Abstract
Background/Aims: The aim of this study was to determine if VSMC ASIC-like currents are regulated by oxidative state. Methods: We used whole-cell patch clamp of isolated mouse cerebral VSMCs to determine if 1) reducing agents, such as DTT and GSH, and 2) inhibition of endogenous oxidase activity from NADPH and Xanthine oxidases potentiate active currents and activate electrically silent currents. Results: Pretreatment with 2 mM DTT or GSH, increased the mean peak amplitude of ASIC-like currents evoked by pH 6.0 from 0.4 ± 0.1 to 14.9 ± 3.6 pA/pF, and from 0.9 ± 0.3 to 11.3 ± 2.4 pA/pF, respectively. Pretreatment with apocynin, a NADPH oxidase inhibitor, mimics the effect of the reducing agents, with the mean peak current amplitude increased from 0.9 ± 0.5 to 7.0 ± 2.6 pA/pF and from 0.5 ± 0.2 to 26.4 ± 6.8 pA/pF by 50 and 200 μM apocynin, respectively. Pretreatment with allopurinol, a xanthine oxidase inhibitor, also potentiates the VSMC ASIC-like activity. Conclusion: These findings suggest that VSMC ASIC-like channels are regulated by oxidative state and may be inhibited by basal endogenous oxidative sources such as NADPH and xanthine oxidase.
Key Words: Extracellular acid gated currents, Degenerin, Vascular smooth muscle, Oxidation-reduction
Introduction
Acid-sensing ion channels (ASICs) are members of the degenerin (DEG)/Epithelial Na+ channel (ENaC) ion channel family [1-3]. Gated by extracellular [H+], ASIC channels are permeable to cations (Na+>Ca2+>K+), and are sensitive to amiloride inhibition. So far, at least 4 ASIC genes (ASIC1, ASIC2, ASIC3, and ASIC4), with 2 splice variants (a, b) for ASIC1 and ASIC2 have been identified. ASIC proteins are predominantly expressed in neurons, glia and sensory epithelia, and may contribute to nociception, acid sensation, learning, proprioception and mechanosensation. Recent studies demonstrate that ASIC proteins are also expressed in vascular smooth muscle cells (VSMCs), and may play a role in regulating vascular tone and chemotactic migration during wound healing [4-9].
In a previous study, we showed ASIC-like channels in cerebral VSMCs tend to be electrically silent although the underlying reason is unclear [10]. Recent reports suggest ASIC currents are modulated by oxidation-reduction; reducing agents potentiate and oxidizing agents inhibit certain ASIC currents [11-13]. Since oxidative potential in VSMCs is high due to nicotine amide dinucleotide phosphate (NADPH) oxidases (NOXs) and xanthine oxidase (XOs) activity, we hypothesized that VSMC ASIC-like currents are suppressed in vitro by endogenous oxidation produced by NOX and XO [14-16]. To address this hypothesis, we first determined if reducing agents dithiothreitol (DTT) and glutathione (GSH) potentiate cerebral VSMC ASIC-like currents. We then determined if inhibition of NOX or XO potentiate ASIC-like currents. Our data suggest that VSMC ASIC-like channels are electrically silent due to oxidative stress.
Materials and Methods
All protocols and procedures used in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Preparation of VSMCs
C57BL6 mice (6–12 wk of age, Jackson Laboratory, Bar Harbor, ME) were anesthetized with isoflurane and immediately decapitated. Cerebral arteries were dissected from the brain and collected into an ice-cold cell isolation solution containing (in mM) 140NaC1, 5KCl, 0.15 CaCl2, 1MgCl2, 10HEPES, and 5.5 D-glucose, pH 7.4. To isolate VSMCs appropriate for evaluating the effect of reducing agents, we modified our previous cell isolation method by replacing papain, which requires the reducing agent DTT for activation, with protease Type XIV [10]. Briefly, cells were first incubated with 1 mg/ml Protease Type XIV (Sigma-Aldrich, St. Louis, MO) and 2 mg/ml Collagenase Type IV (Worthington) for 5 min, followed by 2 mg/ml Collagenase Type IV for 5 min at room temperature. VSMCs were washed using ice-cold cell isolation solution, and single VSMCs were released by gently triturating the digested vessels with a fire-polished Pasteur pipette. Isolated VSMCs were maintained in low Ca2+ external solution at 4°C and used within 12 h after isolation.
Patch-clamp recording
For patch-clamp recording, single, elongated VSMCs in low Ca2+ external solution were plated onto the coverslip of the recording chamber and co-incubated with collagenase (Type IV, 1 mg/ml) for an additional 10-15 min at room temperature. Following cell attachment, the recording chamber was slowly perfused with normal Ca2+ external solution (0.2 ml/min, for 15-20 min) to gradually raise Ca2+ concentration to 1.5 mM in the chamber. The entire recording chamber was then continuously perfused with the normal external solution at about 2 ml/min throughout the experiments. Cells were used within 2 h of plating.
Membrane current was recorded using conventional whole cell patch-clamp techniques with an Axopatch 200B amplifier (Axon Instruments) interfaced with a PC through Digidata 1440A digitizer (Axon Instruments) as previously described (10). Data were sampled at 5 kHz and filtered at 1 kHz using a low-pass Bessel filter. pCLAMP10.0 (Axon Instruments) was used for data acquisition. Patch pipettes were pulled from FLG15 filamentous glass capillaries (Prism, Dagan) using a DMZ-Universal Puller (Zeitz Instruments). The resistances of patch pipettes used ranged from 3.0 to 6.0 MΩ. Fast capacitance transients were compensated before forming the whole cell configuration. Series resistances were below 20 MΩ and were not compensated.
Command voltage for the voltage-clamp mode was −40 mV. The external and internal solutions were designed to create a Cl– Nernst potential (EC1) close to the command voltage to minimize the interference from the spontaneous activity of Ca2+-activated Cl– currents [10]. The internal (pipette) solution contained (in mM) 106 K-gluconate, 25 KCl, 4 NaCl, 1 MgCl2,20 MOPS, 0.1 EGTA, 4 ATP-Na2, and 0.05 GTP-Tris, with pH adjusted to 7.2 using KOH. The external bathing solution contained the same ingredients as aforementioned cell isolation solution except Ca2+ was increased to 1.5 mM. In the acidic external solution (pH 6.0), HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] was replaced with MES [2-(N-morpholino)ethanesulfonic acid]. Targeted VSMCs were perfused locally using a DAD-12 superfusion system (ALA Scientific Instruments) with control or treatment solutions continuously delivered through a 100 μm quartz capillary. The tip of the capillary was positioned within 300 μm from the targeted cell.
Oxidation-reduction status on ASIC-like currents
After forming whole-cell patch configuration, cells were equilibrated for 3-5 min before being exposed to the first control stimulus of extracellular [H+] (pH 6.0 for 15 sec). Cells were then washed with the pH 7.4, 1.5 mM Ca2+ external solution for 30 sec. Under these external Ca2+ conditions, H+ evoked currents are small, ∼1-2 pA/pF. To determine the effect of reducing agents on ASIC-like currents, cells were treated with dithiothreitol (DTT, 0.5 and 2 mM) or reduced L-Glutathione (GSH, 0.5 and 2 mM) for 5-6 minutes before the second stimulus of pH 6.0. To determine if the effect of DTT is attributed to its reducing power, cells were treated with the oxidizing agent 5,5′Dithio-Bis(2-Nitrobenzoic Acid) (DTNB, 1 mM) to reverse the action of DTT (1 mM). Cells were preincubated with the ASIC blocker amiloride (100 μM, Sigma Chemicals) for 45s to confirm DTT potentiated currents were generated by ASIC-like channels. To determine the importance of endogenous oxidase activity on ASIC activity, VSMCs were pretreated with NOX inhibitor apocynin (50, 200 μM), and xanthine oxidase inhibitor allopurinol (50, 200 μM) for 5-6 min before the second stimulus of pH 6.0. Since a limited number of reports suggest apocynin may not be specific for NOX, to further examine the role of NOX, we used Rac1 inhibitor NSC23766 (100 μM, Tocris, Ellisville, MO) [17, 18]. For these experiments, NSC23766 was included in the pipette solution, and its effect on ASIC-like currents was evaluated in VSMCs exposed to 1 mM DTT for 1 min. To determine recovery, VSMCs were washed for 5 min with pH 7.4 normal external solution, and then challenged again with extracellular [H+] (pH 6.0) solution to evoke ASIC-like currents. Except where noted, all reagents were obtained from Sigma Chemicals (St. Louis, MO). All stock solutions were prepared fresh in external bathing solution (pH 7.4), except for allopurinol and amiloride, which were prepared in DMSO (100 mM).
Data analysis
Patch-clamp data were analyzed off-line using Clampfit 10 (Axon Instruments) and SigmaStat (SPSS Inc). Currents were normalized to membrane capacitance of each cell. In neurons or heterologous systems, a typical ASIC current consists of an initial fast transient that is rapidly inactivated and usually transitions smoothly into a smaller, sustained component of current. However, in VSMCs, the sustained component takes on a more stochastic appearance, presumably due to the relatively lower density or activity of the channels. To further characterize the inactivation feature of these channels, we analyzed the accumulated current, or area under curve (AUC) of the current, in addition to the peak current. All data are presented as means ± SE, and n is the number of cells examined. Data were compared using paired t-test, ANOVA, or repeated-measures ANO VA with Student-Newman-Keuls post hoc test where appropriate. Statistical difference was considered significant at P < 0.05.
Results
Reducing agents potentiate acid-induced currents in freshly isolated VSMCs
We demonstrated previously that ASIC-like currents could be evoked in freshly isolated cerebral VSMCs by extracellular [H+][10]. However, under normal external Ca2+, the current amplitudes are low and the percentage of cells responsive small. Thus, the currents appear to be electrically silent. Since reducing agents have been reported to potentiate ASIC currents both in neurons and
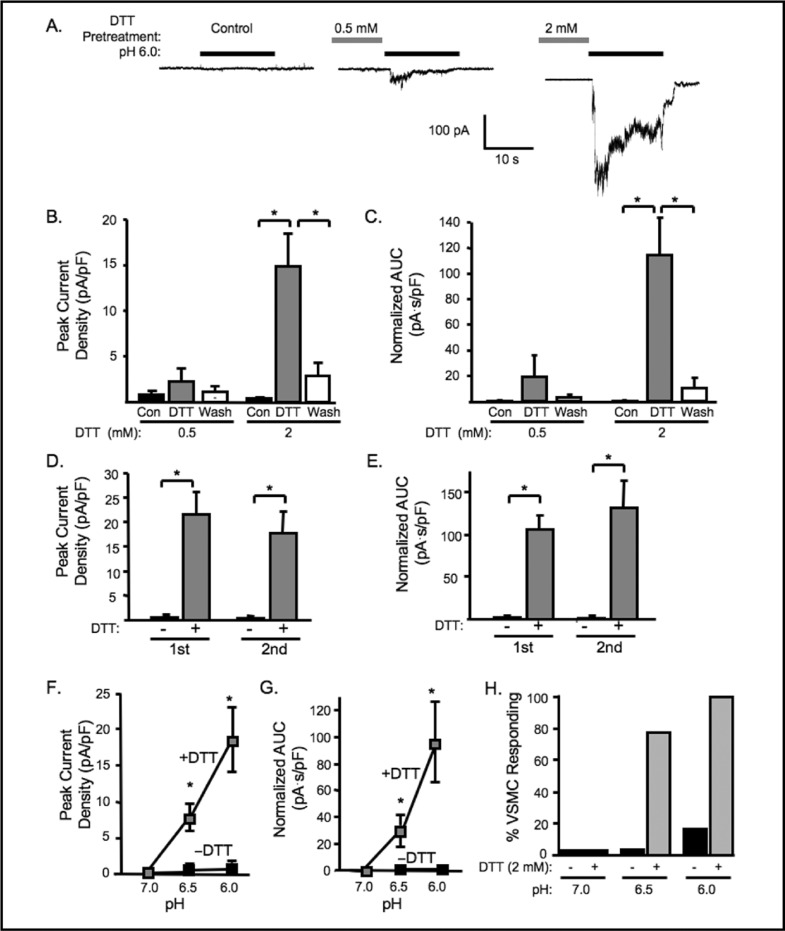
Redox Regulation of Cerebral VSMC ASIC-like Currents heterologous expression systems, we determined if reducing agents could potentiate ASIC-like currents in VSMCs [11-13]. As shown in Fig. 1,5-6 min pretreatment with DTT either potentiated the extracellular [FT] currents or turned non-responsive cells into responsive ones. Representative traces of cells pretreated with 2 mM DTT are shown in Fig. 1A. Pretreatment with 0.5 mM DTT increased the percentage of responding VSMCs from12.5 to 37.5%, but did not significantly increase the mean peak amplitude of currents evoked by pH 6.0 (from 0.8 ± 0.4, to 2.3 ± 1.4 pA/pF, n=8, Fig. IB). In contrast, 2 mM DTT increased the percentage of responding VSMCs from 0 to 100% and the mean peak amplitude of extracellular [FT] gated currents from 0.4 ± 0.1 to 14.9 ± 3.6 pA/pF (n=7) (Fig. IB, right). The accumulated current (area under curve, AUC) during pH 6.0 exposure was also significantly increased (Fig. 1C). The effect of DTT potentiation is reversible (Fig. 1B, C) and reproducible within 10 −15 minutes (Fig. 1D, E). To determine if DTT might alter the [H+] threshold for activation, in a small group of VSMCs, we tested whether or not DTT could enhance currents at lower extracellular [H+] (pH 6.5 and 7.0). We found that DTT pretreatment tended to enhance the magnitude and probability of response to pH 6.5, but not pH 7.0 (n = 4-8) (Fig. 1F, G and H). In fact, our findings suggest a reducing environment of some degree is required for [H+] gating of VSMC ASIC-like channels.
Fig. 1.
DTT pretreatment potentiates acid-induced currents. A. Representative traces of currents evoked by extracellular [H+] (pH 6.0) in VSMCs before DTT pretreatment (control), and after 5-6 min of 0.5 and 2 mM DTT pretreatment. Group data of the effect of DTT (0.5 or 2 mM) pretreatment on the normalized peak amplitude (B) and accumulated currents (C, area under curve, AUC) induced by extracellular [H+], pH 6.0 (n=8 and 7, respectively). Reproducibility of the potentiation effect of DTT (2 mM) on the same cell after wash-off (10-15 min). Peak current (D) and AUC (E) are not different between first and second DTT exposure (n=5). Effect of DTT (2 mM) on pH dose response for peak current (F), AUC (G), and percent responding VSMCs (H) (n=4-8). ∗Significantly different, p<0.05.
Fig. 5.
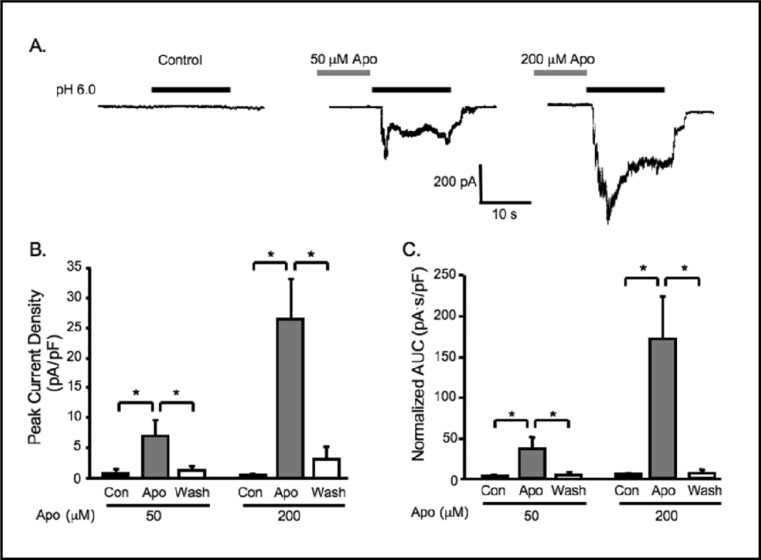
Inhibition of NOX with apocynin (Apo) potentiates extracellular [H+] gated currents. A. Representative traces of currents evoked by extracellular [H+] (pH 6.0) in VSMCs before Apo pretreatment (control, con), and after 5-6 min of 50 or 200 mM Apo pretreatment. Group data of the effect of Apo (50, or 200 mM) pretreatment on normalized peak current amplitude (B) and accumulated currents (C, area under curve, AUC) induced by extracellular [H+] (n=5-14). ∗Significantly different, p<0.05.
Fig. 6.
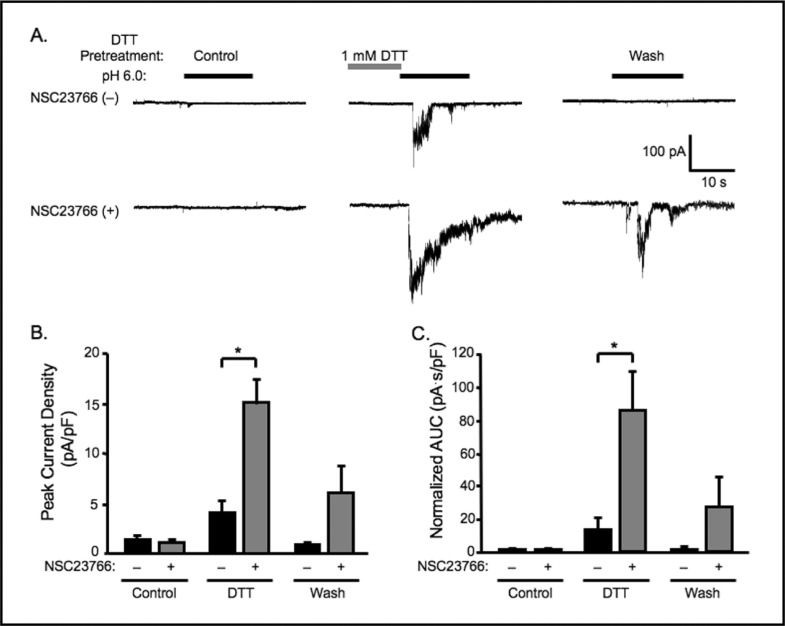
Rac 1 inhibitor NSC23766 (100 μM) potentiates the effect of DTT (1 mM) on extracellular [H+] gated currents. A. Representative traces of currents evoked by extracellular [H+] (pH 6.0) in VSMCs before DTT pretreatment (control, con), right after 1 min of DTT pretreatment, and after 5 min wash-off of DTT, with (+) or without (-) NSC23766 in the pipette solution. Group data of the effect of 1 mM DTT pretreatment for 1 min on normalized peak amplitude (B) and accumulated currents (C, area under curve, AUC) induced by extracellular [H+] without NSC23766, as compared to those with (+) NSC23766 in the pipette solution. (n=11 − 12). ∗Significantly different, p<0.05.
The oxidizing agent DTNB reverses effect of DTT
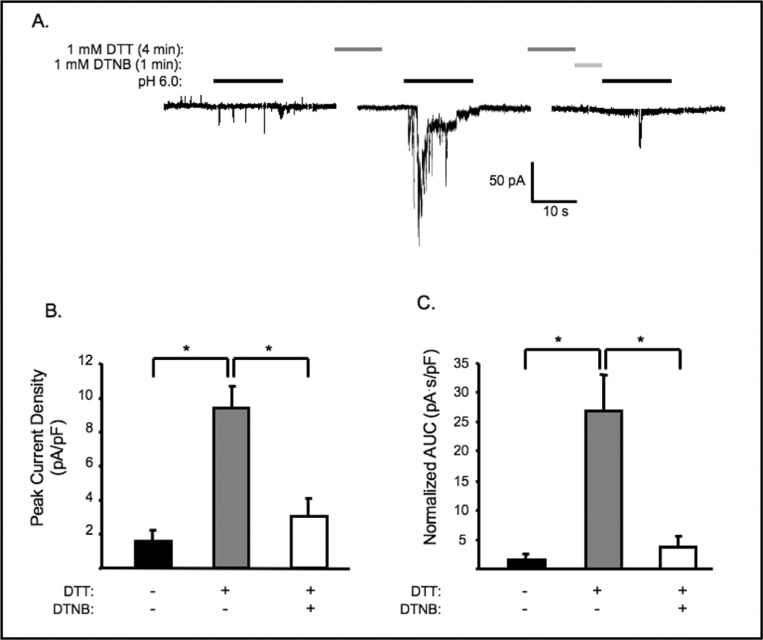
To determine if the potentiation effect of DTT is attributed to its reducing power, we examined the effect of DTNB, an oxidizing agent, on the DTT potentiated currents. Cells pretreated with 1 mM of DTT for 4 min were subsequently washed with normal external solution with or without 1 mM DTNB for another minute before exposure to pH 6.0. As shown in Fig. 2A and B, the potentiated currents were significantly decreased by DTNB, indicating that the effect of DTT may be reversed by an oxidizing agent. This finding suggests that the potentiation effect of DTT results from its reducing power, and that current activity of the channel may depend on the redox status of the cell.
Fig. 2.
DTT potentiation is reversed by oxidizing agent DTNB. A. Representative traces of currents evoked by extracellular [H+] (pH 6.0) in VSMCs before and after DTT pretreatment and following sequential treatment with 1 mM DTT and 1 mM DTNB (time bar not drawn to scale). Group data of the effect of DTT followed by DTNB pretreatment on the normalized peak amplitude (B) and accumulated currents (C, area under curve, AUC) induced by extracellular [H+], (n= 6, 7 and 7, respectively). ∗Significantly different, p<0.05.
ASIC channel blockade inhibits DTT potentiated currents
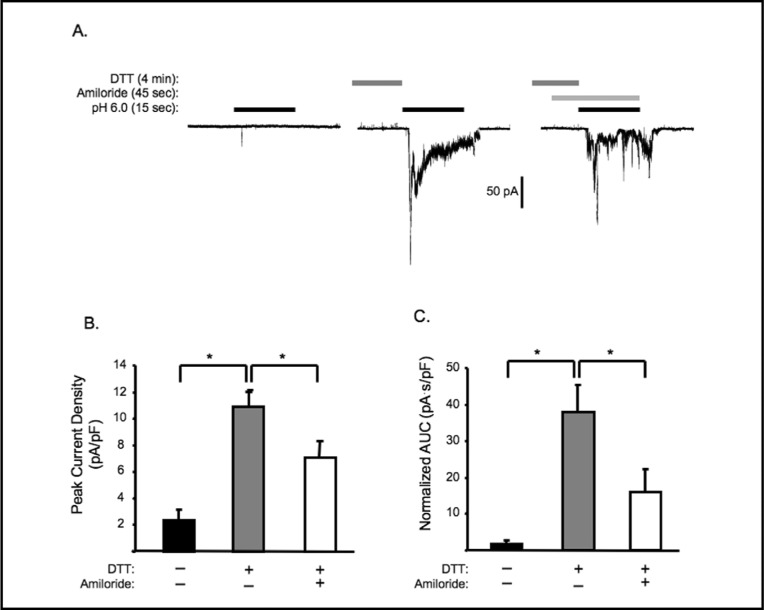
To demonstrate the DTT potentiated currents are mediated by ASIC-like channels, we examined the effect of the broad spectrum ASIC inhibitor amiloride (100 μM) on the currents. As shown in Fig. 3, amiloride significantly decreaseded the peak and AUC of DTT potentiated currents, suggesting that these currents are susceptible to amiloride inhibition.
Fig. 3.
The ASIC channel blocker amiloride inhibits extracellular [H+] evoked currents potentiated by DTT. A. Representative traces. VSMCs pretreated with 1mM DTT for 4 min were exposed to normal external solution ± 100 μM amiloride for 45 sec before exposure to extracellular [H+] (pH 6.0). Group data of the effect of amiloride on DTT potentiation of extracellular [H+] evoked currents on the normalized peak amplitude (B) and accumulated currents (C, area under curve AUC), (n= 7-9 cells). Note that time references are not drawn to scale. ∗Significantly different, p<0.05.
Endogenous reducing agent GSH potentiates acid-induced currents
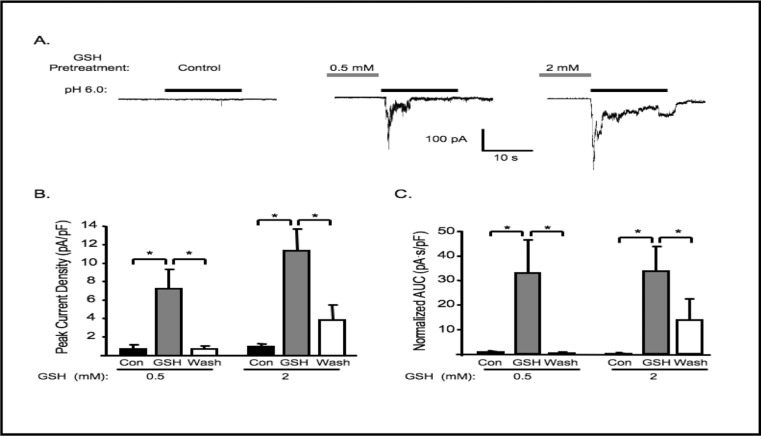
To determine if similar potentiation effects can be observed with intrinsic reducing agents, we used reduced glutathione (GSH, 0.5 and 2 mM) in place of DTT and the same pretreatment protocol. Pretreatment with 0.5 or 2 mM GSH significantly increased the mean peak amplitude and AUC of extracellular [H+] gated currents (Fig. 4). Effects of GSH are also reversible.
Fig. 4.
GSH pretreatment potentiates acid-induced currents. A. Representative traces of currents evoked by extracellular [H+] (pH 6.0) in VSMCs before GSH pretreatment (control), and after 5-6 min GSH 0.5 and 2 mM pretreatment (n=9 and 10, respectively). Group data of the peak amplitude (B), and normalized accumulated current (C, area under curve, AUC) induced by extracellular [H+]. ∗Significantly different, p<0.05.
Inhibition of NADPH and Xanthine oxidase potentiates acid-induced currents VSMCs
Since VSMCs are reported to have high levels of oxidase enzyme activity, attributed to NOX and XO, we considered the possibility that endogenous oxidase activity suppresses ASIC–like currents in VSMCs. To address this possibility, we pretreated VSMCs with the NOX inhibitor apocynin (50 and 200 μM, Fig. 5) using the same protocol as was used in our studies with DTT and GSH. Representative traces are shown in Fig. 5A. Inhibition of NOX with 50 or 200 μM apocynin significantly potentiated extracellular [H+] gated currents (from 0.9 ± 0.5 to 7.0 ± 2.6 pA/pF, n=14, and from 0.5 ± 0.2 to 26.4 ± 6.8 pA/pF, n=11, respectively) and increased the percentage of responding cells from 7 to 57% and 0 to 100%, respectively.
To further support a role for NOX we evaluated the effect of Rac1 inhibition. Rac1 is a component of the NOX complex. Interaction of Rac1 with guanine nucleotide exchange factor (GEF) is required for NOX activation that can be blocked with NSC23766 [19-22]. The effect of Rac1 inhibition on extracellular [H+] gated currents is shown in Fig. 6. Representative traces are shown in Panel 6A and group data in 6B and 6C. NSC23766 alone (in the pipette) did not potentiate extracellular [H+] gated currents, however, it did potentiate the effect of 1 mM DTT (from 4.0 ± 1.3 to 15.0 ± 2.6 pA/pF for peak current, and from 13.1 ± 6.7 to 84.8 ± 24.4 pA.s/pF, n=11-12, for AUC) (Fig. 6B, C). With NSC23766, the percentage of DTT treated cells responding to extracellular [H+] also increased from 55 (n=11) to 92 (n=12) %. These findings provide further support for the importance of endogenous NOX activity in silencing ASIC-like currents in cerebral VSMCs.
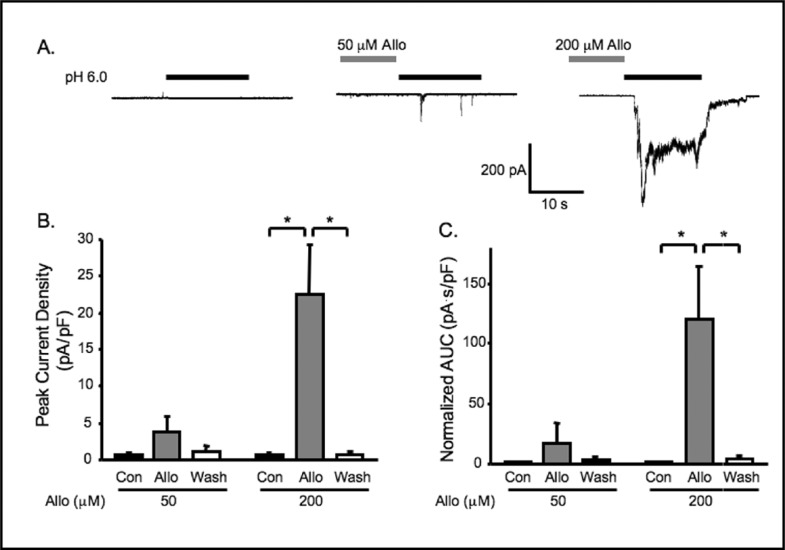
Although NOX is a major oxidase enzyme in VSMCs, XO is also reported to be an important source for vascular oxidative stress [15, 23, 24]. To determine the importance of XO on ASIC-like channel activity, we treated cerebral VSMCs with allopurinol, an inhibitor of XO (50, 200 μM, Fig. 7). Representative traces are shown in Fig. 7A. Exposure to 50 μM allopurinol did not significantly increase extracellular [H+] gated currents (from 0.6 ± 0.3 to 3.7 ± 2.3 pA/pF, n=6); however, it increased the percentage of responding cells from 0 to 50%. With the higher concentration, allopurinol (200 μM) significantly increased extracellular [H+] gated currents from 0.6 ± 0.4 to 22.6 ± 6.8 pA/pF (n=6), and increased the percentage of responding VSMCs from 0 to 100% (Fig 7B). These findings suggest that activity of endogenous XO may also suppress ASIC like channels in cerebral VSMCs.
Fig. 7.
Inhibition of xanthine oxidase using allopurinol (Alio) potentiates extracellular [H+] gated currents. A. Representative traces of currents evoked by extracellular [H+] (pH 6.0) in VSMCs before Alio pretreatment (control, con), and after 5-6 min of 50 or 200 mM Alio pretreatment. Group data of the effect of 5 min Alio (50, 200 mM) pretreatment on normalized peak amplitude (B) and normalized accumulated currents (C, area under curve, AUC) induced by extracellular [H+] (n=5-6). ∗ Significantly different, p<0.05.
Discussion
ASIC channels are a family of inward cation channels that are gated by extracellular [H+]. The channels were first identified in neurons and sensory epithelia. ASIC channels may play a role in learning and neuronal injury following ischemia [2, 3, 25]. We recently demonstrated that ASIC-like channels are also present in cerebral VSMCs. The role of VSMC ASIC channels is unclear; however, they may play a role in pressure-induced constriction and VSMC migration [4-9, 14]. In a previous study, we demonstrated that most VSMC ASIC-like currents are electrically silent [10]. The goal of the current study was to understand possible mechanism(s) that might lead to the reduced activity of VSMC ASIC-like channels.
Reducing agents potentiate ASIC-like currents in cerebral VSMCs
Previous studies demonstrate reducing agents enhance and oxidative agents inhibit ASIC currents in sensory neurons, hippocampal neurons and heterologous expression systems [11-13]. Since VSMCs are known to have high levels of oxidative potential, we considered the possibility that the oxidation-reduction status might contribute to the suppression of ASIC-like currents in VSMCs. To address this possibility, we evaluated the effect of reducing agents (DTT and GSH) and inhibition of oxidative enzymes (NADPH and xanthine oxidase) on ASIC-like channel activity in freshly isolated mouse cerebral VSMCs. Consistent with previous reports, we found reducing agents not only potentiated [H+] gated currents, but also increased the probability of eliciting the currents in cerebral VSMCs. The potentiating effect of the reducing agents was reversed by the oxidizing agent DTNB, suggesting that the [H+] gated current activity is dependent on the redox status of the cell.
The [H+] gated currents reported in this study share several characteristics with previous reports. First, the currents are evoked by a rapid change in extracellular [H+]. Second, the response pattern of a rapid transient followed by a smaller sustained current resembles known ASIC currents and ASIC-like currents in VSMCs. Third, the magnitude of potentiated current in VSMCs is also pH dependent. Although the extracellular [H+] threshold needed to gate the current is similar to our previous report, at each given extracellular [H+] above threshold, the magnitude of the currents is larger in the presence of DTT [10]. For example, the potentiated current magnitude evoked at pH 6.0 in this study is similar to those evoked at pH 5.0 in our previous report. This apparent shift in pH-current curve suggests the redox status of the cell may affect the affinity of VSMC ASIC channels for H+. Fourth, the currents are inhibited by the broad spectrum
ASIC inhibitor amiloride. Thus, the features and behavior of [H+] gated currents in the current study findings are consistent with previous findings [10-13].
Although message and protein for ASIC1a, ASIC1b, ASIC2a, ASIC2b, and ASIC3 are present in cerebral VSMCs, the molecular identity of the VSMC ASIC-like channel is not clear [10]. Similar to sensory neurons, VSMC ASIC-like channels may contain different populations of channels where the ASIC channels may be present as heteromultimers [26]. Although one report suggests reducing agent potentiation of ASIC currents is mediated by ASIC1a, another report demonstrates homomeric channels formed by ASIC1a, ASIC1b, ASIC2a or ASIC3 are sensitive to reducing agents [12, 13]. Thus, our present understanding of ASIC channel sensitivity to reducing agents does not provide insight into the molecular identity of VSMC ASIC-like current.
Possible action of reducing agents on ASIC-like channel
The membrane-impermeable nature of GSH and DTNB suggests that the potential site(s) for redox modulation may be located in the extracellular domain [12]. There are several cysteine residues in the extracellular domain of ASIC channels that are likely targets of the reducing agents. However, an intracellular effect on ASIC trafficking or gating cannot be ruled out since it was demonstrated that the reducing power of GSH may be transduced across the plasma membrane via a thio/disulfide exchange mechanism [27-29]. Although our data do not provide further insight into the potential site of action of the reducing agents on ASIC-like channels, our data demonstrate that a reducing environment is required to for the channels to be operational in VSMCs.
In the current study, we found lower concentrations of membrane impermeable GSH had a greater potentiation of ASIC-like currents than membrane permeable DTT. Although DTT carries two thiols per molecule and GSH only carries one, the stronger reducing power of DTT did not translate into higher capability in potentiating ASIC-like activity. The disproportion between the reducing power and current potentiation suggests that the underlying mechanism may not be simply stochastic, chemical reduction, but may also implicate the involvement of enzymes that use GSH as a cofactor, such as glutaredoxins or thioltransferases [30]. Since GSH is present in cerebral extracellular fluid at 1-2 mM [31, 32], levels comparable to the concentrations used in the current study, it raises the possibility that ASIC-like channels in cerebral VSMCs may be active under native conditions. However, the rapid desensitization of these channels to extracellular [H+] will make it technically challenging to determine the contribution of ASIC-like channels to vascular function in-vitro.
Interestingly, we found DTT altered amiloride sensitivity of the [H+] gated currents. In the current study, 100 μM amiloride significantly inhibits the extracellular [H+]-evoked currents potentiated by DTT by ∼40%, however, in our previous study, 20 μM amiloride inhibited extracellular [H+] evoked currents by ∼80%. Thus, amiloride appears less effective in the presence of DTT. There are several possible explanations. One possibility is that reducing agents may decrease the affinity of amiloride for all VSMC ASIC channels, regardless of molecular identity. Another possibility is that reducing agents may potentate a specific population of channels that happen to have a lower affinity for amiloride [33, 34]. Other studies have not reported the effect of reducing agents on amiloride sensitivity.
Endogenous oxidase activity suppresses ASIC-like activity in cerebral VSMCs
While reducing agents potentiate ASIC currents, oxidizing agents, such as H2O2 and DTNB, inhibit ASIC currents [11-13]. In this study, we demonstrate that the potentiation effect of DTT can be reversed by DTNB. Furthermore, we also demonstrate that inhibition of endogenous oxidases significantly enhanced the frequency of observing extracellular [H+] gated currents and the current amplitude.
Since cerebral vessels express several NOX isoforms and have high levels of NOX activity, we first examined the importance of NOX on cerebral VSMC ASIC-like channel activity using abroad spectrum NOX inhibitor, apocynin [35-37]. Apocynin increased the amplitude and probability of eliciting [H+] gated currents in cerebral VSMCs. Although apocynin is a popular inhibitor of NOX, a few reports suggest it may act as a non-specific antioxidant or inhibit Rho kinase [17, 18]. To provide further evidence of a role for NOX in regulating ASIC-like activity in VSMCs, we evaluated the effect of the Rac1 inhibitor NSC23766 (Fig. 4). In our study, NSC23766 alone failed to potentiate extracellular [H+] gated currents (control condition in Fig. 4B, C). However, NSC23766 did enhance DTT potentiation of ASIC-like currents. Our findings suggest that inhibition of Rac1 alone is not sufficient to achieve a critical level of reduction potential needed to unmask ASIC-like channel activity. Taken together, these findings suggest endogenous NOX
activity may contribute to the low amplitude and probability of ASIC-like currents in cerebral VSMCs.
XO is another major source of oxidative stress in VSMCs [15, 16, 23]. To determine if endogenous XO activity also contributes to the suppression of ASIC-like currents in cerebral VSMCs, we evaluated the effect of allopurinol, a XO inhibitor, on extracellular [H+] gated currents. XO inhibition also potentiates ASIC-like current in VSMCs. This finding suggests that at least two sources of endogenous oxidative stress (NOX and XO) may contribute to the suppression of ASIC-like activity in VSMCs.
In the current study, we demonstrate reducing agents unmask electrically silent ASIC-like currents in isolated cerebral VSMCs. Furthermore, endogenous NOX and XO activities contribute to the suppression of ASIC-like currents. Since increased oxidative stress has been implicated in hypertension, our findings raise the possibility that suppression of vascular ASIC channel activity may play a role in hypertension. Further studies are required to understand the importance of VSMC ASIC-like channels in hypertension.
Acknowlegements
The authors would like to thank Rumbidayzi Chiposi and John Michael Barnard for their technical assistance. This work was supported by NIH HL86996 and HL 51971.
References
- 1.Benos DJ, Stanton BA. Functional domains within the degenerin/epithelial sodium channel (Deg/ENaC) superfamily of ion channels. J Physiol. 1999;520:631–644. doi: 10.1111/j.1469-7793.1999.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 3.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Drummond HA, Grifoni SC, Jernigan NL. A new trick for an old dogma: ENaC proteins as mechanotransducers in vascular smooth muscle. Physiology. 2008;23:23–31. doi: 10.1152/physiol.00034.2007. [DOI] [PubMed] [Google Scholar]
- 5.Drummond HA, Jernigan NL, Grifoni SC. Sensing tension: epithelial sodium channel/acid-sensing ion channel proteins in cardiovascular homeostasis. Hypertension. 2008;51:1265–1271. doi: 10.1161/HYPERTENSIONAHA.107.093401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gannon KP, Vanlandingham LG, Jernigan NL, Grifoni SC, Hamilton G, Drummond HA. Impaired pressure-induced constriction in mouse middle cerebral arteries of ASIC2 knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H1793–1803. doi: 10.1152/ajpheart.01380.2007. [DOI] [PubMed] [Google Scholar]
- 7.Grifoni SC, Jernigan NL, Hamilton G, Drummond HA. ASIC proteins regulate smooth muscle cell migration. Microvasc Res. 2008;75:202–210. doi: 10.1016/j.mvr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grifoni SC, McKey SE, Drummond HA. Hsc70 regulates cell surface ASIC2 expression and vascular smooth muscle cell migration. Am J Physiol Heart Circ Physiol. 2008;294:H2022–2030. doi: 10.1152/ajpheart.01271.2007. [DOI] [PubMed] [Google Scholar]
- 9.Jernigan NL, Paffett ML, Walker BR, Resta TC. ASIC1 contributes to pulmonary vascular smooth muscle store-operated Ca(2+) entry. Am J Physiol Lung Cell Mol Physiol. 2009;297:L271–285. doi: 10.1152/ajplung.00020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung WS, Farley JM, Swenson A, Barnard JM, Hamilton G, Chiposi R, Drummond HA. Extracellular acidosis activates ASIC-like channels in freshly isolated cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2010;298:C1198–1208. doi: 10.1152/ajpcell.00511.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrey F, Tsintsadze T, Volkova T, Lozovaya N, Krishtal O. Acid sensing ionic channels: modulation by redox reagents. Biochim Biophys Acta. 2005;1745:1–6. doi: 10.1016/j.bbamcr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Chu XP, Close N, Saugstad JA, Xiong ZG. ASIC1a-specific modulation of acid-sensing ion channels in mouse cortical neurons by redox reagents. J Neurosci. 2006;26:5329–39. doi: 10.1523/JNEUROSCI.0938-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho JH, Askwith CC. Potentiation of acid-sensing ion channels by sulfhydryl compounds. Am J Physiol Cell Physiol. 2007;292:C2161–2174. doi: 10.1152/ajpcell.00598.2006. [DOI] [PubMed] [Google Scholar]
- 14.Selemidis S, Sobey CG, Wingler K, Schmidt HH, Drummond GR. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther. 2008;120:254–291. doi: 10.1016/j.pharmthera.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Wassmann S, Wassmann K, Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension. 2004;44:381–386. doi: 10.1161/01.HYP.0000142232.29764.a7. [DOI] [PubMed] [Google Scholar]
- 16.Wolin MS. Reactive oxygen species and the control of vascular function. Am J Physiol Heart Circ Physiol. 2009;296:H539–549. doi: 10.1152/ajpheart.01167.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 18.Schluter T, Steinbach AC, Steffen A, Rettig R, Grisk O. Apocynin-induced vasodilation involves Rho kinase inhibition but not NADPH oxidase inhibition. Cardiovasc Res. 2008;80:271–279. doi: 10.1093/cvr/cvn185. [DOI] [PubMed] [Google Scholar]
- 19.Nassar N, Cancelas J, Zheng J, Williams DA, Zheng Y. Structure-function based design of small molecule inhibitors targeting Rho family GTPases. Curr Top Med Chem. 2006;6:1109–1116. doi: 10.2174/156802606777812095. [DOI] [PubMed] [Google Scholar]
- 20.Takemura Y, Goodson P, Bao HF, Jain L, Helms MN. Rac1-mediated NADPH oxidase release of O2-regulates epithelial sodium channel activity in the alveolar epithelium. Am J Physiol Lung Cell Mol Physiol. 2010;298:L509–520. doi: 10.1152/ajplung.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whaley-Connell AT, Morris EM, Rehmer N, Yaghoubian JC, Wei Y, Hayden MR, Habibi J, Stump CS, Sowers JR. Albumin activation of NAD(P)H oxidase activity is mediated via Rac1 in proximal tubule cells. Am J Nephrol. 2007;27:15–23. doi: 10.1159/000098432. [DOI] [PubMed] [Google Scholar]
- 22.Moldovan L, Mythreye K, Goldschmidt-Clermont PJ, Satterwhite LL. Reactive oxygen species in vascular endothelial cell motility. Roles of NAD(P)H oxidase and Rac1. Cardiovasc Res. 2006;71:236–246. doi: 10.1016/j.cardiores.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Forstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med. 2008;5:338–349. doi: 10.1038/ncpcardio1211. [DOI] [PubMed] [Google Scholar]
- 24.Rathaus M, Bernheim J. Oxygen species in the microvascular environment: regulation of vascular tone and the development of hypertension. Nephrol Dial Transplant. 2002;17:216–221. doi: 10.1093/ndt/17.2.216. [DOI] [PubMed] [Google Scholar]
- 25.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leyssac PP, Holstein-Rathlou NH. Effects of various transport inhibitors on oscillating TGF pressure responses in the rat. Pflugers Arch. 1986;407:285–291. doi: 10.1007/BF00585304. [DOI] [PubMed] [Google Scholar]
- 28.Fernandes AP, Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 29.Zha XM, Wang R, Collier DM, Snyder PM, Wemmie JA, Welsh MJ. Oxidant regulated inter-subunit disulfide bond formation between ASIC1a subunits. Proc Natl Acad Sci USA. 2009;106:3573–3578. doi: 10.1073/pnas.0813402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciriolo MR, Paci M, Sette M, De Martino A, Bozzi A, Rotilio G. Transduction of reducing power across the plasma membrane by reduced glutathione. A 1HNMR spin-echo study of intact human erythrocytes. Eur J Biochem. 1993;215:711–718. doi: 10.1111/j.1432-1033.1993.tb18083.x. [DOI] [PubMed] [Google Scholar]
- 31.Slivka A, Spina MB, Cohen G. Reduced and oxidized glutathione in human and monkey brain. Neurosci Lett. 1987;74:112–118. doi: 10.1016/0304-3940(87)90061-9. [DOI] [PubMed] [Google Scholar]
- 32.Nanda D, Tolputt J, Collard KJ. Changes in brain glutathione levels during postnatal development in the rat. Brain Res Dev Brain Res. 1996;94:238–241. doi: 10.1016/0165-3806(96)00061-2. [DOI] [PubMed] [Google Scholar]
- 33.Ugawa S, Yamamoto T, Ueda T, Ishida Y, Inagaki A, Nishigaki M, Shimada S. Amiloride-insensitive currents of the acid-sensing ion channel-2a (ASIC2a)/ ASIC2b heteromeric sour-taste receptor channel. J Neurosci. 2003;23:3616–3622. doi: 10.1523/JNEUROSCI.23-09-03616.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babinski K, Catarsi S, Biagini G, Seguela P. Mammalian ASIC2a and ASIC3 subunits co-assemble into heteromeric proton-gated channels sensitive to Gd3+ J Biol Chem. 2000;275:28519–28525. doi: 10.1074/jbc.M004114200. [DOI] [PubMed] [Google Scholar]
- 35.Miller AA, Drummond GR, De Silva TM, Mast AE, Hickey H, Williams JP, Broughton BR, Sobey CG. NADPH oxidase activity is higher in cerebral versus systemic arteries of four animal species: role of Nox2. Am J Physiol Heart Circ Physiol. 2009;296:H220–225. doi: 10.1152/ajpheart.00987.2008. [DOI] [PubMed] [Google Scholar]
- 36.Miller AA, Drummond GR, Schmidt HH, Sobey CG. NADPH oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ Res. 2005;97:1055–1062. doi: 10.1161/01.RES.0000189301.10217.87. [DOI] [PubMed] [Google Scholar]
- 37.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]