical Guide for Applied Research, ed 3. Thousand Oaks: Sage Publications; 2000. [Google Scholar]
- 28.Miller FA, Christensen R, Giacomini M, Robert JS. Duty to disclose what? Querying the putative obligation to return research results to participants. J Med Ethics. 2008;34:210–213. doi: 10.1136/jme.2006.020289. [DOI] [PubMed] [Google Scholar]
- 29.Partridge AH, Wolff AC, Marcom PK, Kaufman PA, Zhang L, Gelman R, Moore C, Lake D, Fleming GF, Rugo HS, Atkins J, Sampson E, Collyar D, Winer EP. The impact of sharing results of a randomized breast cancer clinical trial with study participants. Breast Cancer Res Treat. 2009;115:123–129. doi: 10.1007/s10549-008-0057-7. [DOI] [PubMed] [Google Scholar]
- 30.Schulz CJ, Riddle MP, Valdimirsdottir HB, Abramson DH, Sklar CA. Impact on survivors of retinoblastoma when informed of study results on risk of second cancers. Med Pediatr Oncol. 2003;41:36–43. doi: 10.1002/mpo.10278. [DOI] [PubMed] [Google Scholar]
- 31.MacNeil SD, Fernandez CV. Offering results to research participants. BMJ. 2006;332:188–189. doi: 10.1136/bmj.332.7535.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caulfield T, McGuire AL, Cho M, Buchanan JA, Burgess MM, Danilczyk U, Diaz CM, Fryer-Edwards K, Green SK, Hodosh MA, Juengst ET, Kaye J, Kedes L, Knoppers BM, Lemmens T, Meslin EM, Murphy J, Nussbaum RL, Otlowski M, Pullman D, Ray PN, Sugarman J, Timmons M. Research ethics recommendations for whole-genome research: consensus statement. PLoS Biol. 2008;6:e73. doi: 10.1371/journal.pbio.0060073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Bioethics Advisory Committee, vol 1: Research involving human biological materials: ethical issues and policy guidance, 1999. http://bioethics.georgetown.edu/nbac/pubs.html
- 34.Stanford Working Group on Reporting Results of Genetic Research: Releasing results of genetic testing to research participants: a multidisciplinary consensus statement. American Society of Human Genetics 55th Annu Meet, Salt Lake City, 2005.
- 35.Miller FG, Wertheimer A. Facing up to paternalism in research ethics. Hastings Cent Rep. 2007;37:24–34. doi: 10.1353/hcr.2007.0044. [DOI] [PubMed] [Google Scholar]
- 36.Edwards SJ, Kirchin S, Huxtable R. Research ethics committees and paternalism. J Med Ethics. 2004;30:88–91. doi: 10.1136/jme.2002.000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Partridge AH, Winer EP. Informing clinical trial participants about study results. JAMA. 2002;288:363–365. doi: 10.1001/jama.288.3.363. [DOI] [PubMed] [Google Scholar]
- 38.Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, Tilburt J, Baffi C, Tanpitukpongse TP, Wilson RF, Powe NR, Bass EB. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112:228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 39.Advani AS, Atkeson B, Brown CL, Peterson BL, Fish L, Johnson JL, Gockerman JP, Gautier M. Barriers to the participation of African-American patients with cancer in clinical trials: a pilot study. Cancer. 2003;97:1499–1506. doi: 10.1002/cncr.11213. [DOI] [PubMed] [Google Scholar]
- 40.Furr LA. Perceptions of genetics research as harmful to society: differences among samples of African-Americans and European-Americans. Genet Test. 2002;6:25–30. doi: 10.1089/109065702760093889. [DOI] [PubMed] [Google Scholar]
- 41.Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore) 2008;87:1–9. doi: 10.1097/MD.0b013e3181625d78. [DOI] [PubMed] [Google Scholar]
- 42.Corbie-Smith G, Thomas SB, St George DM. Distrust, race, and research. Arch Intern Med. 2002;162:2458–2463. doi: 10.1001/archinte.162.21.2458. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez CV, Kodish E, Weijer C. Informing study participants of research results: an ethical imperative. IRB. 2003;25:12–19. [PubMed] [Google Scholar]
- 44.Espeland MA, Dotson K, Jaramillo SA, Kahn SE, Harrison B, Montez M, Foreyt JP, Montgomery B, Knowler WC, Look AHEAD research group Consent for genetics studies among clinical trial participants: findings from Action for Health in Diabetes (Look AHEAD) Clin Trials. 2006;3:443–456. doi: 10.1177/1740774506070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Catz DS, Green NS, Tobin JN, Lloyd-Puryear MA, Kyler P, Umemoto A, Cernoch J, Brown R, Wolman F. Attitudes about genetics in underserved, culturally diverse populations. Community Genet. 2005;8:161–172. doi: 10.1159/000086759. [DOI] [PubMed] [Google Scholar]
- 46.McQuillan GM, Porter KS, Agelli M, Kington R. Consent for genetic research in a general population: the NHANES experience. Genet Med. 2003;5:35–42. doi: 10.1097/00125817-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Wang SS, Fridinger F, Sheedy KM, Khoury MJ. Public attitudes regarding the donation and storage of blood specimens for genetic research. Community Genet. 2001;4:18–26. doi: 10.1159/000051152. [DOI] [PubMed] [Google Scholar]
- 48.Mezuk B, Eaton WW, Zandi P. Participant characteristics that influence consent for genetic research in a population-based survey: the Baltimore epidemiologic catchment area follow-up. Community Genet. 2008;11:171–178. doi: 10.1159/000113880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moorman PG, Skinner CS, Evans JP, Newman B, Sorenson JR, Calingaert B, Susswein L, Crankshaw TS, Hoyo C, Schildkraut JM. Racial differences in enrolment in a cancer genetics registry. Cancer Epidemiol Biomarkers Prev. 2004;13:1349–1354. [PubMed] [Google Scholar]
- 50.DiMartino L, Allen KD, Kasarskis E, Lindquist JH, Coffman CJ, Oddone EZ; National Registry of Veterans with ALS. Characteristics associated with participation in DNA banking: The National Registry of Veterans with ALS. Contemp Clin Trials. 2007;28:572–582. doi: 10.1016/j.cct.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Royal C, Baffoe-Bonnie A, Kittles R, Powell I, Bennett J, Hoke G, Pettaway C, Weinrich S, Vijayakumar S, Ahaghotu C, Mason T, Johnson E, Obeikwe M, Simpson C, Mejia R, Boykin W, Roberson P, Frost J, Faison-Smith L, Meegan C, Foster N, Furbert-Harris P, Carpten J, Bailey-Wilson J, Trent J, Berg K, Dunston G, Collins F. Recruitment experience in the first phase of the African American Hereditary Prostate Cancer (AAHPC) study. Ann Epidemiol. 2000;10(suppl 8):S68–77. doi: 10.1016/s1047-2797(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 52.Halbert CH, Kessler L, Stopfer JE, Domchek S, Wileyto EP. Low rates of acceptance of BRCA1 and BRCA2 test results among African American women at increased risk for hereditary breast-ovarian cancer. Genet Med. 2006;8:576–582. doi: 10.1097/01.gim.0000237719.37908.54. [DOI] [PubMed] [Google Scholar]
- 53.Persky S, Kaphingst KA, Condit CM, McBride CM. Assessing hypothetical scenario methodology in genetic susceptibility testing analog studies: a quantitative review. Genet Med. 2007;9:727–738. doi: 10.1097/gim.0b013e318159a344. [DOI] [PMC free article] [PubMed] [Google Scholar]