Abstract
Background/Aims: The mechanism underlying extracellular adenosine-induced caspase-independent apoptosis in HuH-7 human hepatoma cells is not fully understood. The present study investigated the role for apoptosis-inducing factor (AIF)-homologous mitochondrion-associated inducer of death (AMID) in the pathway. Methods: To see the implication of AMID in adenosine-induced HuH-7 cell apoptosis, real-time reverse transcription-polymerase chain reaction (RT-PCR), immunofluorescent cytochemistry, time-laps GFP monitoring, cell cycle analysis, flow cytometry, Western blotting, cell viability assay, and TUNEL staining were carried out. Results: Adenosine upregulated AMID expression in HuH-7 cells, and translocated AMID from the cytosol into the nucleus. Adenosine induced HuH-7 cell apoptosis, and the effect was further enhanced by overexpressing AMID. Adenosine-induced HuH-7 cell apoptosis, alternatively, was inhibited by knocking-down AMID. Conclusion: The results of the present study provide evidence for AMID as a critical factor for adenosine-induced caspase-independent HuH-7 cell apoptosis.
Key Words: Adenosine, AMID, Caspase-independent, Apoptosis, HuH-7 cell
Introduction
Apoptotic cell death is initiated in a caspase-dependent and -independent manner. Death receptors and the mitochondria engage extrinsic and intrinsic caspase activation, respectively. In contrast, AIF, a phylogenetically conserved flavoprotein within the mitochondrial membrane, participates in caspase-independent apoptosis [1]. In response to lethal signals, AIF is released from the mitochondria, translocated into the nucleus, and binds to the nuclear DNA, thereby causing chromosomal condensation, margination, and large-scale DNA fragmentation (approximately 50-kb fragments) [2, 3]. The AIF homologue AMID, that is designated p53-responsive gene 3, is identified as a human pro-apoptotic protein [4-9]. The AMID mRNA levels for tumor cells are lower than the levels for normal cells, suggesting AMID as a tumor suppressor [10]. AMID, that is noncovalently and stoichiometrically associated with 6-hydroxy-FAD, serves as an NADPH-dependent oxidoreductase. AMID is preferentially localized in the outer mitochondrial membrane or the cytoplasm [7-9]. AMID is capable of binding DNAs, without any specific DNA sequence for its binding, and therefore, AMID could also induce DNA fragmentation, i.e., apoptosis, if the protein is translocated into the nucleus [6, 9].
Adenosine, a metabolite of ATP, that is abundantly present inside and outside cells, induces apoptosis in a variety of cancer cells via extrinsic and intrinsic diverse signaling pathways [11-17]. For extrinsic pathways, A2 adenosine receptors linked to Gs protein involving adenylate cyclase activation execute apoptosis in glioma cells, myeloid leukemia cells, mammary carcinoma cells, and colonic cancer cells [17-20]. A3 adenosine receptors are implicated in apoptosis in human lung cancer cells, breast cancer cells, hepatocellular carcinoma cells, and thyroid cancer cells [21-24]. For intrinsic pathways, intracellularly transported adenosine through adenosine transporters induces apoptosis in GT3-TKB human lung cancer cells by activating AMP-activated protein kinase [12]. Adenosine, alternatively, induces apoptosis in HepG2 hepatoma cells by regulating apoptosis-mediator gene transcription, to activate caspase-3, −8, and −9 [15]. In our earlier studies, adenosine induced apoptosis in HuH-7 human hepatoma cells by downregulating expression of Fas-associated death domain protein (FADD)-like interleukin-1β-converting enzyme inhibitory protein (FLIP), causing activation of caspase-8 and the effector caspase-3 [14] or by tuning Bcl-XL/DIABLO/apoptosis protein (IAP) expression, causing activation of caspase-9 followed by caspase-3 [16]. Amazingly, adenosine-induced HuH-7 cell apoptosis was not completely inhibited by a pan-caspase inhibitor [14], suggesting that the adenosine action is not limited to caspase activation. We show here that AMID participates in adenosine-induced caspase-independent HuH-7 cell apoptosis.
Materials and Methods
Cell culture
HuH-7 cells were obtained from RIKEN cell bank (Ibaraki, Japan). Cells were cultured in Dulbecco's Modified Eagles Medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum, penicillin (final concentration, 100 U/ml), and streptomycin (final concentration, 0.1 mg/ml), in a humidified atmosphere of 5% CO2 and 95% air at 37°C.
Coomassie Brilliant Blue staining
HuH-7 cells were lysed in a lysis buffer [50 mM Tris–HCl, 150 mM NaCl, 0.1% (w/v) sodium deoxycholate, 0.1% (v/v) Triton X-100, and protease inhibitor cocktail, pH 7.4] after 4-h treatment with and without adenosine (10 mM). Then, lysates were loaded on 10% (v/v) sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and separated proteins were stained with Coomassie Brilliant Blue.
Plasmid construction and transfection
Nucleotide sequence coding for AMID was cloned into pEGFP-Cl vector (Clontech) at the XhoI and EcoRI site. The plasmid DNA for AMID was transfected into HuH-7 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) or an electroporation system (Optimizor500, BTX, San Diego, USA).
AMID siRNA transfection
The siRNA silencing the AMID-targeted gene, that was purchased from Cosmo Bio (Kyoto, Japan), was transfected using a Nucleofector II device (Amaxa Biosystems, Cologne, Germany).
Real-time RT-PCR
Total cellular RNA from HuH-7 cells was prepared using a Sepasol-RNA I Super kit. The first-strand cDNAs were synthesized using a High-Capacity cDNA Archive Kit. Each cDNA (2 μl) was amplified in a SYBR Green Realtime PCR Master Mix (final volume, 20 μl) and loaded on the Applied Biosystems 7900 Real-time PCR Detection System (ABI, Foster City, USA). Thermal cycling conditions were as follows: the first one step, 94°C for 4 min and the ensuing 40 cycles, 94°C for 1 s, 65°C for 15 s, and 72°C for 30 s. A standard curve was made by amplifying 0.5, 1, 2, 4, and 8 μl of the GAPDH cDNA diluted at 1:250. mRNA quantity for each target gene was calculated from the standard curve using an SDS 2.1 Software (Applied Biosystems, Foster City, USA). The levels for the AMID and AIF mRNA were normalized by the GAPDH mRNA. The primers as shown in Table 1 were used for real-time RT-PCR.
Immunofluorescent cytochemistry
HuH-7 cells expressing HA-AMID were treated with adenosine (10 mM) for 4 h. Subsequently, cells were fixed with methanol and acetone at −20°C, each for 10 min, blocked with 10% (v/v) goat serum in phosphate buffered saline (PBS) containing 0.01% (v/v) Triton-X100 for 1 h, and reacted with an anti-HA antibody (1:500 dilution) at 4°C overnight followed by Texas Red conjugated with a goat anti-mouse IgG antibody (1:250 dilution). The mitochondria and the nucleus were stained with MitoTracker Green FM (1:500 dilution) and DAPI, respectively. Cell imaging was observed under a laser scanning microscopes (LSM 510, Carl Zeiss, Germany) equipped with 100x plan objective lens.
Time-laps monitoring for intracellular AMID mobilizations
HuH-7 cells were transfected with the expression vector for GFP alone or GFP-AMID. AMID mobilizations were monitored by detecting GFP signals with a laser scanning microscopy (LSM 510, Carl Zeiss, Germany) equipped with 100x plan objective lens every 5 min before and after application with adenosine (10 mM).
Separation into nuclear and cytosolic components
Cells were suspended in 400 μl of a cold buffer A [10 mM HEPES, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 1% (v/v) protease inhibitor cocktail, pH 7.4] and lysed with 25 μl of 10% (v/v) Nonidet P-40 (Nacalai Tesque, Kyoto, Japan). After 15,000 × g centrifugation at 4°C for 5 min, the pellet was resuspended in 50 ul of a cold buffer B [20 mM HEPES, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1% (v/v) protease inhibitor cocktail, pH7.9] as a nuclear component and the supernatant was collected as a cytosolic component.
Western blotting
Samples were loaded on 10% (v/v) SDS-PAGE and transferred to polyvinylidene difluoride membrane. After blocking with TBST [20 mM Tris, 150 mM NaCl, 0.1% (v/v) Tween-20] containing 5% (v/v) of bovine serum albumin, blotting membrane was reacted with an anti-AMID antibody (Santa Crutz Biotechnology, Santa Cruz, CA, USA) followed by an HRP-conjugated anti-goat IgG antibody. For β-actin detection, blotting membrane was reacted with an anti-β-actin antibody (SIGMA, Missouri, SL, USA) followed by an HRP-conjugated anti-mouse IgG antibody. Immunoreactivity was detected with an ECL kit (GE Healthcare, NJ, USA) and visualized using a chemiluminescence with an Image Gause software (FUJIFILM, Tokyo, Japan).
Cell cycle analysis
HuH-7 cells were transfected with the expression vector for GFP alone or GFP-AMID. Twenty-four hours later, cells were untreated and treated with adenosine (10 mM) for 24 h. Then, cells were harvested by a trypsinization, fixed with 70% (v/v) ethanol at 4°C overnight. Fixed cells were incubated in PBS containing 1.5 μg/ml RNase A for 1 h at 37°C, followed by staining with 5 μl of propidium iodide (PI) for 20 min on ice. Then, cells were collected on a nylon mesh filter (pore size, 40 μm), and cell cycles including the sub-G0 phase (apoptosis) were assayed in 1 × 104 GFP-positive cells with a flowcytometer (FACSCalibur, Becton Dickinson, USA) at an excitation of 488 nm and an emission of 585 nm, and analyzed using a Mod Fit LT software (Verity Software House Inc., Topshan, USA).
Apoptosis assay
HuH-7 cells were transfected with the expression vector for HA alone or HA-AMID followed by G418 selection by the method as previously described [25]. Twenty-four hours after transfection, cells were untreated and treated with adenosine (10 mM) for 24 h and then, harvested by adding trypsin. Cells were resuspended in a binding buffer and stained with both PI and annexin V-FITC, and loaded on a flow cytometer (FACSCalibur, Becton Dickinson, Franklin Lakes, USA) available for FL1 (annexin V) and FL2 (PI) bivariate analyisis. Data from 20,000 cells/sample were collected, and the quadrants were set according to the population of viable, unstained cells in untreated samples. CellQuest analysis of the data was used to calculate the percentage of the cells in the respective quadrants.
3-(4, 5-Dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) assay
Cell viability was evaluated by a dye staining method using MTT as previously described [14]. MTT-reactive cells were quantified at an absorbance of 570 nm using a micro-plate reader (SPECTRAmax PLUS384, Molecular Devices, Sunnyvale, USA), and percentage of independent basal levels (MTT intensities of cells untreated with any drug) was calculated.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay
Cells were fixed with 4% (v/v) paraformaldehyde. After removing inactivate endogenous peroxidase with methanol containing 0.3% (v/v) H2O2, a Permeabilization Buffer was applied to cells and stood on ice for 5 min. Then, a Labeling Reaction Mixture was added and incubated in a humidified chamber at 37°C for 60 min. Reactive cells were stained with 3% (v/v) methyl green and detected with a light microscope.
Statistical analysis
Statistical analysis was carried out using unpaired t-test.
Results
Adenosine upregulates AMID expression in HuH-7 cells
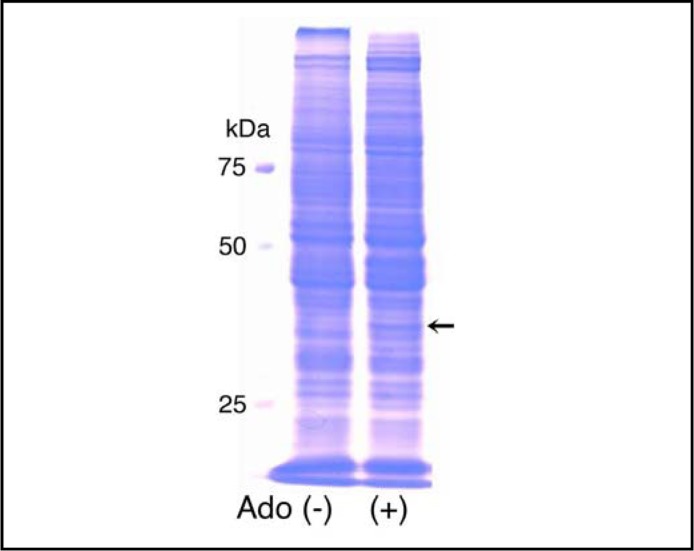
In the SDS-PAGE analysis, 4-h treatment with adenosine (10 mM) produced a new band at 41 kDa (Fig. 1). The protein band was subsequently excised and the amino acid sequence was analyzed with a matrix-assisted laser desorption ionization time of flight mass spectrometry. The amino acid sequence for the protein was well consistent to the sequence for AMID; of total 373 amino acids for AMID the protein contained 98 amino acids identical to AMID (26%).
Fig. 1.
SDS-PAGE. HuH-7 cells were untreated [Ado (+)] and treated with adenosine (10 mM) for 4 h [Ado (+)]. Note a new protein band at 41 kDa after adenosine treatment (arrow).
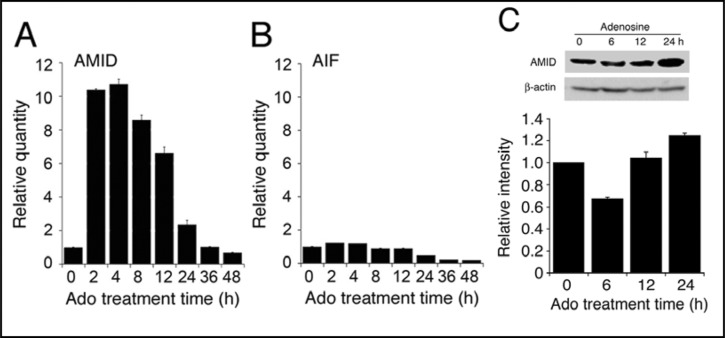
In the real-time RT-PCR analysis, adenosine (10 mM) increased the AMID mRNA in HuH-7 cells in a bell-shaped treatment time (2-48 h)-dependent manner, with a peak at 4-h treatment (Fig. 2A). Adenosine (10 mM) also increased expression of AMID protein at 24-h treatment (Fig. 2C). In contrast, no increase in the AIF mRNA was found with adenosine treatment (Fig. 2B). Taken together, these results indicate that adenosine upregulates AMID expression in HuH-7 cells by tuning the AMID gene transcription. This raises the possibility that AMID might function as a potential effector for adenosine-induced apoptosis.
Fig. 2.
Expression of the AMID mRNA and protein. In the real-time RT-PCR analysis, the mRNAs for AMID (A) and AIF (B) in HuH-7 cells were quantified before and after treatment with adenos-ine (Ado)(10 mM). In the graphs, each column represents the mean (± SEM) ratio against basal levels at 0 h (n=4 independent experiments). (C) Western blotting was carried out using an anti-AMID antibody in cells treated with adenosine (Ado)(10 mM) for periods of time as indicated. Each AMID signal intensity was normalized by β-actin signal intensity. In the graph, each column represents the mean (± SEM) ratio against basal normalized signal intensities at 0 h (n=3 independent experiments).
Adenosine promotes AMID translocation into the nucleus in HuH-7 cells
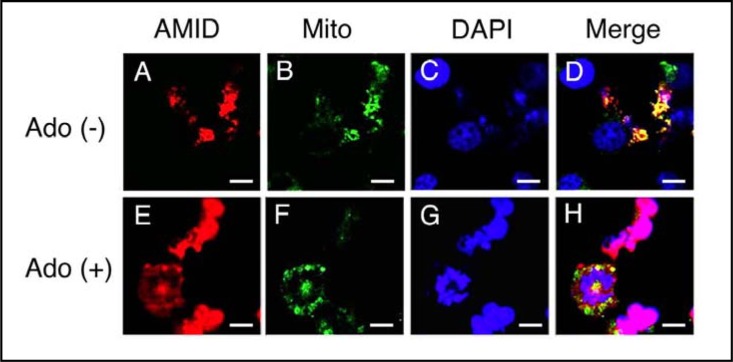
We next monitored intracellular AMID mobilizations in HuH-7 cells. In the immunofluorescent cyotochemical analysis for HuH-7 cells expressing HA-AMID, AMID was distributed in the extra-nuclear region, mainly in the mitochondria, without adenosine treatment (Fig. 3A, B, C, D), but 4-h treatment with adenosine (10 mM) accumulated AMID in the nucleus (Fig. 3E, F, G, H).
Fig. 3.
Immunofluorescent cytochemistry. HuH-7 cells expressing HA-AMID were untreated [Ado (+)] and treated with adenosine (10 mM) for 4 h [Ado (+)]. Then, cells were reacted with an anti-HA antibody for AMID (red), MitoTracker Green FM for the mitochondria (Mito)(green) and DAPI for the nucleus (blue). Similar results were obtained with 4 independent experiments. Bars, 10 μm.
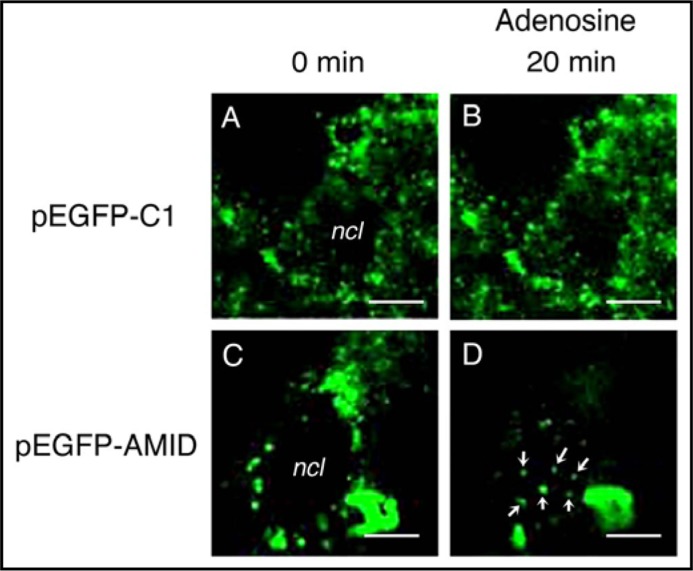
We subsequently carried out time-laps monitoring for intracellular AMID mobilizations in living HuH-7 cells. For cells expressing GFP alone no GFP signal in the nucleus was obtained with adenosine treatment (Fig. 4A, B), but for cells expressing GFP-AMID adenosine (10 mM) generated GFP signals in the nucleus; the number of the signals was enhanced in a treatment time (5-25 min)-dependent manner, with a maximum at 20-min treatment (Fig. 4C, D).
Fig. 4.
Time-laps monitoring for intracellular AMID mobilizations in living HuH-7 cells. Intracellular GFP signals were monitored in cells expressing GFP alone (pEGFP-Cl)(A, B) or GFP-AMID (pEGFP-AMID)(C, D) every 5 min before and after treatment with adenosine (10 mM). Note accumulation of GFP signals in the nucleus (ncl) 20 min after adenosine treatment (arrows) for cells expressing GFP-AMID. Similar results were obtained with 4 independent experiments. Bars, 10 um.
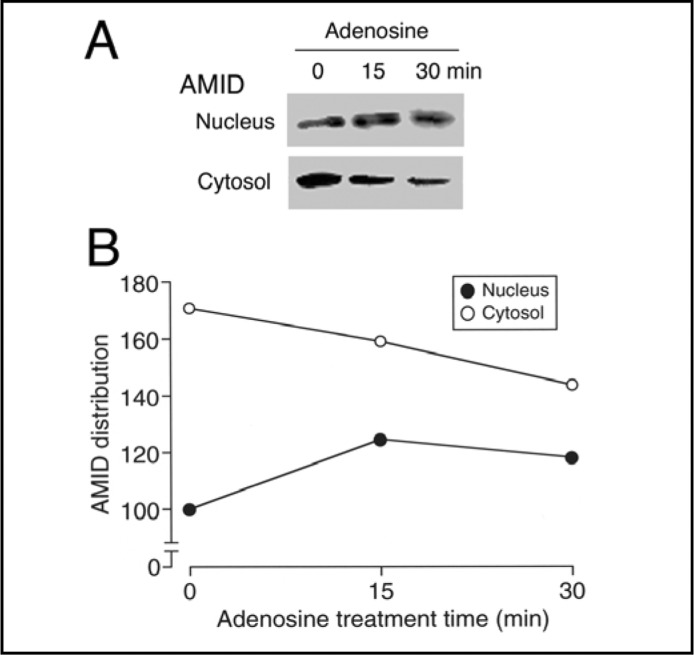
In the Western blot analysis using the cytosolic and nuclear components, adenosine (10 mM) increased presence of AMID in the nuclear component in parallel with the decrease in the cytosolic component in treatment time (15-30 min)-dependent manner (Fig. 5A, B). Collectively, these results confirm that adenosine promotes AMID translocation into the nucleus.
Fig. 5.
Intracellular distribution of AMID. HuH-7 cells, before and after treatment with adenosine (10 mM) for times as indicated, were separated into the cytosolic and nuclear components followed by Western blotting using an anti-AMID antibody (A). (B) In the graph, each point represents the relative AMID signal intensity against the intensity in the nuclear component at 0 min as 100. Note that similar results were obtained with 4 independent experiments.
AMID participates in adenosine-induced HuH-7 cell apoptosis
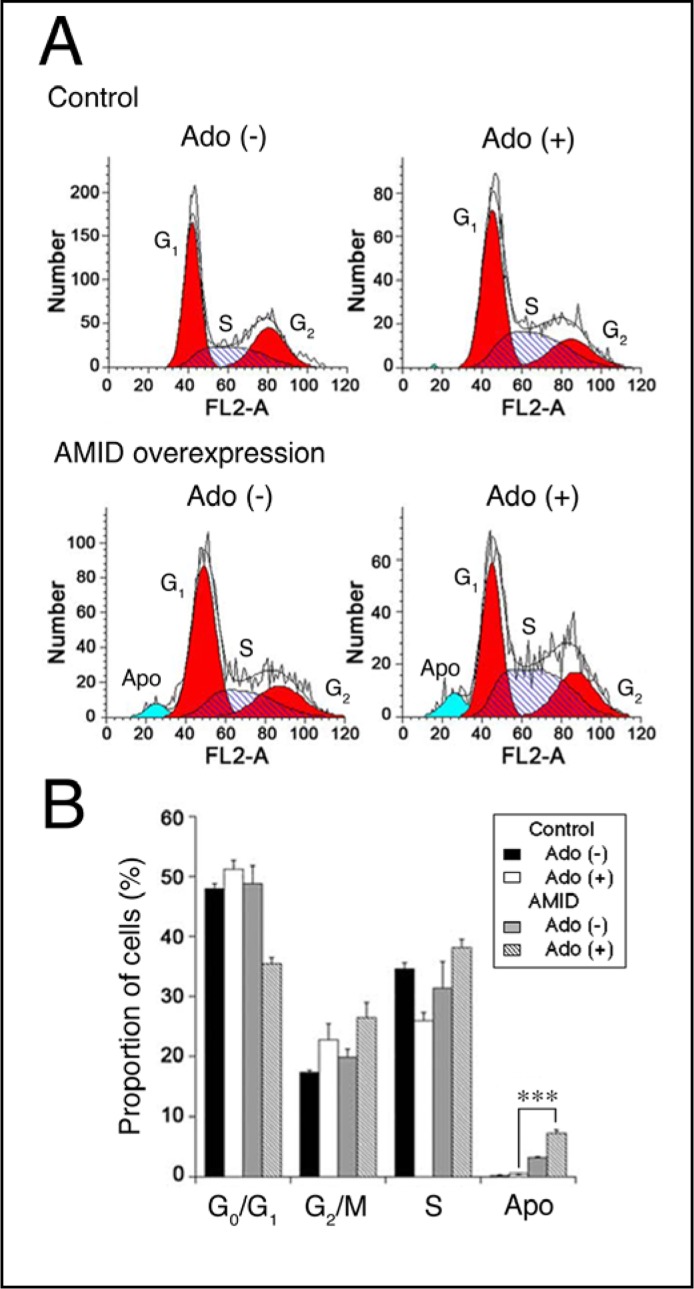
To see the implication of AMID in adenosine-induced HuH-7 cell apoptosis, we carried out cell cycle analysis. For HuH-7 cells overexpressing AMID, 24-h treatment with adenosine (10 mM) decreased the proportion in the G0/G1 phase of cell cycling, but instead increased the proportion in the G2/M and S phase (Fig. 6A, B). In addition adenosine increased the proportion of apoptosis for cells overexpressing AMID, with the extent being significantly larger than the proportion for control cells (Fig. 6A, B). This suggests that AMID serves as a mediator for adenosine-induced HuH-7 cell apoptosis.
Fig. 6.
Cell cycle analysis. HuH-7 cells expressing GFP alone (Control)) or GFP-AMID (AMID overexpression) were untreated [Ado (+)] and treated with adenosine (10 mM) for 24 h [Ado (+)]. Typical profiles are shown in (A). In the graph (B), each column represents the mean (± SEM) percentage (n=4 independent experiments). Apo, apoptosis. ∗∗∗P<0.0001, unpaired t-test.
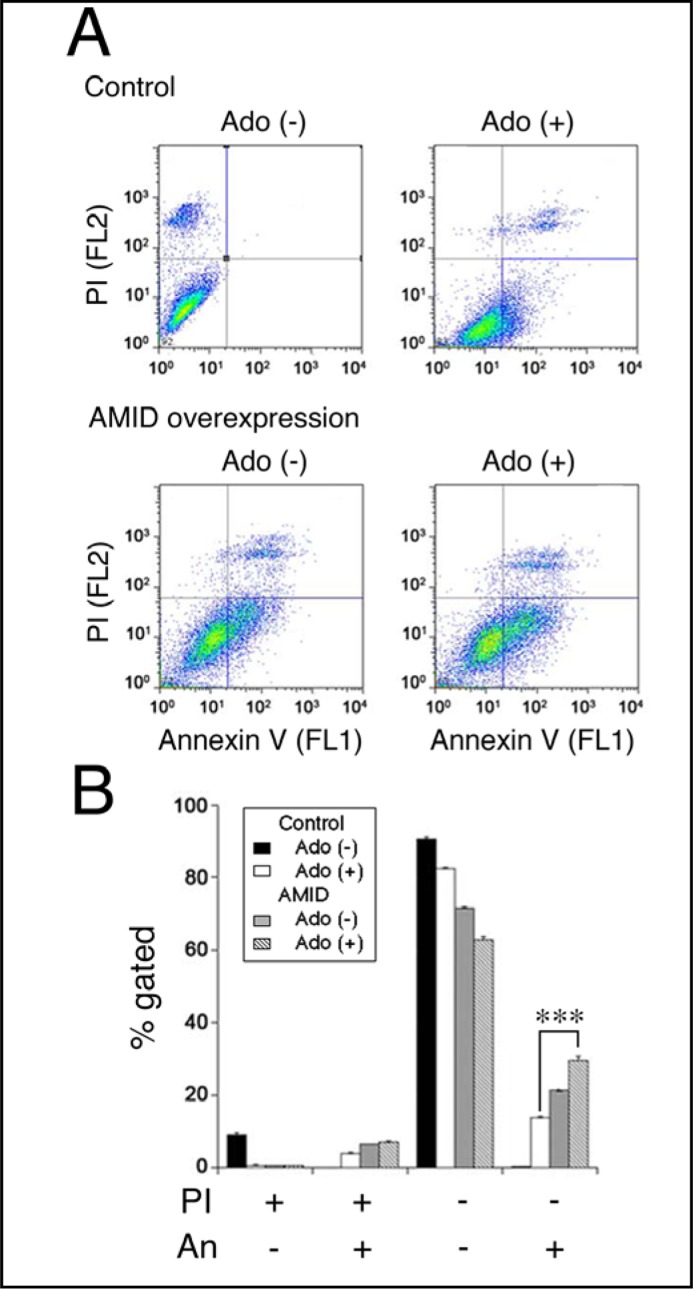
To obtain further evidence for this, we carried out flow cytometry using PI and annexin V-FITC. PI is a marker of dead cells and annexin V, detecting externalized phosphatidylserine residues, is a marker of apoptotic cells [26]. Treatment with adenosine (10 mM) for 24 h increased the population of PI-negative and annexin V-positive cells, that corresponds to early apoptosis [27], for HuH-7 cells overexpressing AMID, with the extent being significantly greater than the population for control cells (Fig. 7A, B). It is indicated from these results that AMID participates in adenosine-induced HuH-7 cell apoptosis.
Fig. 7.
Flowcytometry using PI and annexin V-FITC (An). HuH-7 cells expressing HA alone (Control) or HA-AMID (AMID overexpression) were untreated [Ado (+)] and treated with adenosine (10 mM) for 24 h [Ado (+)]. Typical profiles are shown in (A). In the graph (B), each column represents the mean (± SEM) percentage of cells in 4 fractions against total cells (n=4 independent experiments). ∗∗∗P<0.0001, unpaired t-test.
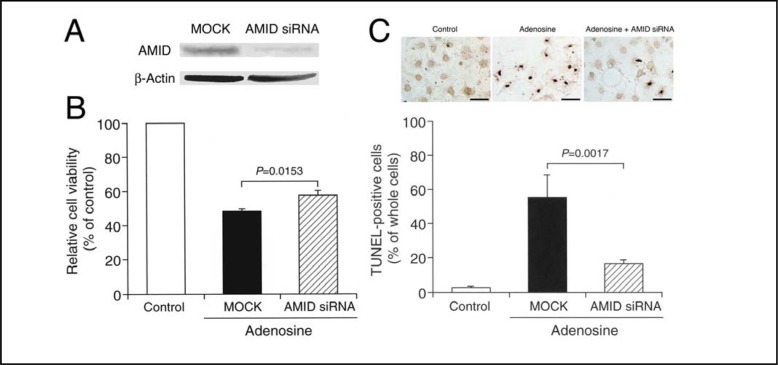
For HuH-7 cells transfected with the AMID siRNA, expression of AMID was clearly suppressed, although expression of β-actin was not affected (Fig. 8A). Treatment with adenosine (10 mM) for 48 h reduced HuH-7 cell viability to nearly 40% of control levels, and the effect was significantly inhibited by knocking-down AMID (Fig. 8B). Moreover, treatment with adenosine (10 mM) for 48 h increased the number of TUNEL-positive cells, and the effect was also prevented by silencing the AMID-targeted gene (Fig. 8C). Overall, these results indicate that adenosine induces HuH-7 cell apoptosis by targeting AMID.
Fig. 8.
AMID knock-down. (A) HuH-7 cells were transfected with MOCK or the AMID siRNA, and 48 h later Western blotting using antibodies against AMID and β-actin was carried out. (B) Cell viability was assayed in cells transfected with MOCK or the AMID siRNA before and after 48-h treatment with adenosine (10 mM). In the graph, each column represents the mean (± SEM) percentage of control levels (MTT intensities for cells untreated with adenosine) (n=8 independent experiments). P value, unpaired t-test. (C) TUNEL staining was carried out in cells transfected with MOCK or the AMID siRNA before and after 48-h treatment with adenosine (10 mM). Control, non-treatment with adenosine for untransfected cells; Adenosine, treatment with adenosine in cells transfected with MOCK; and Adenosine + AMID siRNA, treatment with adenosine in cells transfected with the AMID siRNA. Scale bars, 50 μm. In the graph, each column represents the mean (± SEM) TUNEL-positive cell percentage of whole cells (n=8 independent experiments). P value, unpaired t-test.
Discussion
In our earlier studies, extracellular adenosine induced HuH-7 cell apoptosis by downregulating c-FLIP expression, causing activation of caspase 8 followed by the effector caspase-3 [14]. Intracellularly transported adenosine, alternatively, activated caspase-3 by neutralizing caspase-3 inhibition due to IAP as a result of decreased IAP2 expression and reduced IAP activity in response to increased DIABLO expression and DIABLO release from damaged mitochondria, thereby inducing HuH-7 cell apoptosis [16]. Those pathways are relevant to adenos-ine-induced caspase-dependent HuH-7 cell apoptosis.
The fact that adenosine-induced HuH-7 cell apoptosis was not completely inhibited by a pan-caspase inhibitor [14], however, suggested an additional caspase-independent pathway.
In the present study, adenosine increased expression of the AMID mRNA and protein in HuH-7 cells, and stimulated AMID translocation from the cytosol into the nucleus. Adenosine induced HuH-7 cell apoptosis, and the effect was further enhanced by overexpressing AMID. Adenosine-induced HuH-7 cell apoptosis, alternatively, was inhibited by knocking-down AMID. These results point to AMID as a critical factor in adenosine-induced HuH-7 cell apoptosis. Interestingly, adenosine here decreased the proportion in the G0/G1 phase of cell cycling, but instead increased the proportion in the G2/M and S phase. This suggests that AMID as a target of adenosine governs HuH-7 cell apoptosis via a pathway independent of G2/M cell cycle arrest.
Extracellular adenosine is recognized to induce apoptotic cell death via an extrinsic pathway linked to adenosine receptors and/or an intrinsic pathway linked to adenosine transport into cells. An adenosine-induced increase in the AMID mRNA was not prevented by inhibitors of adenosine receptors (data not shown), ruling out an extrinsic pathway for tuning of the AMID gene transcription. We have found that adenosine induces apoptosis in HepG2 human hepatoma cells by regulating apoptosis mediator gene transcription [15], where GATA-2, a transcriptional factor, was a target of adenosine signal (unpublished data). It is presently unknown whether a similar mechanism underlies adenosine-regulated AMID gene transcription in HuH-7 cells. To address this question, further experiments need to be done.
AIF has a canonical nuclear leading sequence (NLS) and moves into the nucleus, causing chromosomal condensation and DNA fragmentation through some cryptic nuclease activity and/or recruitment of nucleases bearing partial chromatinolysis [1]. In spite of NLS lacking on AMID, adenosine actually promoted AMID translocation into the nucleus in HuH-7 cells. Accumulation of AMID in the nucleus, in the light of the fact that AMID is a DNA-binding protein [6], could cause chromosomal condensation and DNA fragmentation to induce apoptosis, as is the case with AIF. This accounts for the implication of AMID in adenosine-induced caspase-independent HuH-7 cell apoptosis.
In summary, the results of the present study show that AMID serves as a critical effector in adenosine-induced caspase-independent HuH-7 cell apoptosis. This may extend our understanding of adenosine signals for apoptosis.
Acknowledgements
We thank Dr Hong-Bing Shu for providing the c-HA-AMID plasmid, Katelyn Goodman, University of Wisconsin-Madison, for her proof reading of English grammar. This work was supported in part by National Institute of Health R01 grants HL095120 (S.D.).
References
- 1.Cande C, Cecconi F, Dessen P, Kroemer G. Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci. 2002;115:4727–4734. doi: 10.1242/jcs.00210. [DOI] [PubMed] [Google Scholar]
- 2.Daugas E, Susin SA, Zamzami N, Ferri KF, Irinopoulou T, Larochette N, Prévost MC, Leber B, Andrews D, Penning er J, Kroemer G. Mitochondrionuclear translocation of AIF in apoptosis and necrosis. FASEB J. 2000;14:729–739. [PubMed] [Google Scholar]
- 3.Ye H, Cande C, Stephanou NC, Jiang S, Gurbuxani S, Larochette N, Daugas E, Garrido C, Kroemer G, Wu H. DNA binding is required for the apoptogenic action of apoptosis inducing factor. Nat Struct Biol. 2002;9:680–684. doi: 10.1038/nsb836. [DOI] [PubMed] [Google Scholar]
- 4.Bilyy R, Kit Y, Hellman U, Stoika R. AMID: new insights on its intracellular localization and expression at apoptosis. Apoptosis. 2008;13:729–732. doi: 10.1007/s10495-008-0198-5. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Sun L, Liang Q, Wang J, Mo W, Zhou B. Yeast AMID homologue Ndi1p displays respiration-restricted apoptotic activity and is involved in chronological aging. Mol Biol Cell. 2006;17:1802–1811. doi: 10.1091/mbc.E05-04-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall KR, Gong M, Wodke L, Lamb JH, Jones DJ, Farmer PB, Scrutton NS, Munro AW. The human apoptosis-inducing protein AMID is an oxidoreductase with a modified flavin cofactor and DNA binding activity. J Biol Chem. 2005;280:30735–30740. doi: 10.1074/jbc.M414018200. [DOI] [PubMed] [Google Scholar]
- 7.Ohiro Y, Garkavtsev I, Kobayashi S, Sreekumar KR, Nantz R, Higashikubo BT, Duffy SL, Higashikubo R, Usheva A, Gius D, Kley N, Horikoshi N. A novel p53-inducible apoptogenic gene, PRG3, encodes a homologue of the apoptosis-inducing factor (AIF) FEBS Lett. 2002;524:163–171. doi: 10.1016/s0014-5793(02)03049-1. [DOI] [PubMed] [Google Scholar]
- 8.Varecha M, Amrichova J, Zimmermann M, Ulman V, Lukasova E, Kozubek M. Bioinformatic and image analyses of the cellular localization of the apoptotic proteins endonuclease G, AIF, and AMID during apoptosis in human cells. Apoptosis. 2007;12:1155–1171. doi: 10.1007/s10495-007-0061-0. [DOI] [PubMed] [Google Scholar]
- 9.Wu M, Xu LG, Li X, Zhai Z, Shu HB. AMID, an apoptosis-inducing factor-homologous mitochondrion-associated protein, induces caspase-independent apoptosis. J Biol Chem. 2002;277:25617–25623. doi: 10.1074/jbc.M202285200. [DOI] [PubMed] [Google Scholar]
- 10.Wu M, Xu LG, Su T, Tian Y, Zhai Z, Shu HB. AMID is a p53-inducible gene downregulated in tumors. Oncogene. 2004;23:6815–6819. doi: 10.1038/sj.onc.1207909. [DOI] [PubMed] [Google Scholar]
- 11.Sai K, Yang D, Yamamoto H, Fujikawa H, Yamamoto S, Nagata T, Saito M, Yamamura T, Nishizaki T. A1 adenosine receptor signal and AMPK involving caspase-9/-3 activation are responsible for adenosine-induced RCR-1 astrocytoma cell death. Neurotoxicol. 2006;27:458–467. doi: 10.1016/j.neuro.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Saitoh M, Nagai K, Nakagawa K, Yamamura T, Yamamoto S, Nishizaki T. Adenosine induces apoptosis in the human gastric cancer cells via an intrinsic pathway relevant to activation of AMP-activated protein kinase. Biochem Pharmacol. 2004;67:2005–2011. doi: 10.1016/j.bcp.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Saito M, Yaguchi T, Yasuda Y, Nakano T, Nishizaki T. Adenosine suppresses CW2 human colonic cancer growth by inducing apoptosis via A1 adenosine receptors. Cancer Lett. 2010;290:211–215. doi: 10.1016/j.canlet.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Yang D, Yaguchi T, Yamamoto H, Nishizaki T. Intracellularly transported adenosine induces apoptosis in HuH-7 human hepatoma cells by downregulating c-FLIP expression causing caspase-3/-8 activation. Biochem Pharmacol. 2007;73:1665–1675. doi: 10.1016/j.bcp.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Yang D, Yaguchi T, Lim CR, Ishizawa Y, Nakano T, Nishizaki T. Tuning of apoptosis-mediator gene transcription in HepG2 human hepatoma cells through an adenosine signal. Cancer Lett. 2010;291:225–229. doi: 10.1016/j.canlet.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Yang D, Yaguchi T, Nakano T, Nishizaki T. Adenosine-induced caspase-3 activation by tuning Bcl-XL/DIABLO/IAP expression in HuH-7 human hepatoma cells. Cell Biol Toxicol. 2010;26:319–330. doi: 10.1007/s10565-009-9145-7. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda Y, Saito M, Yamamura T, Yaguchi T, Nishizaki T. Extracellular adenosine induces apoptosis in Caco-2 human colonic cancer cells by activating caspase-9/-3 via A2a adenosine receptors. J Gastroenterol. 2009;44:56–65. doi: 10.1007/s00535-008-2273-7. [DOI] [PubMed] [Google Scholar]
- 18.Boe R, Gjertsen BT, Doskeland SO, Vintermyr OK. 8-Chloro-cAMP induces apoptotic cell death in a human mammary carcinoma cell (MCF-7) line. Br J Cancer. 1995;72:1151–1159. doi: 10.1038/bjc.1995.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pratt RM, Martin GR. Epithelial cell death and cyclic AMP increase during palatal development. Proc Natl Acad Sci USA. 1975;72:874–877. doi: 10.1073/pnas.72.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vintermyr OK, Gjertsen BT, Lanotte M, Doskeland SO. Microinjected catalytic subunit of cAMP-dependent protein kinase induces apoptosis in myeloid leukemia (IPC-81) cells. Exp Cell Res. 1993;206:157–161. doi: 10.1006/excr.1993.1132. [DOI] [PubMed] [Google Scholar]
- 21.Bar-Yehuda S, Stemmer SM, Madi L, Castel D, Ochaion A, Cohen S, Barer F, Zabutti A, Perez-Liz G, Del Valle L, Fishman P. The A3 adenosine receptor agonist CF102 induces apoptosis of hepatocellular carcinoma via de-regulation of the Wnt and NF-(B signal transduction pathways. Int J Oncol. 2008;33:287–295. [PubMed] [Google Scholar]
- 22.Kim SJ, Min HY, Chung HJ, Park EJ, Hong JY, Kang YJ, Shin DH, Jeong LS, Lee SK. Inhibition of cell proliferation through cell cycle arrest and apoptosis by thio-Cl-IB-MECA, a novel A3 adenosine receptor agonist, in human lung cancer cells. Cancer Lett. 2008;264:309–315. doi: 10.1016/j.canlet.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 23.Morello S, Sorrentino R, Porta A, Forte G, Popolo A, Petrella A, Pinto A. Cl-IB-MECA enhances TRAIL-induced apoptosis via the modulation of NF-(B signalling pathway in thyroid cancer cells. J Cell Physiol. 2009;221:378–386. doi: 10.1002/jcp.21863. [DOI] [PubMed] [Google Scholar]
- 24.Panjehpour M, Karami-Tehrani F. Adenosine modulates cell growth in the human breast cancer cells via adenosine receptors. Oncol Res. 2007;16:575–585. doi: 10.3727/000000007783629981. [DOI] [PubMed] [Google Scholar]
- 25.Song C, Satoh T, Edamatsu H, Wu D, Tadano M, Gao X, Kataoka T. Differential roles of Ras and Rap1 in growth factor-dependent activation of phospholipase C( Oncogene. 2002;21:8105–8113. doi: 10.1038/sj.onc.1206003. [DOI] [PubMed] [Google Scholar]
- 26.Vanags DM, Porn-Ares MI, Coppola S, Burgess DH, Orrenius S. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J Biol Chem. 1996;271:31075–31085. doi: 10.1074/jbc.271.49.31075. [DOI] [PubMed] [Google Scholar]
- 27.Pietra G, Mortarini R, Parmiani G, Anichini A. Phases of apoptosis of melanoma cells, but not of normal melanocytes, differently affect maturation of myeloid dendritic cells. Cancer Res. 2001;61:8218–8226. [PubMed] [Google Scholar]