Abstract
Background/Aims
Ischemia induced by large-vessel obstruction or vascular injury induces a complex cascade of vasodilatory, remodeling and inflammatory pathways; coordination of these processes by vascular endothelium is likely to involve endothelial gap junctions. Vascular endothelium predominantly expresses two connexin (Cx) isoforms: Cx37 and Cx40. The relevance of these Cxs to postischemic limb recovery remains unclear.
Methods
In this study, we use a well-established, severe femoral-saphenous artery-vein pair resection model of unilateral hindlimb ischemia to test the relevance of Cx37 and Cx40 to postischemic tissue survival and recovery of limb perfusion.
Results
Cx40-deficient animals (Cx40–/–) experienced a severe reduction in limb perfusion relative to wild-type (WT) animals and exhibited profound and rapid failure of ischemic limb survival. By contrast, the deficit in limb perfusion was less severe in Cx37-ablated (Cx37–/–) animals compared to WT, corresponding with more rapid recovery of limb appearance and use. These results demonstrate that Cx40 is necessary for postischemic limb survival and reperfusion, whereas Cx37 deletion reduces the extent of ischemia in the same model.
Conclusion
In summary, we present evidence demonstrating that Cx37 and Cx40 uniquely regulate postischemic limb perfusion, altering the severity of ischemic insult and consequent postischemic survival.
Key Words: Ischemia, Gap junction, Cx37, Cx40
Introduction
Acute vascular obstruction and injury, as occurs in coronary and peripheral artery disease or blunt trauma, acutely reduces downstream blood flow, resulting in localized ischemia and cellular damage that is reversed over time by complex repair and remodeling processes. Survival of distal tissue depends, in part, upon acute vasodilatory mechanisms to maintain sufficient residual perfusion to prevent distal tissue death, and prolonged vascular remodeling processes that restore peripheral flow towards preinjury levels [1,2]. Coordination of the complex vasomotor, injury and repair signaling cascades activated by ischemia likely involves vascular gap junctions, plaques of intercellular channels that mediate electrical and chemical coupling between neighboring endothelial cells (ECs) and between ECs and smooth muscle cells. Gap junctions may also interact with and modulate mediators of key signaling pathways via communication-independent mechanisms [3]. In the vasculature, intercellular coupling of the endothelium is believed to be necessary for dynamic regulation of flow; the absence of gap junction-mediated EC–EC communication promotes shunting (and consequently distal hypoxia) in a mathematical model of the microvasculature [4].
Gap junction channels are formed by the docking of two hexameric complexes of connexin (Cx) proteins. Of the 21 different Cx isoforms identified in the human genome, Cx37 and Cx40 are strongly expressed whereas Cx43 is minimally expressed in vascular endothelium under laminar flow conditions [5,6]. Studies of Cx-deficient animals have provided evidence suggesting the importance of vascular Cxs in regulating responses to vascular injury or disease. Ablation of Cx40 disrupts intercellular dye transfer in intact endothelium [7], reduces conducted vasomotor responses in isolated arterioles [8,9] and increases susceptibility to vascular inflammatory disease [10], suggesting a critical involvement of Cx40 in EC–EC coupling and endothelial barrier function. Interestingly, while intercellular dye transfer is reduced in either Cx40- or Cx37-deficient endothelium [7], upstream conduction of vasomotor signals appears to be largely unaffected in isolated arterioles obtained from Cx37-deficient animals [9]. These studies suggest that, despite their observed colocalization at EC–EC contacts [5], Cx37 and Cx40 may regulate different, if overlapping, functions in the vasculature. More specifically, Cx40 appears to be the Cx primarily responsible for mediating upstream communication of vasodilatory signals in the vasculature while the role of Cx37 in the vasculature remains more elusive.
Interestingly, Cx37 – but not Cx40 – expression is downregulated (and Cx43 upregulated) in the endothelium of aorta subjected to coarctation [5], a model that induces a hundred-fold increase in EC proliferation at the site of flow disruption [11]. Recently, we showed that Cx37 expression in highly proliferative cancer cells (rat insulinoma, Rin) limits cell proliferation by inducing G1/S arrest to produce a 4.5-fold increase in cell cycle duration [12]; no such growth suppression was observed in Rin cells expressing either Cx40 or Cx43. Growth suppression by the 319P human Cx37 (hCx37) polymorphic isoform, but not the 319S polymorph, has also been reported in Sk-Hep-1 and HeLa cells [13]. Further, Cx37 expression is absent, and Cx43 expression abundant, in subconfluent, proliferating microvascular ECs whereas the opposite is true when these cells achieve confluence [14]. Taken together, these studies support the hypothesis that Cx37 may function in the vasculature to regulate and limit vascular growth by limiting EC proliferation, a process required for vascular remodeling and repair following blood vessel injury or obstruction. This growth-suppressive function may not, however, be shared by other vascular Cxs, including Cx40.
While the studies described above suggest isoform-specific, and important, roles for Cx37 and Cx40 in regulating specific mechanisms necessary for the maintenance of vascular health, no studies have specifically demonstrated and compared the importance of either Cx37 or Cx40 in models of ischemic vascular injury or disease. For this study, we use Cx37–/– and Cx40–/– mice to determine the requirement for either Cx in the survival, repair and remodeling processes. To insure the detection of possible beneficial as well as detrimental effects of gene deletion, we used a severe form of unilateral hindlimb ischemia that wild-type (WT) animals typically recover from over a 2- to 3-week period [15,16]. Tissue survival and repair in models of hindlimb ischemia depend on both acute vasodilatory mechanisms and long-term vascular remodeling processes [1,2,15,17]. We demonstrate that limb recovery and survival are compromised in Cx40–/–, but accelerated in Cx37–/– animals.
Methods
Animals
Two- to six-month-old WT C57Bl/6 animals were compared to age-matched and sex-matched animals bearing a systemic deletion of either Cx37 (Cx37–/–) [18] or Cx40 (Cx40–/–) [19]. Animals were provided access to food (Harlan No. 7013 NIH-31) and water ad libitum, and studies were conducted in accordance with the University of Arizona's Institutional Animal Care and Use Committee.
Femoral-Saphenous Artery-Vein Pair Resection
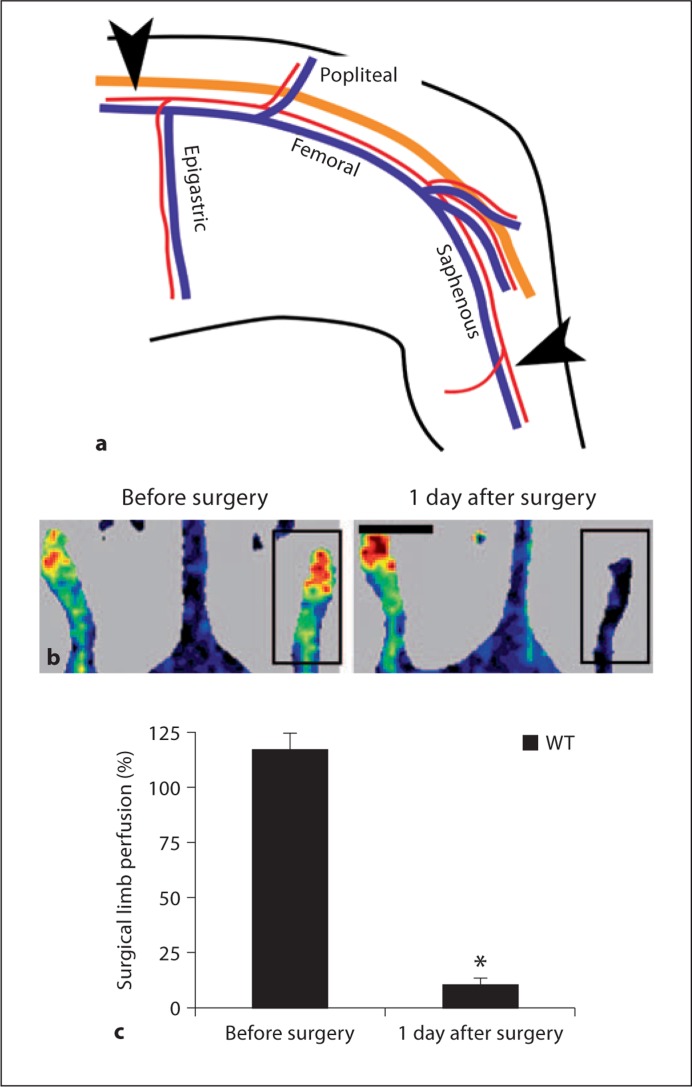
Two models for induction of hindlimb ischemia are commonly used: femoral artery occlusion [2,20] and femoral-saphenous artery-vein pair resection (FSAVPR) [15,17]. The WT C57Bl/6 mouse recovers blood flow to the ischemic hindlimb over a course of 14 days with the more severe form of ischemia, a period that is long enough to enable the detection of potential beneficial as well as detrimental effects of gene deletion. Consequently, to maximize our chances of observing improved as well as compromised recovery in Cx-ablated animals we applied the FSAVPR model for these studies. Ischemia was induced as previously described (fig. 1a) [15]. In brief, animals were anesthetized with 1.5–5% isoflurane in O2 until the toe pinch reflex was lost. The femoral-saphenous artery-vein pair of the left hindlimb was exposed and dissected away from the overlying nerve under sterile conditions, and the epigastric fat pad was removed by cauterization to reveal the upstream ligation site. 6-0 silk sutures were placed around the femoral artery-vein pair upstream of the epigastric branch and downstream on the saphenous artery-vein pair midway between the knee and ankle. The intervening portion of blood vessel was resected, and the wound was closed using 7-0 absorbable prolene suture. The animals received two injections of an analgesic (Buprenorphine, 10 mg/kg), one immediately and the other 24 h after surgery.
Fig. 1.
a Ischemia was surgically induced in experimental animals by FSAVPR. In brief, the femoral and saphenous artery (red)-vein (blue) pair was dissected away from the overlying nerve (orange), sutures were placed as indicated (arrowheads), and the intervening length of vessel was resected. b Unilateral loss of limb flow was confirmed by laser Doppler imaging of the surgical (box) and control limb before and 1 day after surgery (scale bar = 1 cm). c A significant decrease in mean surgical limb perfusion (vs. control limb) was observed relative to presurgical scans (∗, n = 8).
Assessment of Surgical Limb Survival and Recovery
At multiple time points following FSAVPR, an investigator blinded to animal genotype and surgical manipulation scored the appearance and use of the surgical limb according to the descriptors in tables 1 and 2 (modified from Stabile et al. [21]). Animals experiencing severe necrosis or autoamputation were sacrificed at the time of detection, which in some cases occurred prior to the end of the standard 14-day recovery period. For those animals, appearance scores subsequent to the time of euthanasia were assigned a value of −4 based on the assumption that distal tissue death or autoamputation is an irreversible event.
Table 1.
Descriptors for scoring the appearance of the ischemic versus nonischemic contralateral limb
| Score | Description |
|---|---|
| 0 | No difference |
| −1 | Mild redness |
| −2 | Dark redness |
| −3 | Purple coloration and/or mild necrosis |
| −4 | Severe necrosis and/or autoamputation of distal surgical limb |
Table 2.
Descriptors for scoring the use of the ischemic versus nonischemic contralateral limb
| Score | Description |
|---|---|
| 0 | Plantar flexion, toes grip with gentle traction of tail |
| −1 | Plantar flexion, toes do not grip with gentle traction of tail |
| −2 | No plantar flexion, some weight placed on foot |
| −3 | No plantar flexion, movement in hip |
| −4 | No use, dragging of foot |
Laser Doppler Scanning
Animals were anesthetized with 1.5–5% isoflurane in O2 until loss of righting reflex. The hindquarters of the animals were then depilated, and the animals were placed supine on a heated block to maintain body temperature at 37°C. A Periflux Pim II (Perimed) small-animal laser Doppler scanner was used to assess the perfusion of surgical and control limbs [15]. Scanning range was between 5 and 10 V, and the threshold was set at 5.6 V based on scans made of a dead animal wherein flow was absent. Pixel intensities above threshold were converted by LDPIWin software (Perimed, version 2.3.14) to a perfusion value within a range of 0–10 V. Surgical and control limb perfusion were quantified from each scan by creating an 11 × 24 pixel region of interest around each limb, and determining the average perfusion voltage of above-threshold pixels contained therein. Mean surgical limb perfusion was calculated for each image by normalizing surgical limb pixel intensities to that of the control limb in each image. Animals were scanned in triplicate over a 10- to 15-min period, and the mean surgical limb perfusion for the three scans was calculated and expressed as surgical limb perfusion (%) for each animal at each time point. The perfused area (%) of the surgical limb was determined as the average across three scans of the number of above-threshold pixels within the region of interest of the surgical limb and normalized to the same value calculated for the control limb. Total limb flow (%) was calculated as the product of limb perfusion level and perfused limb area for each image and averaged across three scans. Graphs plot the means of the indicated parameter for all animals for each time point; perfusion level and area for animals sacrificed prior to day 14 were assumed to be zero for all time points after sacrifice.
Histology
Experimental animals were euthanized and gastrocnemius skeletal muscle was harvested from control and surgical limbs and fixed in 4% paraformaldehyde (in PBS) for 24 h. Tissue was dehydrated by progressive alcohol washes, cleared with xylene, and embedded in paraffin. Seven-micrometer-thick cross-sections were stained using a standard hematoxylin and eosin staining protocol to assess the morphological characteristics of the tissue.
Statistics
Nonparametric hindlimb use and appearance scores are expressed as median values as a function of time, and were compared using a rank-sum test. All other comparisons were performed using Student's t test. In all cases, α was set at 0.05.
Results
In this study, we tested the hypothesis that Cx37 and Cx40 are necessary for survival and recovery of the hindlimb following femoral-saphenous artery-vein ligation and resection. We induced ischemia in WT, Cx37–/– and Cx40–/– animals by unilateral FSAVPR (fig. 1a), a well-established surgical model of peripheral artery disease that requires native and remodeled vasculature to maintain sufficient perfusion to preserve downstream tissue health [15]. We confirmed induction of ischemia by laser Doppler scanning of control and surgical limbs before surgery and 24 h after surgery (fig. 1b). Both mean (fig. 1c) and total (data not shown) surgical limb perfusion of WT animals were significantly reduced compared to presurgical scans, demonstrating that FSAVPR produced an acute ischemic insult that decreased subcutaneous blood flow by about 90%. Although older animals fare less well than juveniles in similar models of hindlimb ischemia [22], no differences in postischemic limb flow were detected at 24 h after surgery between younger (8- to 12-week-old) and older (16- to 25-week-old) WT animals within the 8- to 25-week age range (online suppl. fig. 1; for all online supplementary material see, www.karger.com/doi/10.1159/000329616), suggesting that age-dependent differences within our experimental group are unlikely to alter postischemic outcome in this model.
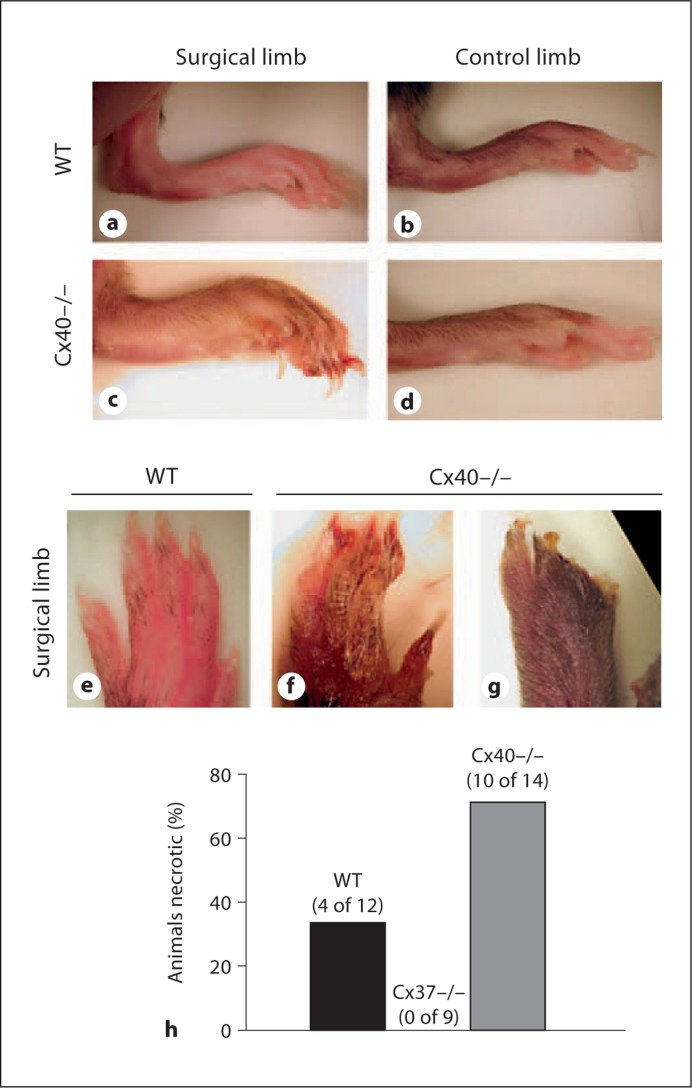
No differences in initial animal weight were detected across animal genotype (online suppl. fig. 2A). Following induction of unilateral ischemia by FSAVPR, limb recovery and overall animal health were assessed for 14 days. FSAVPR induced a temporary decrease in animal weight that recovered in all three genotypes by day 14 (online suppl. fig. 2B). At most postischemic time points, no genotype-specific differences were detected, indicating that overall animal health was not significantly or differentially compromised in any of our experimental groups. Indeed, the initial reduction in, and subsequent recovery of, whole-animal weight was observed to be modestly improved for Cx37–/– animals, suggesting improved resilience to FSAVPR in these animals. Additionally, macroscopic assessment of overall surgical limb appearance in WT, Cx37–/– and Cx40–/– animals during the 14-day recovery period revealed obvious genotype-specific differences in post-FSAVPR limb recovery. From gross images of the distal surgical limb of Cx40–/– mice at day 7 (fig. 2), we observed morphological characteristics consistent with failure of this tissue to recover following ischemia, including distal edema (fig. 2c), necrosis (fig. 2f) and autoamputation of the toes (fig. 2g). By contrast, the surgical limbs of WT (fig. 2a, e) and Cx37–/– (not shown) animals typically appeared indistinguishable from control limbs (fig. 2b) by day 7. By 2 weeks following FSAVPR, 71% (10 of 14) of Cx40–/– animals experienced distal limb necrosis and autoamputation, compared to 0% (0 of 9) of Cx37–/– and 33% (4 of 12) in WT (fig. 2h), suggesting that Cx40, but not Cx37, is required for postischemic limb survival.
Fig. 2.
a–d Representative images taken on day 7 after FSAVPR of the surgical and control limbs of Cx40–/– and WT animals, which demonstrate failure of the Cx40–/– surgical limb to recover. Compared to WT (e), images of the surgical paw of Cx40–/– reveal distal necrosis (f) and autoamputation (g). h Incidence of severe distal necrosis was significantly increased in Cx40–/– animals (71%, 10 of 14) compared to WT (33%, 4 of 12). Necrosis was not observed in any Cx37–/– animals (0%, 0 of 9) following FSAVPR.
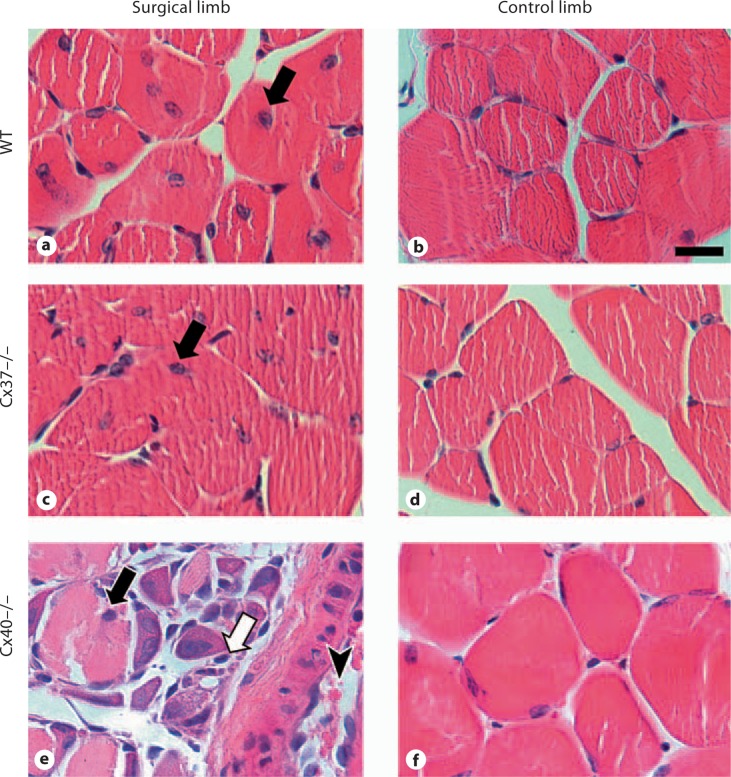
To determine the extent of ischemic damage in the surgical limb, cross-sections of control and surgical limb gastrocnemius skeletal muscle were assessed for morphological characteristics of ischemia and muscle fiber regeneration at day 14 after FSAVPR (fig. 3). The gastrocnemius muscle was chosen for further analysis because it is located proximally to the regions of gross necrosis and autoamputation observed in Cx40–/– mice (fig. 2), and consequently this tissue was predicted to survive despite downstream tissue death. In all three genotypes, we observed centrally located muscle fiber nuclei, a hallmark of muscle fiber injury and regeneration, in muscle harvested from surgical limbs, confirming the effect of FSAVPR on this tissue. No centrally located muscle fiber nuclei were seen in tissue obtained from control limbs. The increased severity of FSAVPR-induced ischemic damage in Cx40–/– animals was also evident in these sections, which displayed hemorrhage, increased interfiber eosinophilic staining, and profound tissue disorganization (fig. 3e) suggestive of increased ischemic damage and a subsequently aggressive inflammatory infiltration in these animals; no such characteristics were observed in skeletal muscle sections obtained from the surgical limbs of WT (fig. 3a) or Cx37–/– (fig. 3c) animals, or in tissue harvested from control limbs (fig. 3b, d, f).
Fig. 3.
Representative images of cross-sections of the gastrocnemius muscle stained with hematoxylin and eosin and harvested from the surgical and control limbs of WT (a, b), Cx37–/– (c, d) and Cx40–/– (e, f) animals at day 14 after FSAVPR (scale bar = 20 μm). Centrally located muscle fiber nuclei (black arrows) were observed in tissue obtained from the surgical limbs of all genotypes, indicating muscle fiber damage and regeneration. Additionally, tissue from the surgical limbs of Cx40–/– animals exhibited substantial disorganization, hemorrhage (arrowhead), and interfiber eosinophilic staining (white arrow), indicative of the increased severity of ischemic damage in these animals.
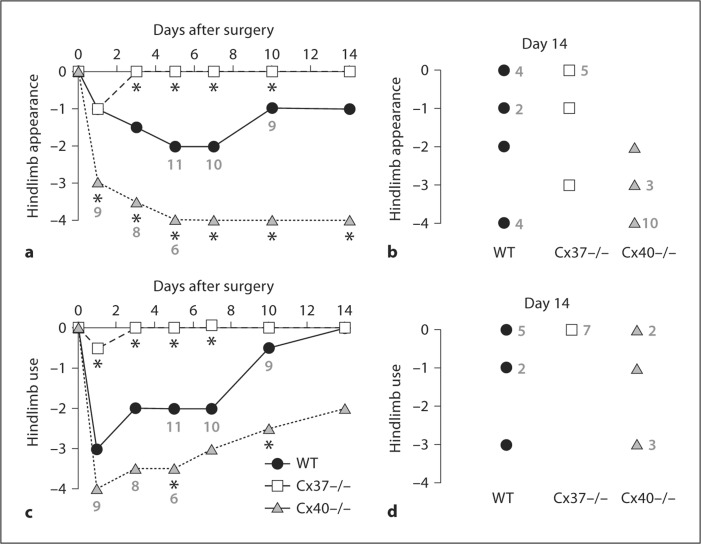
The heightened incidence of postischemic necrosis in Cx40–/– mice relative to WT prompted us to examine limb appearance and use in greater detail. Limb appearance was assessed by qualitatively scoring appearance (table 1) at multiple time points following FSAVPR. As expected in the FSAVPR model, WT animals experienced moderate discoloration of the surgical limb immediately following surgery that improved over the subsequent 14 days (fig. 4a, b), indicative of initial ischemic injury and subsequent recovery. By contrast, tissue appearance scores for Cx40–/– animals were significantly more negative immediately after FSAVPR relative to WT, and failed to improve at any of the subsequent time points. Consistent with the reduced incidence of necrosis in Cx37–/– animals (fig. 2h), appearance scores for the surgical limbs of Cx37–/– animals demonstrated only a mild impact of FSAVPR on limb appearance at day 1 after surgery, and by day 3, the appearance of the surgical limbs of most Cx37–/– animals was indistinguishable from that of control limbs. Appearance scores for Cx37–/– mice were significantly better than those of WT by day 3 and at most subsequent time points. Thus, the lack of Cx40 leads to a more severe injury whereas the lack of Cx37 lessens the injury induced by FSAVPR.
Fig. 4.
Surgical limb appearance (a, b) and use (c, d) scores were collected at multiple time points after FSAVPR in WT (n = 12), Cx37–/– (n = 9) and Cx40–/– (n = 14) animals. Median appearance (a) and use (c) scores are shown as a function of time following FSAVPR. Asterisks indicate a significant difference in scores relative to WT at the specified time point, and premature sacrifice of necrotic animals is denoted by the new sample sizes indicated in gray. For WT (n = 11 total, n = 8 surviving to day 14), Cx37–/– (n = 7) and Cx40–/– (n = 14, n = 6 surviving to day 14) animals, appearance (b) and use (d) scores for each animal at day 14 are shown; frequency of scores are denoted in gray. In Cx37–/– animals, both median hindlimb appearance and use were only mildly affected by FSAVPR at day 1 and recovered rapidly by day 3, whereas median limb appearance and use scores for Cx40–/– mice were profoundly more negative relative to WT at most time points, indicating rapid necrosis and autoamputation in these animals.
Based on our finding that postischemic limb appearance is improved in Cx37–/– animals and impaired in Cx40–/– animals, we investigated the functional impact of Cx37 or Cx40 ablation on the recovery of surgical hindlimb use. WT, Cx37–/– and Cx40–/– animals were assessed for recovery of normal gait at multiple time points following FSAVPR using the scores shown in table 2 (fig. 4c, d). As expected, WT animals experienced moderate impairment of ambulation immediately following FSAVPR that fully recovered by day 14. Hindlimb use was significantly more impaired in Cx40–/– animals relative to WT at later time points, consistent with failure of the limb to recover following proximal ischemic damage (fig. 3), and distal necrosis and autoamputation (fig. 2) although some improvement of hindlimb use was still observed in Cx40–/– animals that survived to day 14. By contrast, Cx37–/– animals experienced significantly improved hindlimb use at day 1 relative to WT, and displayed no detectable impairment in surgical limb use by day 3 after FSAVPR. Taken together, these findings are consistent with our hypothesis that Cx40 deletion limits ischemic limb survival whereas Cx37 deletion improves postischemic limb recovery.
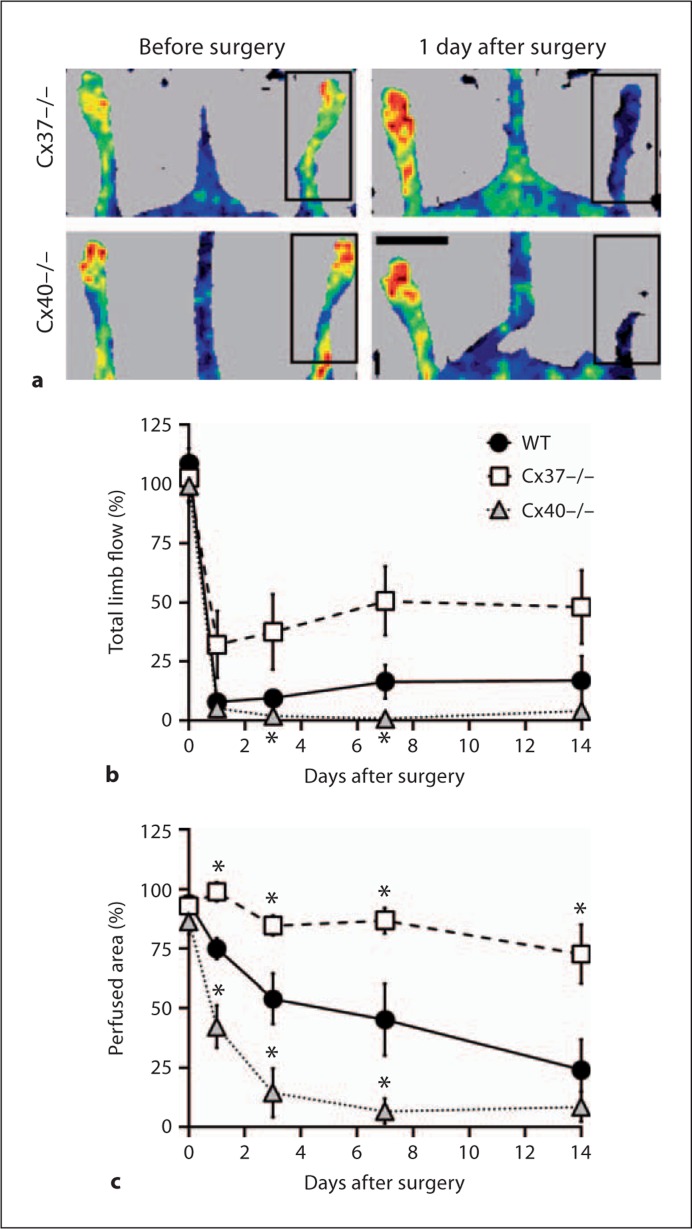
The reduced impact of FSAVPR on surgical limb use and appearance in Cx37–/– animals and the rapid onset of necrosis in Cx40–/– animals suggest that differences in postischemic limb perfusion may underlie these genotype-specific differences. To test this possibility, laser Doppler perfusion imaging was used to assess recovery of subcutaneous distal limb blood flow following FSAVPR in each genotype, and to calculate total limb perfusion at multiple time points during the recovery period. Representative laser Doppler scans of Cx37–/– and Cx40–/– animals before and 1 day after surgery confirm induction of ischemia (fig. 5a), and suggest that immediate postischemic limb flow may be enhanced in the Cx37–/– animal and reduced in the Cx40–/– animal relative to similar scans obtained from WT controls (fig. 1b). Calculation of total limb perfusion confirmed a significant reduction at days 3 and 5 in Cx40–/– animals relative to WT whereas surgical limb perfusion appeared to be increased in Cx37–/– animals at multiple time points during the recovery period (fig. 5b). The genotype-specific differences in recovery of total ischemic limb flow seemed to be due primarily to differences in the size of the perfused area. Although no significant differences could be detected between genotypes in mean blood flow to the ischemic limb at any postischemic time point (data not shown), figure 5c shows genotype-specific differences in the size of the perfused area at 24 h following ischemic insult and at subsequent time points. Whereas Cx40–/– animals experienced a significant reduction (vs. WT) in postischemic perfused area at days 1–7, the perfused area was unaffected by FSAVPR in Cx37–/– animals, and significantly elevated at all time points relative to WT. These data demonstrate that ablation of either Cx37 or Cx40 affects immediate and prolonged post-FSAVPR limb flow, thereby altering the degree of ischemia induced by the surgical model employed herein. Consequently, Cx37–/– animals experience a reduced sensitivity to FSAVPR whereas the effect of FSAVPR is heightened in the Cx40–/– mouse.
Fig. 5.
a Representative laser Doppler images of Cx37–/– and Cx40–/– animals were obtained before and 1 day after surgery of surgical (box) and control limbs (scale bar = 1 cm). Total limb flow (b) and size (c) of perfused areas (both parameters normalized to the control limb) were elevated in Cx37–/– mice (n = 8) and reduced in Cx40–/– mice (n = 12) relative to WT (n = 8) throughout the 14-day recovery period. Asterisks denote a significant difference versus WT at the specified time point.
Discussion
Cx37 and Cx40 are coexpressed at high levels by ECs where they colocalize to cell–cell junctions. Despite the potential for redundant functions, recent evidence increasingly suggests unique contributions of these Cxs to vascular development and response to injury and disease [3,5,10,12,23]. The endothelium and Cxs expressed therein support conducted dilatory responses [11] and form a critical component of the blood-tissue barrier [10], and thus are likely critically involved in coordinating the ischemic responses induced following large-vessel ligation or obstruction. The aim of this study was to test the necessity of Cx37 and Cx40 in postischemic tissue survival and recovery, a process requiring acute and sustained vascular dilation, remodeling and repair. Using a surgical model of unilateral hindlimb ischemia (FSAVPR), we demonstrate that compared to WT mice, Cx40–/– mice fail to recover ischemic tissue, suffering severely decreased limb perfusion that leads to profound distal limb edema, necrosis and autoamputation by day 7 after surgery. More proximal tissue also exhibits characteristics of profound ischemic damage, suggesting an increased severity of FSAVPR in this animal model. Thus, Cx40 is clearly essential for limb survival and recovery following ischemic insult. By contrast, accelerated recovery of surgical limb perfusion, appearance and use in the Cx37–/– mouse all suggest that Cx37 is not necessary for postischemic limb survival, but instead may function to limit survival and recovery of ischemic tissue in the WT animal.
The best predictor of poor postischemic outcome was the magnitude of the deficit in the perfused limb area at day 1 after FSAVPR and throughout the subsequent recovery period. In WT animals, the perfused area was noticeably decreased 24 h after FSAVPR and continued to decline throughout the recovery period. By contrast, the perfused area was largely unaffected in Cx37–/– animals, and was significantly elevated relative to WT at all assessed time points. In stark contrast, the perfused area in Cx40–/– animals was substantially decreased at all assessed time points following FSAVPR, including at day 1. These data indicate that Cx37–/– animals are capable of rapidly restoring flow to distal tissue following FSAVPR, thereby limiting the severity of induced ischemia and promoting rapid downstream recovery. By contrast, Cx40 appears to be necessary to return sufficient blood flow to ischemic tissue, and deletion of this Cx exacerbates post-FSAVPR ischemia resulting in rapid downstream tissue death.
While our data demonstrate the involvement of both Cx40 and Cx37 in ischemia-induced responses, the mechanism(s) affected by Cx deletion that alters postischemic limb perfusion requires additional study and consideration. These mechanisms might include: (1) vasodilatory signaling to upstream collaterals, (2) number of upstream collaterals, (3) the inflammatory response, and (4) renin-angiotensin signaling; dysregulation of one or more of these pathways might be theorized to produce the observed postischemic outcomes in Cx37–/– and Cx40–/– mice. We discuss below the existing evidence supporting or refuting a possible involvement of each of these mechanisms in the postischemic outcomes of Cx37–/– and Cx40–/– mice.
EC–EC coupling via gap junctions is thought to be responsible for upstream conduction of the vasodilatory responses that allow for dynamic coordination of distal flow consequent to changes in metabolic demand [4]. Although Cx37 and Cx40 colocalize at sites of EC–EC cell contact, and both proteins form functional intercellular channels, deletion of Cx40, but not Cx37, reduces conducted vasodilatory responses in isolated arterioles [9], which suggests that Cx40 may be the endothelial Cx principally involved in mediating intercellular communication of vasomotor signals. Vasodilatory agents are typically released locally at the onset of ischemia, and their local dilatory effects are conducted upstream via EC gap junctions to dilate upstream feed arteries and possibly collateral vessels – arteriolar-arteriolar connections that typically serve to shunt blood around an obstruction or injury to preserve downstream flow. Thus, although the local release of dilatory signals could differ between genotypes [24,25], conduction of these signals is predicted to be severely limited by reduced EC coupling in the Cx40–/– mouse. Consistent with this possibility, we report a significant reduction in distal limb blood flow at 24 h after FSAVPR in the Cx40–/– animal relative to WT. This result is consistent with recent findings by Buschmann et al. [20], who reported reduced peripheral limb flow as early as 4 h after surgery using a milder unilateral hindlimb ischemia model. Thus, reduced vasodilatory signaling associated with decreased gap junction coupling in the endothelium of the Cx40–/– mouse could contribute to the observed postischemic failure in this animal. In contrast, dysregulation of conducted vasodilatory responses are unlikely to explain the accelerated postischemic recovery reported in the Cx37–/– mouse. Deletion of Cx37 does not detectably alter conducted vasodilatory responses in isolated arterioles [9], and has been shown to reduce rather than enhance, intercellular dye coupling in intact endothelium. Neither of these observations would be predicted to improve postischemic outcome in the Cx37–/– mouse, suggesting that the involvement of Cx37 in mechanisms activated following FSAVPR may not involve regulation of gap junction-mediated conducted vasodilation to upstream feed or collateral vessels. Recently, the C-terminal domain of Cx37 was shown to interact with and limit the activity of eNOS [24]; improved nitric oxide production in the Cx37–/– mouse could improve acute vasodilation of upstream vessels, and thereby improve postischemic outcome by reducing the severity of the ischemic insult to affected tissue. Consistent with this hypothesis, we observed a significant increase in the perfused area of the surgical limb at 24 h after FSAVPR in the Cx37–/– mouse relative to WT, associated with immediate improvements in limb appearance and limb use.
Acute and long-term redistribution of blood flow to ischemic tissue also depends upon the number of available upstream collateral vessels and their capacity for outward remodeling. There are virtually no data available on these issues for the Cx40- or Cx37-deficient animals. Preliminary data provided in supplemental material by Buschmann et al. [20] showed that collateral number and remodeling capacity were reduced in the Cx40–/– mouse. Although it remains unclear how Cx40 deficiency might limit collateral development and postnatal remodeling, reduced native collateral number would be reasonably predicted to reduce postischemic limb flow in the Cx40–/– mouse, an effect that would have a profound impact on tissue survival in our model due to the severe nature of the surgery. Comparable data for collateral density and capacity for remodeling in Cx37–/– mice are not yet available. Nevertheless, based on the growth-suppressive effects of Cx37 on proliferation of tumor cells [12,13], we hypothesize that expression of Cx37 may also affect collateral number, but in this case by limiting collateral growth and remodeling. Several studies are consistent with a growth-suppressive role for Cx37 in the vasculature. Cx37 expression is not observed in subconfluent primary cultures of ECs although its expression returns when cells reach confluence [14], suggesting an effect of proliferation and/or cell–cell contact on Cx37 expression in this cell type. In addition, expression of Cx37 – but not Cx40 – is altered by coarctation of the aorta [5], a model shown to significantly enhance EC proliferation rates [26]. Recently, we reported that Cx37 expression suppresses the growth of highly proliferative cancer cells (Rin), an observation confirmed by Morel et al. [13] in SkHep1 and HeLa cells. The growth-suppressive effect in Rin cells was unique to Cx37 of the three vascular Cxs assessed [12]. If Cx37 does indeed exert a growth-suppressive effect in the vascular endothelium, then Cx37–/– animals might be predicted to have an increased number of collaterals and an increased remodeling capacity, which could support an increase in flow to ischemic tissue acutely, and throughout the recovery period, as suggested by our data. Deletion of Cx37 would be predicted to alleviate its growth-suppressive effect on the vasculature, enhancing both the native and remodeled collateral number in the Cx37–/– mouse and thereby improving immediate and long-term postischemic outcome.
Dysregulation of the inflammatory system might also be predicted to affect post-FSAVPR limb survival and recovery. While a regulated inflammatory response is critical for postischemic vascular remodeling and consequent tissue survival and repair (see Silvestre et al. [27] for a review) an overly aggressive inflammatory response could limit (or potentially enhance) recovery through direct cytotoxic effects on distal tissue or dysregulated production of pro- or antiangiogenic factors. Recent reports suggest that the inflammatory response is modified in both Cx37–/– and Cx40–/– animals. Chadjichristos et al. [10] demonstrated that endothelial Cx40 regulates and limits macrophage adhesion via a CD73-dependent mechanism; deletion of this Cx from the vascular endothelium resulted in increased macrophage infiltration into atherosclerotic plaques. Our data showing severe proximal and distal limb injury in the Cx40–/– mouse are consistent with an aggressive inflammatory response in these animals. Deletion of Cx37 has also been reported to enhance monocyte adhesion and invasion, although this response associates with Cx37 expression by the monocyte, not the EC [23]. Several inflammatory mediators promote angiogenesis and arteriogenesis under ischemic conditions; an effect of Cx37 deletion on these regulatory roles of the inflammatory system on these vascular remodeling processes might facilitate postischemic limb recovery. However, we were unable to resolve any differences between Cx37–/– mice and WT in the extent of postschemic inflammatory response: increased numbers of infiltrated monocytes were not evident in histological sections obtained from the surgical limbs of Cx37–/– mice at 14 days after FSAVPR, suggesting that if such infiltration occurred, it had already fully resolved without detriment to gastrocnemius skeletal muscle prior to the time of animal sacrifice and tissue harvest.
Finally, in addition to the mechanisms discussed above, systemic hypertension (or signaling pathways involved in blood pressure regulation) may also impact postischemic limb flow or other processes. The Cx40–/– animal has been demonstrated to suffer systemic hypertension, associated with dysregulation of the renin-producing cells of the kidney [28]. Both the hypertension and the underlying hyperactivity of the renin-angiotensin system could have a profound impact on the vasculature that could affect postischemic outcome. For instance, both systemic hypertension [29] and transgenic deletion of the angiotensin II type IA receptor [16] produces microvascular rarefaction. Further, genomic deletion of the angiotensin II type IA receptor limits collateral and microvascular remodeling following hindlimb ischemia, independent of blood pressure changes [16]. It is unclear what the combined effects of high blood pressure and excessive angiotensin II signaling will have on the microvasculature of the Cx40–/– animal; however, reduced microvascular density would likely increase sensitivity of Cx40–/– animals to ischemia induced by FSAVPR, promoting tissue death. By contrast, no significant effects of Cx37 deletion on systemic blood pressure [[9] and unpublished data] or renin-angiotensin signaling [30] have thus far been reported in the Cx37–/– mouse, although acute disruption of Cx37 junctions by mimetic peptide infusion of the kidney results in acute elevation of renin, angiotensin II and blood pressure. The latter study suggests that Cx37 may regulate renin-angiotensin signaling when normally expressed, but apparently is not necessary for normal renin secretion if genomically deleted [31].
In summary, although EC–EC coupling via gap junctions contributes critically to coordinated blood flow distribution [4], the roles of specific vascular Cxs in regulation of the blood flow changes required for the maintenance of vascular health and disrupted in disease progression remain unclear. In this study, we demonstrate the importance of both Cx37 and Cx40 in regulating postischemic responses in a model of vascular obstruction. We show that Cx40 is necessary for postischemic tissue survival, and that ablation of this Cx results in reduced postischemic limb perfusion, and frequently distal necrosis and death of the ischemic limb. By contrast, Cx37–/– mice experience enhanced postischemic recovery of limb perfusion and improved ischemic limb outcome, indicating that Cx37 is unnecessary for postischemic limb survival, and instead appears to limit postischemic recovery in the WT mouse. Taken together, these studies demonstrate the importance of both vascular Cxs in postischemic responses, and further suggest that, despite their coexpression and colocalization at the site of EC–EC contact, Cx37 and Cx40 play distinct roles in regulating vascular physiology.
Disclosure Statement
None.
Supplementary Material
Supplemental Figures
Acknowledgements
The authors would like to thank Dr. James Hoying and Dr. David Kurjiaka for their assistance in establishing many of the experimental procedures, and would also like to acknowledge Ms. Miranda Good for her occasional assistance. This work was supported in part by the American Heart Association (550158Z, 0715532Z and 09PRE2060122) and the National Institutes of Health (HL064232, HL058732).
References
- 1.Qian HS, Liu P, Huw LY, Orme A, Halks-Miller M, Hill SM, Jin F, Kretschmer P, Blasko E, Cashion L, Szymanski P, Vergona R, Harkins R, Yu J, Sessa WC, Dole WP, Rubanyi GM, Kauser K. Effective treatment of vascular endothelial growth factor refractory hindlimb ischemia by a mutant endothelial nitric oxide synthase gene. Gene Ther. 2006;13:1342–1350. doi: 10.1038/sj.gt.3302781. [DOI] [PubMed] [Google Scholar]
- 2.Chalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics. 2007;30:179–191. doi: 10.1152/physiolgenomics.00047.2007. [DOI] [PubMed] [Google Scholar]
- 3.Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid Redox Signal. 2009;11:251–266. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pries AR, Hopfner M, le Noble F, Dewhirst MW, Secomb TW. The shunt problem: control of functional shunting in normal and tumour vasculature. Nat Rev Cancer. 2010;10:587–593. doi: 10.1038/nrc2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ Res. 1998;83:636–643. doi: 10.1161/01.res.83.6.636. [DOI] [PubMed] [Google Scholar]
- 6.Looft-Wilson RC, Payne GW, Segal SS. Connexin expression and conducted vasodilation along arteriolar endothelium in mouse skeletal muscle. J Appl Physiol. 2004;97:1152–1158. doi: 10.1152/japplphysiol.00133.2004. [DOI] [PubMed] [Google Scholar]
- 7.Simon AM, McWhorter AR. Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. J Cell Sci. 2003;116:2223–2236. doi: 10.1242/jcs.00429. [DOI] [PubMed] [Google Scholar]
- 8.de Wit C, Roos F, Bolz SS, Kirchhoff S, Krüger O, Willecke K, Pohl U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res. 2000;86:649–655. doi: 10.1161/01.res.86.6.649. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa XF, Duling BR. Dissection of two Cx37-independent conducted vasodilator mechanisms by deletion of Cx40: electrotonic versus regenerative conduction. Am J Physiol Heart Circ Physiol. 2008;295:H2001–2007. doi: 10.1152/ajpheart.00063.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chadjichristos CE, Scheckenbach KE, van Veen TA, Richani Sarieddine MZ, de Wit C, Yang Z, Roth I, Bacchetta M, Viswambharan H, Foglia B, Dudez T, van Kempen MJ, Coenjaerts FE, Miquerol L, Deutsch U, Jongsma HJ, Chanson M, Kwak BR. Endothelial-specific deletion of connexin40 promotes atherosclerosis by increasing CD73-dependent leukocyte adhesion. Circulation. 2010;121:123–131. doi: 10.1161/CIRCULATIONAHA.109.867176. [DOI] [PubMed] [Google Scholar]
- 11.Langille BL, O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- 12.Burt JM, Nelson TK, Simon AM, Fang JS. Connexin 37 profoundly slows cell cycle progression in rat insulinoma cells. Am J Physiol Cell Physiol. 2008;295:C1103–C1112. doi: 10.1152/ajpcell.299.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morel S, Burnier L, Roatti A, Chassot A, Roth I, Sutter E, Galan K, Pfenniger A, Chanson M, Kwak BR. Unexpected role for the human Cx37 C1019T polymorphism in tumour cell proliferation. Carcinogenesis. 2010;31:1922–1931. doi: 10.1093/carcin/bgq170. [DOI] [PubMed] [Google Scholar]
- 14.Larson DM, Wrobleski MJ, Sagar GD, Westphale EM, Beyer EC. Differential regulation of connexin43 and connexin37 in endothelial cells by cell density, growth, and TGF-β1. Am J Physiol. 1997;272:C405–C415. doi: 10.1152/ajpcell.1997.272.2.C405. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan CJ, Doetschman T, Hoying JB. Targeted disruption of the Fgf2 gene does not affect vascular growth in the mouse ischemic hindlimb. J Appl Physiol. 2002;93:2009–2017. doi: 10.1152/japplphysiol.00451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki K, Murohara T, Ikeda H, Sugaya T, Shimada T, Shintani S, Imaizumi T. Evidence for the importance of angiotensin II type 1 receptor in ischemia-induced angiogenesis. J Clin Invest. 2002;109:603–611. doi: 10.1172/JCI13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature. 1997;385:525–529. doi: 10.1038/385525a0. [DOI] [PubMed] [Google Scholar]
- 19.Simon AM, Goodenough DA, Paul DL. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr Biol. 1998;8:295–298. doi: 10.1016/s0960-9822(98)70113-7. [DOI] [PubMed] [Google Scholar]
- 20.Buschmann I, Pries A, Styp-Rekowska B, Hillmeister P, Loufrani L, Henrion D, Shi Y, Duelsner A, Hoefer I, Gatzke N, Wang H, Lehmann K, Ulm L, Ritter Z, Hauff P, Hlushchuk R, Djonov V, van Veen T, le Noble F. Pulsatile shear and Gja5 modulate arterial identity and remodeling events during flow-driven arteriogenesis. Development. 2010;137:2187–2196. doi: 10.1242/dev.045351. [DOI] [PubMed] [Google Scholar]
- 21.Stabile E, Burnett MS, Watkins C, Kinnaird T, Bachis A, la Sala A, Miller JM, Shou M, Epstein SE, Fuchs S. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation. 2003;108:205–210. doi: 10.1161/01.CIR.0000079225.50817.71. [DOI] [PubMed] [Google Scholar]
- 22.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 23.Wong CW, Christen T, Roth I, Chadjichristos CE, Derouette JP, Foglia BF, Chanson M, Goodenough DA, Kwak BR. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med. 2006;12:950–954. doi: 10.1038/nm1441. [DOI] [PubMed] [Google Scholar]
- 24.Pfenniger A, Derouette JP, Verma V, Lin X, Foglia B, Coombs W, Roth I, Satta N, Dunoyer-Geindre S, Sorgen P, Taffet S, Kwak BR, Delmar M. Gap junction protein Cx37 interacts with endothelial nitric oxide synthase in endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30:827–834. doi: 10.1161/ATVBAHA.109.200816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alonso F, Boittin FX, Beny JL, Haefliger JA. Loss of connexin40 is associated with decreased endothelium-dependent relaxations and eNOS levels in the mouse aorta. Am J Physiol Heart Circ Physiol. 2010;299:H1365–H1373. doi: 10.1152/ajpheart.00029.2010. [DOI] [PubMed] [Google Scholar]
- 26.Langille BL, Reidy MA, Kline RL. Injury and repair of endothelium at sites of flow disturbances near abdominal aortic coarctations in rabbits. Arteriosclerosis. 1986;6:146–154. doi: 10.1161/01.atv.6.2.146. [DOI] [PubMed] [Google Scholar]
- 27.Silvestre JS, Mallat Z, Tedgui A, Levy BI. Post-ischaemic neovascularization and inflammation. Cardiovasc Res. 2008;78:242–249. doi: 10.1093/cvr/cvn027. [DOI] [PubMed] [Google Scholar]
- 28.Wagner C, de Wit C, Kurtz L, Grunberger C, Kurtz A, Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res. 2007;100:556–563. doi: 10.1161/01.RES.0000258856.19922.45. [DOI] [PubMed] [Google Scholar]
- 29.Paiardi S, Rodella LF, De Ciuceis C, Porteri E, Boari GE, Rezzani R, Rizzardi N, Platto C, Tiberio GA, Giulini SM, Rizzoni D, Agabiti-Rosei E. Immunohistochemical evaluation of microvascular rarefaction in hypertensive humans and in spontaneously hypertensive rats. Clin Hemorheol Microcirc. 2009;42:259–268. doi: 10.3233/CH-2009-1195. [DOI] [PubMed] [Google Scholar]
- 30.Wagner C, Kurtz L, Schweda F, Simon AM, Kurtz A. Connexin 37 is dispensable for the control of the renin system and for positioning of renin-producing cells in the kidney. Pflügers Arch. 2009;459:151–158. doi: 10.1007/s00424-009-0707-6. [DOI] [PubMed] [Google Scholar]
- 31.Takenaka T, Inoue T, Kanno Y, Okada H, Meaney KR, Hill CE, Suzuki H. Expression and role of connexins in the rat renal vasculature. Kidney Int. 2008;73:415–422. doi: 10.1038/sj.ki.5002673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figures