Abstract
Background/Aims: While the Cl- efflux assays are relatively straightforward, their ability to assess the efficacy of phenotypic correction in cystic fibrosis (CF) tissue or cells may be limited. Accurate assessment of therapeutic efficacy, i.e., correlating wild type CF transmembrane conductance regulator (CFTR) levels with phenotypic correction in tissue or individual cells, requires a sensitive assay. Methods: Radioactive chloride (36Cl) efflux was compared to Ussing chamber analysis for measuring cAMP-dependent Cl- transport in mixtures of human normal (16HBE14o-) and cystic fibrosis (CF) (CFTE29o- or CFBE41o-, respectively) airway epithelial cells. Cell mixtures with decreasing amounts of 16HBE14o- cells were evaluated. Results: Efflux and Ussing chamber studies on mixed populations of normal and CF airway epithelial cells showed that, as the number of CF cells within the population was progressively increased, the cAMP-dependent Cl- decreased. The 36Cl efflux assay was effective for measuring Cl- transport when ≥ 25% of the cells were normal. If < 25% of the cells were phenotypically wild-type (wt), the 36Cl efflux assay was no longer reliable. Polarized CFBE41o-cells, also homozygous for the ΔF508 mutation, were used in the Ussing chamber studies. Ussing analysis detected cAMP-dependent Cl- currents in mixtures with ≥1% wild-type cells indicating that Ussing analysis is more sensitive than 36Cl efflux analysis for detection of functional CFTR. Conclusions: Assessment of CFTR function by Ussing analysis is more sensitive than 36Cl efflux analysis. Ussing analysis indicates that cell mixtures containing 10% 16HBE14o- cells showed 40–50% of normal cAMP-dependent Cl- transport that drops off exponentially between 10-1% wild-type cells.
Key Words: cAMP-dependent Cl- ion transport, Cystic fibrosis, CF therapies, 36Cl efflux, Ussing chamber analysis, Wild-type CFTR efficacy
Introduction
The development of methods for rapidly measuring Cl- ion transport has been important for the characterization of CF epithelial cells [1-6], especially in the context of developing novel genetic and pharmacological therapies for CF [7-15]. The implementation of strategies for transferring wild-type (wt) CF transmembrane conductance regulator (CFTR) sequences into the CF epithelial cells or the development of agents that activate mutant CFTR has made it increasingly important to have a means to efficiently evaluate Cl- ion transport [7, 8, 10-12, 14, 15]. In particular, it will be important to determine whether the transfection of the wtCFTR into CF epithelial cells and the level of CFTR expression is adequate for correcting the Cl- ion transport phenotype in the cells [16]. One approach that has been effective uses radioactive chloride (36Cl) efflux and has been effective to evaluate cAMP-, calcium-(Ca) [1, 17], and swelling-dependent [1, 6, 18] Cl- transport in epithelial cells. The efflux of radioactive Cl- is appealing when compared to the other methods, because it is not only rapid, but does not require complicated and expensive equipment outside of the standard equipment present in most laboratories. In addition, it does not require cell polarity and the interpretation of the data is straightforward.
Measurement of radioactive Cl- (36Cl) efflux has been useful to verify whether CF epithelial cells have been functionally complemented after the introduction of wtCFTR cDNA [19]. However, the use of this technique as the sole measure of Cl- ion transport may be shortsighted, since it is not clear how the level of wtCFTR expression correlates with detectable 36Cl efflux and whether this approach is adequate for assessing the efficacy of a genetic or pharmacological therapy for CF. The studies presented here comparing Ussing chamber analysis to 36Cl efflux suggest that an adjuvant measure is necessary to provide a more comprehensive overview of cAMP-dependent Cl- ion transport characteristics of in vitro cell systems.
Materials and Methods
Cell culture and cell lines
The cells used in this study have been described previously and were isolated from airways of a normal and CF (ΔF508/ΔF508) individuals and then used to generate the immortalized cell lines 16HBE14o- [3], CFTE29o- [20], and CFBE410- [21-24]. The 16HBE14o- cells have intact cAMP-dependent Cl- ion transport, are polar, and express abundant levels of endogenous CFTR mRNA and protein [3]. The CFTE290- and CFBE41o-cells are homozygous for the ΔF508 mutation, express CFTR mRNA and protein, and are defective in cAMP-regulated Cl- ion transport [20, 21, 23, 25]. In contrast to CFTE29o-cells, the CFBE41o-cells have retained their ability to form tight epithelial monolayers and are suitable for transepithelial experiments in Ussing chambers [23, 24]. While ΔF508-CFTR mRNA expression is detectable by PCR, the levels of expression are low when compared to 16HBE14o-cells [20-22, 24] (∼1.6% of 16HBE14o-levels, unpublished observations). Stock cultures of all cell types were grown in Eagle's minimal essential medium (MEM) supplemented with 10% fetal calf serum (FCS) and antibiotics on plasticware or Snapwell inserts coated with a cocktail of fibronectin (FN)/ Vitrogen (V)/bovine serum albumin (BSA) [26, 27]. Stock cultures were maintained until the experiments were initiated and all cells were grown under humidified conditions in an atmosphere of 5% CO2.
36Cl efflux measurements
Stock cultures were trypsinized, resuspended in fresh MEM, and counted on a hemocytometer. The 16HBE14o- cells were then mixed with CFTE29o-cells at different ratios (100:0, 75:25, 50:50, 25:75, 10:90, 1:99, respectively), and plated on 35 mm dishes in triplicate. Approximately 3 × 105 cells total were plated onto each dish. Cultures were grown until they had just reached confluence (∼24-48 h) and then labeled with 36Cl. The efflux of Cl- was measured after a 2 h loading with 2 μCi of 36Cl as previously described [2, 3]. Briefly, the cells were grown to confluence. The growth medium was removed and cultures were rinsed twice with 2 ml efflux buffer (140 mM NaCl, 3.3 mM KH2PO4, 0.83 mM K2HPO4, 1 mM CaSO4, 1 mM MgSO4, 10mM HEPES, pH 7.4, 10 mM glucose). After rinsing, fresh efflux buffer (1 ml) containing 2 μCi/ml 36Cl was added to each dish for a 2 h incubation at 37°C. Each dish was then washed by dipping into 2 beakers with 200 ml efflux buffer; for a total wash time of 8-10 sec. Following washing, 1 ml of fresh efflux buffer was added and the cells were again incubated at 37°C. Efflux buffer was removed and replaced with fresh medium at 1 min intervals. After 3 min, the Cl- secretagogue, forskolin (10−5 M) was added to stimulate Cl- efflux. Additional buffer samples were removed and replaced at the subsequent 1 min intervals. At the end of the experiment, the 36Cl remaining in the cells was extracted with 1 ml of 0.1 N HCl overnight at 4°C. Samples were added to 4 ml scintillation cocktail and counted.
The % efflux per one min timepoint was calculated as follows: % efflux/min = cpm for sample/total cpm available at this time.
Transepithelial Cl- current measurements
Stock cultures of cells were trypsinized, resuspended in fresh MEM, and counted on a particle counter (Coulter Z Series; Coulter Inc., Miami, Fl). The 16HBE14o- cells were labeled with green fluorescent protein (GFP) using an adenovirus (Ad5-GFP, University of Iowa, 100 MOI), added to CFBE41o-cells at increasing ratios (0.01%, 0.1%, 1%, 10%, respectively), and then plated on 12 mm Costar Snapwell inserts (Corning, Lowell, MA) in triplicate. Approximately 105 cells total were plated onto each insert. Mixed cultures were grown for 6-8 days and inserts were mounted onto Easy Mount Ussing chambers (Physiologic Instruments, San Diego, CA). Transepithelial short circuit current (Isc) was measured using a voltage clamp (VCC MC6, Physiologic Instruments, Can Diego, CA) with Ag/AgCl electrodes (World Precision Instruments, Sarasota, Florida) connected to the chamber solutions through 3% agar bridges containing 1 M KCl. A basolateral-to-apical Cl- gradient was established to increase the electrochemical driving force for Cl- secretion across the apical membrane. The basolateral solution was composed of: (in mM; 120 NaCl, 25 NaHCO3, 5 KCl, 1.2 NaH2PO4, 5.6 glucose, 1.0 CaCl2, and 1.2 MgCl2), while the apical Cl--free solution was composed of: (in mM; 120 Na-gluconate, 20 NaHCO3, 5 KHCO3, 1.2 NaH2PO4, 5.6 glucose, 2.5 Ca(gluconate)2, and 1.2 MgSO4). Both chamber compartments were separately perfused with 5 ml of each solution at 37°C, respectively, and gassed with 5% CO2 in air to give a pH of 7.4. Positive currents were defined as the movement of anions in the basolateral-to-apical direction. Transepithelial Isc measured under these conditions is indicated as ICl and was recorded at 5 Hz by an analog-to-digital board (DATAQ Instruments, Inc. Akron, OH) interfaced with a computer.
Fluorescence microscopy
Mixed monolayer cultures of CFBE41o- and 16HBE14o-were grown on Snapwell filters and evaluated ∼2 h prior to Ussing chamber analysis on an inverted fluorescence microscope (Olympus IMT-2) equipped with a Lambda LS xenon arc lamp (Sutter Instruments, Novato, CA), and a cooled charge-coupled device camera (Photometrics Coolsnap HQ, Roper Scientific) that is controlled by a computerized image acquisition system (Metafluor version 6.0, Universal Imaging Corp.). Samples were observed with a 10x objective and excited at 470 nm. Fluorescence was collected at >515 nm. The number of GFP-expressing cells within the mixed cell populations, i.e., non-fluorescing CFBE41o-cells mixed with GFP-expressing 16HBE14o-cells was estimated from the pixel count above background and relative to the count observed from monolayers of pure GFP-expressing 16HBE14o- cells. Image analysis was carried out using ImageJ (version 1.43u, http://rsb.info.nih.gov/ij).
Chemicals
Unless otherwise specified, reagents and chemicals were obtained from Sigma (St. Louis, MO). Genistein and CFTR-inh172 were prepared as a 20 mM stock solution in DMSO. The adenylate cyclase activator forskolin (Calbiochem, La Jolla, CA) was prepared in DMSO as a 20 mM stock solution and used at a final concentration of 20 μM; 1-Ethyl-2-benzimidazolinone (EBIO) (Aldrich Chemical Company, Inc, Milwaukee, WI) was prepared as a 1 M stock solution in DMSO and used at a final concentration of 1 mM.
Results
36Cl efflux measurements
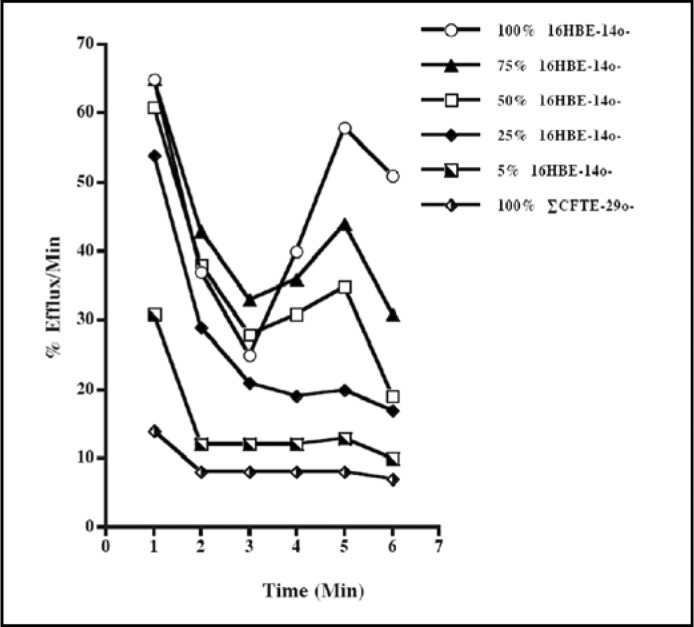
The different mixed cell populations were assayed at 1 min time intervals. Forskolin (10−5 M) was added 3 min after the initiation of the experiment. There was a progressive increase in the amount of 36Cl released from the cells in response to forskolin stimulation that appeared to be directly correlated with the proportion of wild-type cells within the population (Fig. 1). When the fraction of cells expressing the wild-type CFTR was ≤ 25%, the assay was unable to detect any cAMP-dependent Cl- transport. Previous studies showed that the 16HBE14o- cells were able to secrete Cl- in response to forskolin stimulation [3] and that the CFTE29o-cells were defective in cAMP-dependent Cl- transport [20, 25]. The results presented here are consistent with those findings.
Fig. 1.
A representative example of the changes in the rate of Cl- efflux following stimulation with the cAMP agonist, forskolin. Mixed populations of cells were plated in 35 mm dishes, incubated in the presence of 36Cl-, and then assayed for cAMP-dependent 36Cl- efflux. The rate of 36Cl- efflux was measured a 1 min intervals. Forskolin (10−5 M) was added 3 min after the beginning of the experiment as indicated by the arrow. The proportion of normal, 16HBE14o-, cells within the population is indicated at the right. Error bars indicate ± SEM. Each data point is the mean of 3 independent measurements, i.e., n = 3.
Transepithelial CFTR Cl current measurements
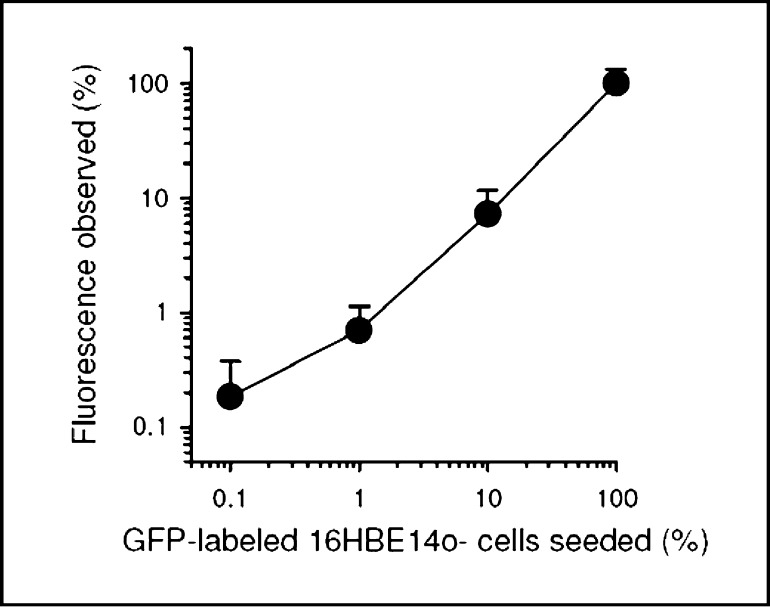
Mixed cultures of CFBE41o- and 16HBE14o- cells were obtained by serial dilutions from a stock of lxlO5 GFP-labeled 16HBE14o- cells. Confluent monolayers were sequentially exposed to forskolin (2×10−5 M; serosal), EBIO (10−3 M; serosal), and genistein (10−5 M, apical) to stimulate maximum CFTR Cl- transport. The CFTR blocker, CFTR(inh)-172 (2×10−5M, mucosal), was used to identify and quantify the magnitude of CFTR-mediated Cl- currents in each monolayer. The original traces of transepithelial Cl- currents in pure CFBE41o- monolayers (0% 16HBE14o-), and mixed cultures of CFBE41o- cells containing 0.1% 16HBE14o-, 1% 16HBE14o-, and 10% 16HBE14o-, and pure 16HBE41o- monolayers (0% CFBE41o-) are shown in Fig. 2 A-E, respectively. The relative proportion of wtCFTR expressing, GFP-labeled 16HBE14o- cells can be visualized by GFP fluorescence (Fig. 2 F-J). As a basis for comparison, visual light images of the cells in the fluorescent field (Fig. 2 K-O) are adjacent to the GFP images. The fluorescence obtained from mixed monolayers is shown as a function of the GFP-labeled 16HBE14o- cells seeded (Fig. 3). The fluorescence increased as a function of increasing numbers of GFP-labeled cells that were seeded. The slope of the graph is close to unity (0.92±0.09; n=2), thereby indicating that the two cells lines used in the Ussing chamber analyses can be quantitatively mixed and without any appreciable change in the relative proportion of each cell line within the mixed cultures during the culture period prior to the measurement of short circuit current.
Fig. 2.
Ussing chamber analysis of CFTR Cl- transport in mixed monolayer cultures of CFBE41o-/16HBE41o-cells. Original short-circuit current traces (A-E) of transepithelial Cl- currents (ICl) in (A) pure cultures of CFBE410- monolayers (100% CFBE41o-/0% 16HBE14o-), mixed cultures of CFBE41o-/16HBE14o- cells with increasing numbers (B) 0.1%, (C) 1%, and (D) 10%, of GFP-labeled 16HBE14o- cells, and (E) pure cultures of 16HBE140- monolayers (100% 16HBE14o-/0% CFBE41o-). CFTR Cl currents were step-wise stimulated by sequential additions of the CFTR Cl channel openers forskolin, 1-ethyl-benzimidazolinone (EBIO), and genistein. The CFTR blocker CFTR(inh)-172 was used to quantify CFTR-mediated ICl. Even though different y-axis scales are used (A-C vs D and E), a small (∼0.4 μA/cm2) inhibitory effect of CFTR(inh)-172 was detected, suggesting residual ΔF508-CFTR activity in 100% CFBE41o- cultures. Corresponding live cell GFP fluorescent images (F-J) of pure and mixed cell populations provide visual evidence of the relative proportion of GFP-labeled, wtCFTR expressing 16HBE14o- cells within each population; (F) 100% CFBE41o-cells, 16HBE14o-/CFBE41o-cultures containing (G) 0.1%, (H) 1%, (I) 10% GFP-labeled 16HBE14o- cells, and (J) 100% 16HBE14o- cells. (K-O) Bright-field images of corresponding monolayers. (magnification: x100).
Fig. 3.
Analysis of GFP-fluorescence observed in mixed CFBE410- and 16HBE14o-monolayers. Fluorescence was quantified in images shown in Fig. 2F-J and plotted as a function of the percentage of the GFP-labeled 16HBE14o- cells seeded. Observed fluorescence above background is expressed as the fluorescence relative to that obtained from monolayers of pure 16HBE140- cells (=100%) expressing GFP. Note log-log scaling. Correlation is significant, p=0.001, r=0.99, slope=0.92±0.09.
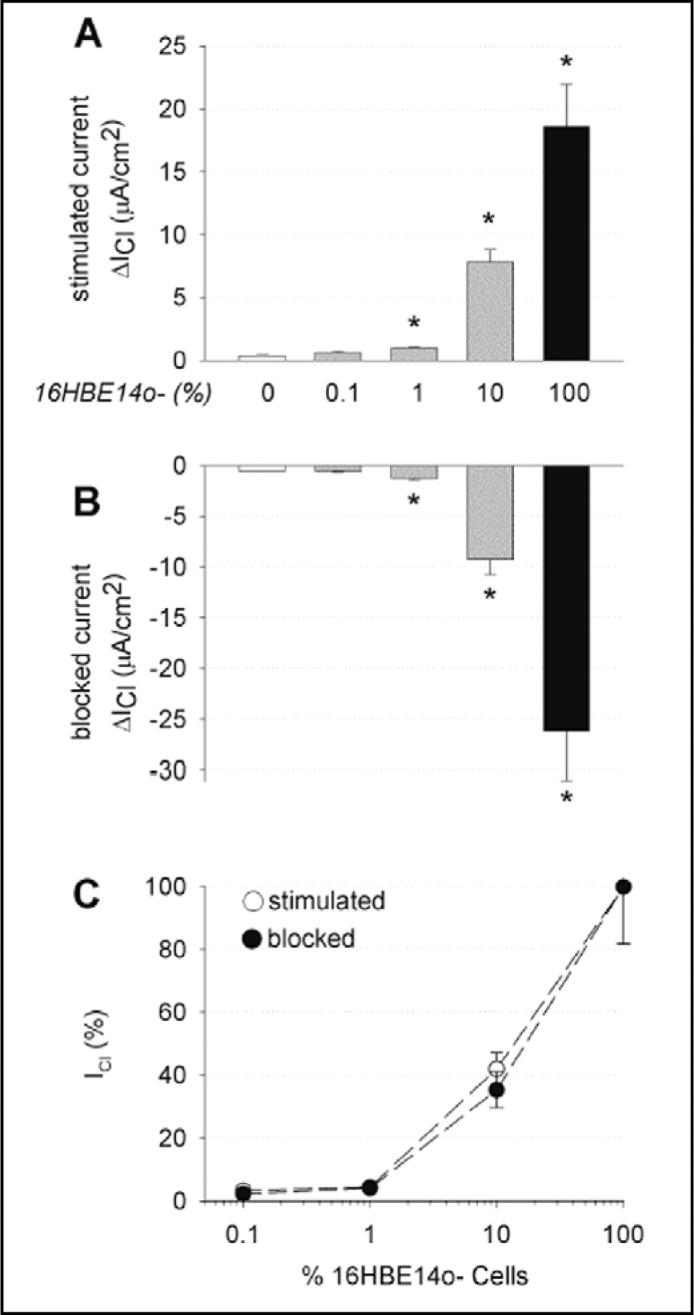
Changes in transepithelial Cl current (ΔIC1) after stimulation with a cocktail of the CFTR transport enhancers forskolin, EBIO, and genistein (Fig. 4A) and subsequent inhibition with the CFTR blocker CFTR(inh)-172 (Fig. 4B) were significantly increased in mixed CFBE41o- monolayers containing 1% and 10% 16HBE41o- cells. These studies suggest that the addition of 1,000-10,000 cells, respectively, expressing native wtCFTR, to 105 CFBE410- cells displayed detectable CFTR Cl- currents with the Ussing chamber assay and are summarized in Fig. 4C. Lower numbers of cells expressing native wtCFTR (10 cells/cm2 (data not shown), or 100 cells/cm2, Fig. 4B), when added to the CFBE41 o-cells, did not result in significant current changes. These data and the concomitant changes in transepithelial resistance (Rt) at baseline, after stimulation and inhibition are summarized in Table 1.
Fig. 4.
CFTR Cl- currents in polarized monolayer mixtures of CFBE41o-/16HBE14o-. Changes in transepithelial Cl- current (ΔICl) upon: (A) stimulation with a cocktail of CFTR transport enhancers (forskolin, 2×10−5 M; EBIO, 10−3M; genistein, 10−5 M), and (B) inhibition with the CFTR blocker CFTR (inh)-172 (2×10−5 M, mucosal). The portion of normal, 16HBE14o-, cells within the population is indicated. Stimulated and blocked CFTR Cl- currents were significantly increased in mixed monolayers containing 1%, 10%, and 100% 16HBE14o- cells, respectively. (C) Graphic representation of stimulated and CFTR(inh)-172-sensitive ICl of CF cultures with varying proportions of non-CF bronchial epithelial cells. Data are presented as a percentage of the changes in ICl measured in cultures containing 100% 16HBE140- cells. Note that mixed CF monolayers with ∼10% normal cells generated ∼35-40% of the ICl observed for monolayers containing 100% normal CFTR cells. Data are expressed as mean values ± SEM; n = 2-5 experiments per group.
Table 1.
Transepithelial Cl Current and Resistance in mixed CFBE41o-/16HBE14o- cultures. Legend: The table shows the transepithelial resistance (Rt) and short-circuit Cl current (Icl) values before and after stimulation with forskolin (20 μM), EBIO (1 mM) and genistein (10 μM), and after inhibition with CFTR-inhl72 (20 μM). The changes in Icl after stimulation (ΔIstim) and inhibition (ΔIinh) are also indicated. Values are expressed as the mean ± standard error of the mean (SEM); n = the # of measurements.
| Percent 16HBE14o- | Before Stimulation | After Stimulation | After Inhibition | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Icl (μA/cm2) | Rt (Ω cm2) | Icl (μA/cm2) | Rt (Ω cm2) | Icl (μA/cm2) | Rt (Ω cm2) | ΔIstim (μA/cm2) | ΔIinh (μA/cm2) | n | |
| 0% | 13.4±1.6 | 609±44 | 13.8±1.6 | 607±78 | 13.2±1.6 | 590±95 | 0.4±0.0 | −0.6±0.0 | 2 |
| 0.1% | 5.1±0.5 | 1211±84 | 5.8±0.5 | 1240±83 | 5.2±0.4 | 1176±49 | 0.6±0.1 | −0.7±0.1 | 3 |
| 1% | 5.6±0.7 | 1320±116 | 6.4±0.5 | 1335±160 | 5.3±0.7 | 1347±176 | 1.0±0.1 | −1.3±0.2 | 2 |
| 10% | 35.9±21.3 | 343±203 | 43.7±22.3 | 326±185 | 34.5±20.8 | 344±202 | 7.8±1.0 | −9.2±1.5 | 2 |
| 100% | 84.7±10.4 | 96±14 | 103.3±9.0 | 91±11 | 77.1±9.4 | 100±14 | 18.6±3.4 | −26.2±4.8 | 5 |
Discussion
The cAMP-dependent Cl- ion transport defect is one of the most well defined biochemical feature characterizing CF. Correction of this Cl- transport defect in CF epithelial cells is an integral component of CF therapy, whether it is genetic, pharmacological, and/or cellular [12, 15, 28-31]. Therefore, an effective means to measure changes in this biological endpoint will be an important factor in determining the extent and duration of correction in tissue and cells. Among the assays currently in use for the measurement of Cl- ion transport, the efflux of 36Cl has a particular appeal. However, there has been little work done to determine the sensitivity of this assay. The results presented here indicate that this assay has some limitations for assessing the effectiveness of gene complementation or correction as well as CFTR activation within mixed populations of cells and implies that either the proportion of cells expressing wtCFTR or the level of wtCFTR needs to be at minimal level to be detectable.
The measurements made with the Cl- efflux assay indicated no significant cAMP-dependent Cl- transport when the proportion of wild-type cells within the population was ≤ 25%. This is in conflict with a previous study that showed 20% wtCFTR expressing cells resulted in ∼70% of the Cl- current observed in the wild-type cells alone [16]. On the other hand, transepithelial Cl- current measurements on mixed monolayers indicated detectable cAMP-dependent Cl- currents when the fraction of wild-type cells within the CF population was 1%. Furthermore, transepithelial ICl with 10% wild-type cells resulted in a ICl that was 40-50% of the cAMP-dependent Cl- observed in the wild-type cells (Fig. 3) and is consistent with previous studies assessing transepithelial current in freshly isolated epithelial cells [32] or in CF epithelial cells complemented with a wtCFTR cDNA [16]. Furthermore, there does not appear to be any apparent functional differences in the ion transport characteristics of heterozygotes that carry only one wtCFTR allele [33].
One interesting aspect of the studies presented here is the exponential decrease in Cl- secretion between 10%-1% normal cells. This suggests that the pathways underlying processing and recycling of CFTR may become saturated as the level of wtCFTR approaches 10%. This is also consistent with a recent study that showed normal ion transport properties and normalized IL-8 secretion in mixed cultures containing 90% CF and 10% non-CF human bronchial epithelial cells [34]. While it has been suggested that these observations indicate involvement of gap-junctional communication via connexins [35-37], the underlying mechanism that links wtCFTR function to IL-8 secretion is not known and further investigation will be required to dissect the pathways involved.
The discrepancy between the 36Cl efflux assay and transepithelial ICl could be due to the relative sensitivity of the assays. This difference may also be a reflection of the nature of the monolayers, in that, the lack of polarity in the CFTE29o- cell mixtures might also alter the cell-cell communication networks that influence global Cl- ion transport [35, 36, 38].
Recent studies have also shown a positive correlation between the expression of wtCFTR and the formation of tight junctions [39-41]. It is difficult to assess the effect of wtCFTR in tight junction formation from the studies presented here, since the wild-type 16HBE14o- cell monolayers have a lower baseline Rt than the CFBE41 o-cells (Table 1). This is likely due to the junctional properties characteristic of each cell line at the time of the studies. The apparent increases in Rt when there is 0.1 or 1% 16HBE14o- cells within the mixtures may reflect an increase due to wtCFTR. However, this observation will require more detailed analysis in multiple polar cell systems.
The observations made in this study demonstrate the variability between different assay systems and the data that can be generated. These findings are not only significant for gene or stem cell-based therapies, but also for therapies that rely on the enhancement of the endogenous mutant CFTR expression. Both the number of cells expressing wtCFTR or the level of functional or partially functional CFTR expression on a per cell basis will require more than 36Cl efflux if the amount of functional CFTR is ≤ 25%.
While the results presented in this study do not show the cooperation between adjacent cells as a potential mechanism to account for this far reaching effect, the possibility that the two different cell lines tested are refractory to such interaction cannot be excluded. This may be due, in part, to gap junction interactions and the connexin profiles of the individual cell lines [35, 37].
Gene, stem cell or pharmacological therapy strategies that result in < 25% effective CFTR correction, as has been detected in various adenovirus, adeno-associated virus, and liposome-mediated clinical trials [10, 42-44], as well as gene targeting strategies for CF showing correction in ∼ 1% of cultured cells, will require other electrophysiological assays such as Ussing chamber analysis to determine their effectiveness [11, 25]. This will require that the cells analyzed are able to form and maintain tight junctions and close cell-to cell contact and will be a critical element for the design of pharmacological and cell therapy trials for CF lung disease. In addition, studies will be needed to ascertain whether the overall effectiveness of gene therapy that corrects cAMP-dependent Cl- ion transport will depend on the connexin profiles of the treated cells [35, 37].
Acknowledgements
We would like to thank Luz Feeney and Linda Escobar for their expert technical assistance. This work was supported by NIH grants DK46002, DK46677 (DCG, DL), and HL86323 (HF), a grant from the Cystic Fibrosis Foundation (Illek08G0), as well as grants from Cystic Fibrosis Research, Inc. grant #09-004 (DCG, BI) and Pennsylvania Cystic Fibrosis, Inc (DCG).
References
- 1.Cliff WH, Frizzell RA. Separate Cl-conductances activated by cAMP and Ca2+ in Cl--secreting epithelial cells. Proc Natl Acad Sci USA. 1990;87:4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cozens AL, Yezzi MJ, Chin L, Simon EM, Finkbeiner WE, Wagner JA, Gruenert DC. Characterization of immortal cystic fibrosis tracheobronchial gland epithelial cells. Proc Natl Acad Sci USA. 1992;89:5171–5175. doi: 10.1073/pnas.89.11.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 4.Illsley NP, Verkman AS. Membrane chloride transport measured using a chloride-sensitive fluorescent probe. Biochemistry. 1987;26:1215–1219. doi: 10.1021/bi00379a002. [DOI] [PubMed] [Google Scholar]
- 5.Munkonge F, Alton EW, Andersson C, Davidson H, Dragomir A, Edelman A, Farley R, Hjelte L, McLachlan G, Stern M, Roomans GM. Measurement of halide efflux from cultured and primary airway epithelial cells using fluorescence indicators. J Cyst Fibros. 2004;3:171–176. doi: 10.1016/j.jcf.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 6.Rugolo M, Romeo G, Lenaz G. Kinetic analysis of chloride efflux from normal and cystic fibrosis fibroblasts. Biochem Biophys Res Commun. 1986;134:233–239. doi: 10.1016/0006-291x(86)90552-8. [DOI] [PubMed] [Google Scholar]
- 7.Amaral MD, Kunzelmann K. Molecular targeting of CFTR as a therapeutic approach to cystic fibrosis. Trends Pharmacol Sci. 2007;28:334–341. doi: 10.1016/j.tips.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Buscher R, Grasemann H. Disease modifying genes in cystic fibrosis: Therapeutic option or one-way road? Naunyn Schmiedebergs Arch Pharmacol. 2006;374:65–77. doi: 10.1007/s00210-006-0101-2. [DOI] [PubMed] [Google Scholar]
- 9.Cormet-Boyaka E, Jablonsky M, Naren AP, Jackson PL, Muccio DD, Kirk KL. Rescuing cystic fibrosis transmembrane conductance regulator (CFTR)-processing mutants by transcomplementation. Proc Natl Acad Sci USA. 2004;101:8221–8226. doi: 10.1073/pnas.0400459101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griesenbach U, Alton EW. Gene transfer to the lung: Lessons learned from more than 2 decades of CF gene therapy. Adv Drug Deliv Rev. 2009;61:128–139. doi: 10.1016/j.addr.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Gruenert DC, Bruscia E, Novelli G, Colosimo A, Dallapiccola B, Sangiuolo F, Goncz KK. Sequence-specific modification of genomic DNA by small DNA fragments. J Clin Invest. 2003;112:637–641. doi: 10.1172/JCI19773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreindler JL. Cystic fibrosis: Exploiting its genetic basis in the hunt for new therapies. Pharmacol Ther. 2010;125:219–229. doi: 10.1016/j.pharmthera.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenecker J, Huth S, Rudolph C. Gene therapy for cystic fibrosis lung disease: Current status and future perspectives. Curr Opin Mol Ther. 2006;8:439–445. [PubMed] [Google Scholar]
- 14.Rowe SM, Clancy JP. Pharmaceuticals targeting nonsense mutations in genetic diseases: Progress in development. BioDrugs. 2009;23:165–174. doi: 10.2165/00063030-200923030-00003. [DOI] [PubMed] [Google Scholar]
- 15.Rubenstein RC. Targeted therapy for cystic fibrosis: Cystic fibrosis transmembrane conductance regulator mutation-specific pharmacologic strategies. Mol Diagn Ther. 2006;10:293–301. doi: 10.1007/BF03256204. [DOI] [PubMed] [Google Scholar]
- 16.Farmen SL, Karp PH, Ng P, Palmer DJ, Koehler DR, Hu J, Beaudet AL, Zabner J, Welsh MJ. Gene transfer of CFTR to airway epithelia: Low levels of expression are sufficient to correct Cl- transport and overexpression can generate basolateral CFTR. Am J Physiol Lung Cell Mol Physiol. 2005;289(6):L1123–1130. doi: 10.1152/ajplung.00049.2005. [DOI] [PubMed] [Google Scholar]
- 17.Wagner JA, Cozens AL, Schulman H, Gruenert DC, Stryer L, Gardner P. Activation of chloride channels in normal and cystic fibrosis airway epithelial cells by multifunctional calcium/calmodulin-dependent protein kinase. Nature. 1991;349:793–796. doi: 10.1038/349793a0. [DOI] [PubMed] [Google Scholar]
- 18.Braunstein GM, Zsembery A, Tucker TA, Schwiebert EM. Purinergic signaling underlies CFTR control of human airway epithelial cell volume. J Cyst Fibros. 2004;3:99–117. doi: 10.1016/j.jcf.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Lei DC, Kunzelmann K, Koslowsky T, Yezzi MJ, Escobar LC, Xu Z, Ellison AR, Rommens JM, Tsui LC, Tykocinski M, Gruenert DC. Episomal expression of wild-type CFTR corrects cAMP-dependent chloride transport in respiratory epithelial cells. Gene Ther. 1996;3:427–436. [PubMed] [Google Scholar]
- 20.Kunzelmann K, Schwiebert EM, Zeitlin PL, Kuo WL, Stanton BA, Gruenert DC. An immortalized cystic fibrosis tracheal epithelial cell line homozygous for the delta F508 CFTR mutation. Am J Respir Cell Mol Biol. 1993;8:522–529. doi: 10.1165/ajrcmb/8.5.522. [DOI] [PubMed] [Google Scholar]
- 21.Bruscia E, Sangiuolo F, Sinibaldi P, Goncz KK, Novelli G, Gruenert DC. Isolation of CF cell lines corrected at deltaF508-CFTR locus by SFHR-mediated targeting. Gene Ther. 2002;9:683–685. doi: 10.1038/sj.gt.3301741. [DOI] [PubMed] [Google Scholar]
- 22.Ehrhardt C, Collnot EM, Baldes C, Becker U, Laue M, Kim KJ, Lehr CM. Towards an in vitro model of cystic fibrosis small airway epithelium: Characterisation of the human bronchial epithelial cell line CFBE41o- Cell Tissue Res. 2006;323:405–415. doi: 10.1007/s00441-005-0062-7. [DOI] [PubMed] [Google Scholar]
- 23.Gruenert DC, Willems M, Cassiman JJ, Frizzell RA. Established cell lines used in cystic fibrosis research. J Cyst Fibros. 2004;3:191–196. doi: 10.1016/j.jcf.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 24.Illek B, Maurisse R, Wahler L, Kunzelmann K, Fischer H, Gruenert DC. Cl transport in complemented CF bronchial epithelial cells correlates with CFTR mRNA expression levels. Cell Physiol Biochem. 2008;22:57–68. doi: 10.1159/000149783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunzelmann K, Legendre JY, Knoell DL, Escobar LC, Xu Z, Gruenert DC. Gene targeting of CFTR DNA in CF epithelial cells. Gene Ther. 1996;3:859–867. [PubMed] [Google Scholar]
- 26.Gruenert DC, Basbaum CB, Welsh MJ, Li M, Finkbeiner WE, Nadel JA. Characterization of human tracheal epithelial cells transformed by an origin-defective simian virus 40. Proc Natl Acad Sci USA. 1988;85:5951–5955. doi: 10.1073/pnas.85.16.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gruenert DC, Basbaum CB, Widdicombe JH. Long-term culture of normal and cystic fibrosis epithelial cells grown under serum-free conditions. In Vitro. Cell Dev Biol. 1990;26:411–418. doi: 10.1007/BF02623833. [DOI] [PubMed] [Google Scholar]
- 28.Bruscia EM, Price JE, Cheng EC, Weiner S, Caputo C, Ferreira EC, Egan ME, Krause DS. Assessment of cystic fibrosis transmembrane conductance regulator (CFTR) activity in CFTR-null mice after bone marrow transplantation. Proc Natl Acad Sci USA. 2006;103:2965–2970. doi: 10.1073/pnas.0510758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galietta LV, Jayaraman S, Verkman AS. Cell-based assay for high-throughput quantitative screening of CFTR chloride transport agonists. Am J Physiol Cell Physiol. 2001;281:C1734–1742. doi: 10.1152/ajpcell.2001.281.5.C1734. [DOI] [PubMed] [Google Scholar]
- 30.Lee J, Wen J, Park JY, Kim SA, Lee EJ, Song SY. Reversal of diabetes in rats using GLP-1-expressing adult pancreatic ductlike precursor cells transformed from acinar to ductal cells. Stem Cells Dev. 2009;18:991–1002. doi: 10.1089/scd.2008.0107. [DOI] [PubMed] [Google Scholar]
- 31.Zhang LN, Karp P, Gerard CJ, Pastor E, Laux D, Munson K, Yan Z, Liu X, Godwin S, Thomas CP, Zabner J, Shi H, Caldwell CW, Peluso R, Carter B, Engelhardt JF. Dual therapeutic utility of proteasome modulating agents for pharmaco-gene therapy of the cystic fibrosis airway. Mol Ther. 2004;10:990–1002. doi: 10.1016/j.ymthe.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Johnson LG, Olsen JC, Sarkadi B, Moore KL, Swanstrom R, Boucher RC. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat Genet. 1992;2:21–25. doi: 10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- 33.Johnson JP, Louie E, Lewiston NJ, Wine JJ. Beta-adrenergic sweat responses in cystic fibrosis heterozygotes with and without the deltaF508 allele. Pediatr Res. 1991;29:525–528. doi: 10.1203/00006450-199106010-00001. [DOI] [PubMed] [Google Scholar]
- 34.Dannhoffer L, Blouquit-Laye S, Regnier A, Chinet T. Functional properties of mixed cystic fibrosis and normal bronchial epithelial cell cultures. Am J Respir Cell Mol Biol. 2009;40:717–723. doi: 10.1165/rcmb.2008-0018OC. [DOI] [PubMed] [Google Scholar]
- 35.Chanson M, Berclaz PY, Scerri I, Dudez T, Wernke-Dollries K, Pizurki L, Pavirani A, Fiedler MA, Suter S. Regulation of gap junctional communication by a pro-inflammatory cytokine in cystic fibrosis transmembrane conductance regulator-expressing but not cystic fibrosis airway cells. Am J Pathol. 2001;158:1775–1784. doi: 10.1016/S0002-9440(10)64133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chanson M, Kotsias BA, Peracchia C, O'Grady SM. Interactions of connexins with other membrane channels and transporters. Prog Biophys Mol Biol. 2007;94:233–244. doi: 10.1016/j.pbiomolbio.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang S, Jornot L, Wiszniewski L, Rochat T, Suter S, Lacroix JS, Chanson M. Src signaling links mediators of inflammation to CX43 gap junction channels in primary and transformed CFTR-expressing airway cells. Cell Commun Adhes. 2003;10:279–285. doi: 10.1080/cac.10.4-6.279.285. [DOI] [PubMed] [Google Scholar]
- 38.Kotsias BA, Salim M, Peracchia LL, Peracchia C. Interplay between cystic fibrosis transmembrane regulator and gap junction channels made of connexins 45, 40, 32 and 50 expressed in oocytes. J Membr Biol. 2006;214:1–8. doi: 10.1007/s00232-006-0064-8. [DOI] [PubMed] [Google Scholar]
- 39.Baldursson O. Regulating the barrier function of airway epithelia. A novel role for CFTR – does it make a difference this time? J Physiol. 2010;588:1385. doi: 10.1113/jphysiol.2010.189894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LeSimple P, Liao J, Robert R, Gruenert DC, Hanrahan JW. Cystic fibrosis transmembrane conductance regulator trafficking modulates the barrier function of airway epithelial cell monolayers. J Physiol. 2010;588:1195–1209. doi: 10.1113/jphysiol.2009.182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nilsson HE, Dragomir A, Lazorova L, Johannesson M, Roomans GM. CFTR and tight junctions in cultured bronchial epithelial cells. Exp Mol Pathol. 2010;88:118–127. doi: 10.1016/j.yexmp.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Griesenbach U, Ferrari S, Geddes DM, Alton EW. Gene therapy progress and prospects: Cystic fibrosis. Gene Ther. 2002;9:1344–1350. doi: 10.1038/sj.gt.3301791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griesenbach U, Geddes DM, Alton EW. Update on gene therapy for cystic fibrosis. Curr Opin Mol Ther. 2003;5:489–494. [PubMed] [Google Scholar]
- 44.Caplen NJ, Alton EW, Middleton PG, Dorin JR, Stevenson BJ, Gao X, Durham SR, Jeffery PK, Hodson ME, Coutelle C, et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med. 1995;1:39–46. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]