Abstract
Epithelial-to-Mesenchymal Transition (EMT) is an important pathogenic mechanism mediating glomerular injury or sclerosis in a variety of renal and systemic diseases such as hyperhomocysteinemia (hHcys). The present study was designed to test whether Hcys-induced EMT in podocytes is reversed by growth hormone (GH), a hormone regulating cell differentiation and growth and to explore the cellular and molecular mechanism mediating its action. It was found that Hcys induced significant EMT in podocytes, as shown by marked decreases in slit diaphragm-associated protein P-cadherin and zonula occludens-1 as epithelial markers and by dramatic increases in the expression of mesenchymal markers, fibroblast specific protein-1 and α-smooth muscle actin, which were detected by all examinations via immunocytochemistry, real time RT-PCR and Western blot analysis. When podocytes were treated with GH at 25 ng/mL, however, Hcys failed to induce podocyte EMT. Using electromagnetic spin resonance spectrometry, Hcys-induced superoxide (O2.−) production via NADPH oxidase was found to be significantly inhibited by GH (66%). Functionally, GH was shown to substantially inhibit Hcys-induced increases in the permeability of podocyte monolayers and to block the decrease in podocin expression in these cells. In addition, NADPH oxidase subunit, gp91phox and GH receptors aggregated in membrane raft clusters, which produced O2.− in response to Hcys and could be blocked by GH, membrane raft disruptors filipin and MCD or NADPH oxidase inhibitor, apocynin. It is concluded that Hcys-induced podocyte EMT is associated with transmembrane membrane raft-redox signaling and that GH reverses this Hcys-induced EMT protecting podocytes from functional disturbance.
Key Words: Homocysteinemia, Growth hormone therapy, Reactive oxygen species, Transdifferentiation, Membrane microdomains
Introduction
Hyperhomocysteinemia (hHcys) has been known as a risk factor for many diseases such as cardiovascular diseases, stroke, neurodegenerative diseases, end-stage renal disease, multiple sclerosis, and osteoporotic fractures [1-4]. Studies from our laboratory [5, 6] and by others [7] have demonstrated that Hcys induces extracellular matrix accumulation in mesangial cells and podocyte injury, which lead to glomerulosclerosis and loss of renal function, resulting in end-stage renal disease (ESRD). The podocyte, as an important structure of the glomerular filtration barrier, is a dynamic cell involved in several important cellular or physiological processes determining glomerular function such as glomerular filtration, maintenance of the glomerular basement membrane (GBM), regulation of the shape and integrity of the capillaries, and signal transduction. These podocytes are characterized by extensive interdigitating foot processes containing actin filaments [8]. Recent studies have indicated that podocyte injury is an important early event leading to glomerulosclerosis in both genetic and nongenetic glomerular diseases [9]. Indeed, our laboratory and others have shown that the podocyte injury also occurred during hyperhomocysteinemia (hHcys) via nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation and subsequent Superoxide (O2−) generation, which resulted in glomerular sclerosis. It has also been demonstrated that Hcys-induced epithelial-to-mesenchymal transition (EMT) of podocytes is a crucial event leading to hHcys-induced glomerulosclerosis [6, 10, 11]. Knocking out the gp91phox gene, one essential catalytic subunit of NADPH oxidase complex, significantly inhibited O2− production and EMT induced by hHcys and protected podocytes from Hcys-induced injury [11]. These results indicated that O2− production via NADPH oxidase and associated EMT in podocytes may be an important target for treatment or prevention of hHcys-induced glomerular sclerosis.
In this regard, growth hormone (GH) as a therapeutic strategy under different pathological conditions has been demonstrated to decrease oxidative stress and recover antioxidant defenses via reduced cellular ROS generation through various pathways such as upregulation of the expression of Mn-SOD, Cu, Zn-SOD, GPx-1 and eNOS in endothelial cells [12, 13]. In addition, a growth hormone-releasing peptide, ghrelin has also been found to have protective effects from Hcys-induced coronary endothelial dysfunction by increasing expression of endothelial nitric oxide synthase and reducing local oxidative stress [14-16]. However, it remains unknown whether GH is able to protect podocytes or glomeruli from hHcys-induced injury.
In the present study, we performed a series of studies to test the hypothesis that GH protects podocytes from Hcys-induced injury through abrogation of enhanced EMT associated with NADPH oxidase activation. We first determined the effects of GH on Hcys-induced changes in expression of EMT markers including the slit diaphragm-associated proteins P-cadherin and zonula occludens-1 (ZO-1) as epithelial markers and the mesenchymal markers fibroblast specific protein-1 (FSP-1) and α-smooth muscle actin (α-SMA). We also determined whether GH-induced protective effects are associated with inhibition of Hcys-induced increases in NADPH oxidase-dependent O2 .- production and whether GH indeed restores podocyte function from Hcys-induced impairment that relate to podocin production, cell monolayer permeability, and VEGF production. Then, we went on to explore the mechanisms by which GH exerts its beneficial effect through inhibition of membrane raft (previously referred to as lipid raft) clustering and associated redox signaling. Our results demonstrate that GH is able to abrogate Hcys-induced formation of membrane raft platforms and to block a transmembrane redox signaling pathway, whereby Hcys-induced podocyte EMT is blocked.
Materials and Methods
Cell culture
Conditionally immortalized mouse podocytes cell line, kindly provided by Dr. Klotman PE (Division of Nephrology, Department of Medicine, Mount Sinai School of Medicine, New York, NY, USA), were cultured on collagen I-coated flasks or plates in RPMI 1640 medium supplemented with recombinant mouse interferon–γ at 33°C. After differentiated at 37°C for 10-14 days without interferon–γ, podocytes were used for proposed experiments. The preparation of L-Hcys (a pathogenic form of Hcys) and the concentration and incubation time of L-Hcys treatment were chosen based on our previous studies [17]. In addition, mouse GH (from National Hormone & Peptide Program Harbor-UCLA Medical Center, Torrance, California, USA) was added into the culture medium and then incubated for different time periods. The concentrations used for all protocols were decided based on our preliminary dose-response experiments (from 12.5-50 ng/mL), which showed that 25 ng/mL GH slightly activated EMT in podocytes and had stable effects on Hcys-induced changes in EMT.
Immunofluorescent microscopy
Double-immunofluorescent staining was performed using cultured podocytes on cover slips. After fixation, the cells were incubated with rabbit anti-podocin 1: 200 (Sigma, St. Louis, MO, USA), which was followed by incubation with Alex-488-labeled donkey anti-rabbit secondary antibody. Then, goat anti-FSP-1 (1:50 dilution), goat anti-ZO-1 (1:50 dilution) (Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA), goat anti-P-cadherin (1:25 dilution), or mouse anti-α-SMA (1:300 dilution) (R&D system, Minneapolis, MN, USA) were added to the cell slides and then incubated overnight at 4°C. After washing, the slides were incubated with corresponding Alex-555-labeled secondary antibodies and then mounted and subjected to examinations using a confocal laser scanning microscope (Fluoview FV1000, Olympus, Japan). These double staining experiments were performed to observe the relationship between podocin production and EMT changes during Hcys incubation.
In addition to double staining and confocal microscopy, several groups of podocytes were used for quantitative analysis of expression of EMT markers under fluorescence microscope. In these experiments, podocytes on cover slips were fixed in 4% PFA for 20 minutes. After rinsed with phosphate-buffer saline (PBS), they were incubated with rabbit anti-FSP-1 (1:100, Abcam, Cambridge, MA, USA), rabbit anti-ZO-1 (1:50, Invitrogen, Camarillo, CA, USA), rabbit anti-P-cadherin (1:25), or mouse anti-α-SMA (1:300, R&D system, Minneapolis, MN, USA) antibodies. After washing, the slides were incubated with corresponding Alex-488-labeled secondary antibodies for 1 h at room temperature. After mounted with DAPI-containing mounting solution, the slides were observed under a fluorescence microscope and photos were taken and analyzed.
Real-time reverse transcription polymerase chain reaction (RT-PCR)
Total RNA from cultured podocytes was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA. USA) according to the protocol as described by the manufacturer. Aliquots of total RNA (1 μg) from each sample were reverse-transcribed into cDNA according to the instructions of the first strand cDNA synthesis kit manufacturer (Bio-Rad, Hercules, CA, USA). Equal amounts of the reverse transcriptional products were subjected to PCR amplification using SYBR Green as the fluorescence indicator on a Bio-Rad iCycler system (Bio-Rad, Hercules, CA, USA). The mRNA levels of target genes were normalized to the β-actin mRNA levels. The primers used in this study were synthesized by Operon (Huntsville, AL, USA) and the sequences were: for P-cadherin, sense GTA AGG GCT ACC GCT CAC TC, antisense TGT GAG GCC AAG TGA AAG AC; for ZO-1, sense GAG CTA CGC TTG CCA CAC TGT, antisense TCG GAT CTC CAG GAA GAC ACT T; for FSP-1, sense GTT ACC ATG GCA AGA CCC TT, antisense AAC TTG TCA CCC TCT TTG CC; for α-SMA, sense CAG GAT GCA GAA GGA GAT CA, antisense TCC ACA TCT GCT GGA AGG TA; and for β-actin, sense TCG CTG CGC TGG TCG TC, antisense GGC CTC GTC ACC CAC ATA GGA.
Western blot analysis
Western blot analysis was performed as we described previously [18]. In brief, homogenates from cultured podocytes were prepared using sucrose buffer containing protease inhibitors. After boiling for 5 min at 95°C in a 5× loading buffer, 20 μg of total proteins were subjected to SDS-PAGE, transferred onto a PVDF membrane and blocked by solution with dry milk. Then, the membrane was probed with primary antibodies of anti-ZO-1 (1:500, Invitrogen), anti-P-cadherin (1: 500, R&D System), anti-α-SMA (1:5000, R&D System), anti-FSP-1 (1:500, Abcam) or anti-β-actin (1:5000, Santa Cruz Biotechnology) overnight at 4 °C followed by incubation with horseradish peroxidase-labeled IgG (1:5000). The immunoreactive bands were detected by chemiluminescence methods and visualized on Kodak Omat X-ray films. Densitometric analysis of the images obtained from X-ray films was performed using the Image J software (NIH, Bethesda, MD, USA).
NADH/NADPH oxidase activity in podocytes
Fluorescence spectrometry for O2 .- production in podocytes was performed by using a modification of methods described previously [19]. Briefly, the fluorogenic oxidation of dihydroethidium (DHE) to ethidium (Eth) was used as a measure of O2.- production. The homogenates (10 μg) freshly prepared from podocytes were incubated with DHE (10 μ mol/L) and salmon testes DNA (0.5 mg/mL, Sigma) with or without NADPH (2 mmol/L, Sigma) in a microtiter plate at 37°C for 30 minutes, and then Eth-DNA fluorescence was measured at an excitation of 485 nm and an emission of 590 nm by using a CytoFluor Series 4000-fluorescence microplate reader (Applied Biosystems, Foster City, CA, USA). The Eth fluorescence in podocytes incubated without NADPH was quantified as basal O2.- levels, and NADH/NADPH oxidase activity to produce O2.- examined by addition of NADPH as a substrate in the reaction mixture. Salmon DNA was added to bind to Eth and consequently stabilize Eth fluorescence, thereby increasing the sensitivity of O2.- measurement (>40 fold). The enzyme activity of NADH/NADPH oxidase and O2.- levels were presented as percent increases in Eth fluorescence versus control.
Electromagnetic spin resonance (ESR) analysis of NADPH oxidase-dependent O2.- production
For detection of NADPH oxidase-dependent O2.- production, homogenates from cultured podocytes were extracted using sucrose buffer and re-suspended with modified Kreb's-Hepes buffer containing deferoximine (100 μmol/L, Sigma) and diethyldithiocarbamate (5 μmol/L, Sigma). The NADPH oxidase-dependent O2.- production was examined by addition of 1 mM NADPH (Sigma) as a substrate in 10 μg protein and incubated for 10 min at 37 °C in the presence or absence of SOD (200 U/mL, Sigma), and then supplied with 1 mM O2.- specific spin trap 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH, Noxygen, Elzach, Germany) as we described before [20]. SOD was added to measure SOD-inhibitable signaling of CMH. The mixture was loaded in glass capillaries and immediately analyzed for O2.- production kinetically for 5 min in a Miniscope MS200 ESR spectrometer (Magnettech Ltd, Berlin, Germany). The ESR settings were as follows: biofield, 3350; field sweep, 60 G; microwave frequency, 9.78 GHz; microwave power, 20 mW; modulation amplitude, 3 G; 4,096 points of resolution; receiver gain, 50. The results were expressed as the fold changes of control.
Assay of the permeability through podocytes monolayer
The permeability of podocyte monolayer was measured as we described previously [21]. Briefly, podocytes were seeded in the upper chambers of 0.4μm polycarbonate Transwell filters of a 24-well filtration microplate (Whatman Inc., Florham Park, NJ, USA). After treatment with L-Hcys (40 μmol/L) with or without GH (25 ng/mL) for 48 h, the culture medium was replaced with fresh phenol red-free RPMI 1640 in the presence of Hcys and 70 kD FITC-dextran (2.5 mol/l) in the upper chambers. After 6 h, the filtration microplate was removed, the medium from the lower compartment was collected, and then fluorescence was measured in a spectrofluorometer at an excitation wavelength of 494 nm. The permeable fluorescence intensity was used to represent cell permeability and the values were normalized to that of the control cells.
ELISA for VEGF-A secretion by podocytes
Podocytes were incubated with different stimulations like GH (25 ng/mL) and L-Hcys (40 μmol/L) with or without GH (25 ng/mL) for 24 h. The supernatant was collected for ELISA to measure VEGF-A using a commercially available kit (R&D Systems, Minneapolis, MN, USA).
Confocal microscopic detection of membrane raft platforms and their colocalization with NADPH oxidase subunits or growth hormone receptor (GHR) in podocytes
For confocal microscopic detection of membrane raft clusters or platforms and their associated proteins, podocytes were seeded on poly-L-lysine-coated chambers. The cells were treated with L-Hcys (40 μM) with or without GH (25 ng/mL) or vehicle for 30 min. In additional groups of podocytes, the membrane raft disruptors, methyl-β-cyclodextrin (MCD) at 1 mM (Sigma) and filipin at 1 μg/mL (Sigma), were added to pretreat cells for 30 min before addition of L-Hcys with GH. The formation of membrane raft platforms was detected under confocal microscope as we previously described [22]. Briefly, podocytes were washed with cold PBS, fixed for 20 min in 4% PFA and then blocked with 1% BSA in PBS for 30 min. GM1 gangliosides enriched in membrane rafts were stained with Alexa488-labeled cholera toxin B (Alexa488-CTXB) at 0.5 μg/mL (Molecular Probes, Eugene, OR, USA) for 30 min. For detection of the colocalization of membrane raft platforms and NADPH oxidase subunits gp91phox or GHR, podocytes were incubated overnight with indicated primary monoclonal mouse anti-gp91phox (BD biosciences, San Jose, CA, USA) or anti-GHR (Sigma) at 1:100 followed by incubation with 5 μg/rnL Texas Red-conjugated anti-mouse IgG or Texas Red-conjugated antigoat IgG for an additional 1 h at room temperature. Then, Alexa488-labeled cholera toxin B (Alexa488-CTXB) at 0.5 μg/mL was used to label GM1 gangliosides enriched in membrane raft platforms. After mounting, the slides were observed using a confocal laser scanning microscope (Fluoview FV1000, Olympus, Japan). In each slide, the presence or absence of clusters or platforms in 100 cells was scored by an unwitting researcher after specifying the criteria for positive spots of fluorescence. Podocytes that displayed a homogenous distribution of fluorescence were indicated as negative. Results were given as the percentage of podocytes showing one or more overlaid fluorescent spots or patches vs. total cells observed in each protocol.
Statistical analysis
All of the values are expressed as mean ± SEM. Significant differences among multiple groups were examined using ANOVA followed by a Student-Newman-Keuls test. χ2 test was used to assess the significance of ratio and percentage data. P<0.05 was considered statistically significant.
Results
Hcys-induced changes in EMT markers detected by fluorescent microscopy in the absence and presence of GH
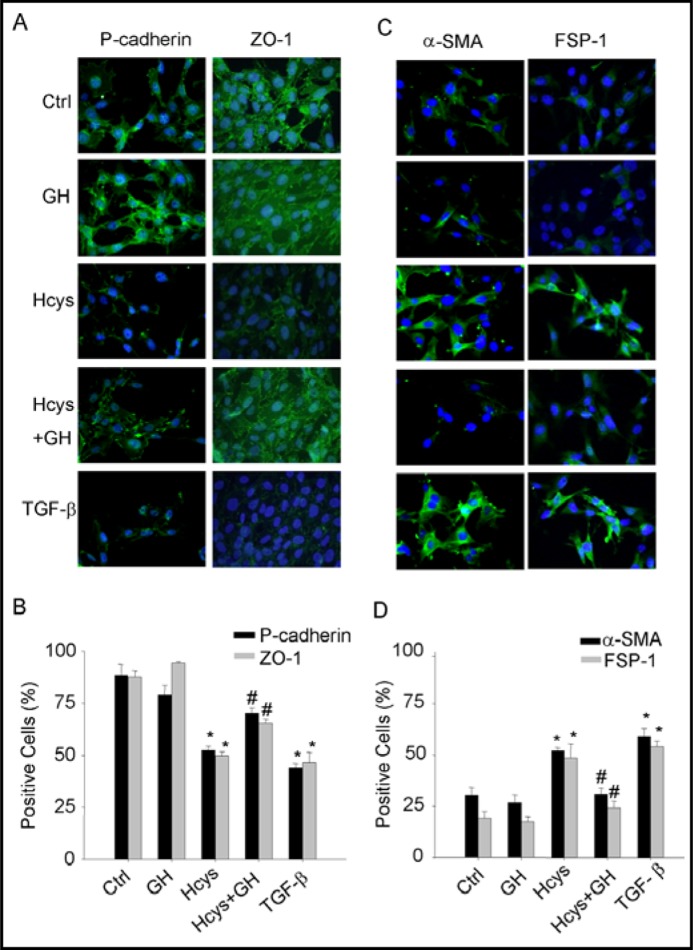
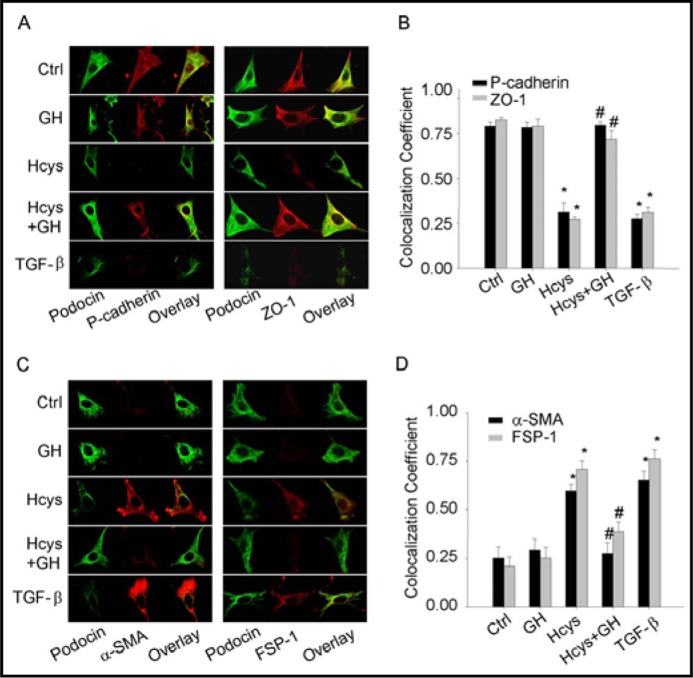
To determine whether GH has effects on Hcys-induced EMT in podocytes, immunostaining analysis of EMT markers was performed in podocytes before and after stimulation of Hcys. As shown in Fig. 1A, under basal condition podocytes were enriched with P-cadherin and ZO-1, two epithelial markers. When these podocytes were treated with L-Hcys (40 μmol/L) or TGF-β as a positive control (2.5 ng/mL), the expression of both P-cadherin and ZO-1 was markedly reduced as shown in decreased green fluorescence in example images and positive stained podocytes (Fig. 1A, 1B). This Hcys-induced reduction of P-cadherin and ZO-1 expression in podocytes was similar to that induced by TGF-β. GH (25 ng/mL) had no significant effect on the expression of P-cadherin and ZO-1, but it almost completely blocked Hcys-induced reduction of the expression of both epithelial markers. In contrast, the abundance of two mesenchymal markers, FSP-1 and α-SMA were very low in podocytes under control condition. When these podocytes were stimulated by L-Hcys, the expression of both FSP-1 and α-SMA was remarkably increased to an extent similar to TGF-β. When podocytes were pretreated with GH, L-Hcys failed to increase FSP-1 and α-SMA expression (Fig. 1C, 1D).
Fig. 1.
Fluorescent microscopic evidence of Hcys-induced epithelial-to-mesenchymal transition (EMT) of podocytes in the absence or presence of GH. Podocytes were stimulated by Hcys (40 μM) with or without GH (25 μg/mL) or TGF-β (2.5 μg/mL) for 24 h. A. Representative images show the expression of P-cadherin and ZO-1 as epithelial markers in different groups. B. Summarized data show the percentage of positive staining cells of P-cadherin and ZO-1 positive staining vs. control group (n=5 patches of podocytes). C. Representative immuno-fluorescent staining shows the expression of α-SMA and FSP-1 as mesenchymal markers in different cell groups. D. Summarized data show the percentage of positive staining cells of α-SMA and FSP-1 vs. control group (n=5). ∗ P<0.05 vs. Ctrl, # P<0.05 vs. Hcys.
Effects of GH on Hcys-induced changes in the expression of EMT markers detected by real time RT-PCR and Western blot analysis
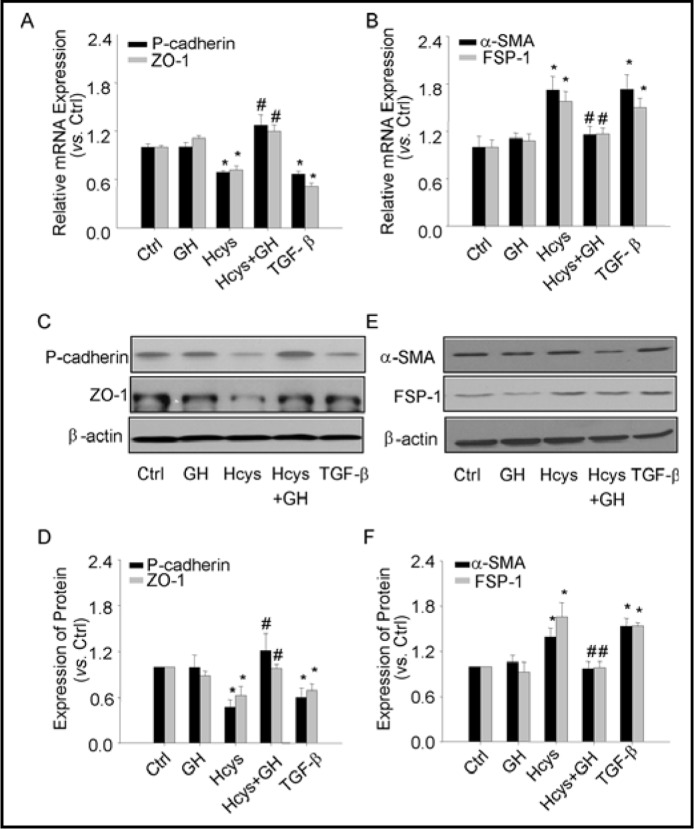
To further confirm the effects of GH on Hcys-induced EMT markers expression, we performed real time RT-PCR and Western blot analysis to quantify the expression of P-cadherin, ZO-1, FSP-1 and α-SMA in podocytes. As shown in Fig. 2A, 2C and 2D, mRNA and protein levels of P-cadherin and ZO-1 were significantly decreased in podocytes treated with Hcys for 24 h, which was similar to that induced by TGF-β, a well-known cytokine that affects EMT. When these podocytes were incubated with GH, no significant changes in the mRNA and protein levels of P-cadherin and ZO-1 were observed. However, co-treatment of GH with L-Hcys significantly reversed the action of L-Hcys on both mRNA and protein levels of P-cadherin and ZO-1. As shown in Fig. 2B, 2E and 2F the changes in mesenchymal markers were detected under different treatments by real time RT-PCR and Western blot analysis. In contrast to its effects on epithelial markers, L-Hcys significantly increased both mRNA and protein levels of FSP-1 and α-SMA, which was similar to the effect of TGF-β. In the presence of GH, this L-Hcys-induced increase in the expression of FSP-1 and α-SMA was substantially blocked.
Fig. 2.
Effects of GH on Hcys-induced changes in mRNA and protein expression of P-cadherin, ZO-1, FSP-1 and a-SMA. A. Relative quantitative data summarizing the effect of Hcys on mRNA expression of epithelial markers, P-cadherin and ZO-1 in podocytes detected by real-time RT-PCR analysis (n=6). B. Relative quantitative data summarizing the effect of Hcys on mRNA expression of mesenchymal markers (α-SMA and FSP-1) in podocytes (n=6). C. Representative gel documents showing the expression of P-cadherin and ZO-1 in different groups. D. Summarized data showing expression of P-cadherin and ZO-1, quantitated as a ratio of detected specific protein band vs. β-actin as loading control (n=4-5). E. Representative gel documents showing the expression of α-SMA and FSP-1 in different groups. F Summarized data showing expression of α-SMA and FSP-1, quantitated as a ratio of detected specific protein band vs. β-actin as loading control (n=4-5). ∗ P<0.05 vs. Ctrl podocytes; # P<0.05 vs. Hcys.
Inhibition of Hcys-induced NADPH oxidase activation in podocytes by GH
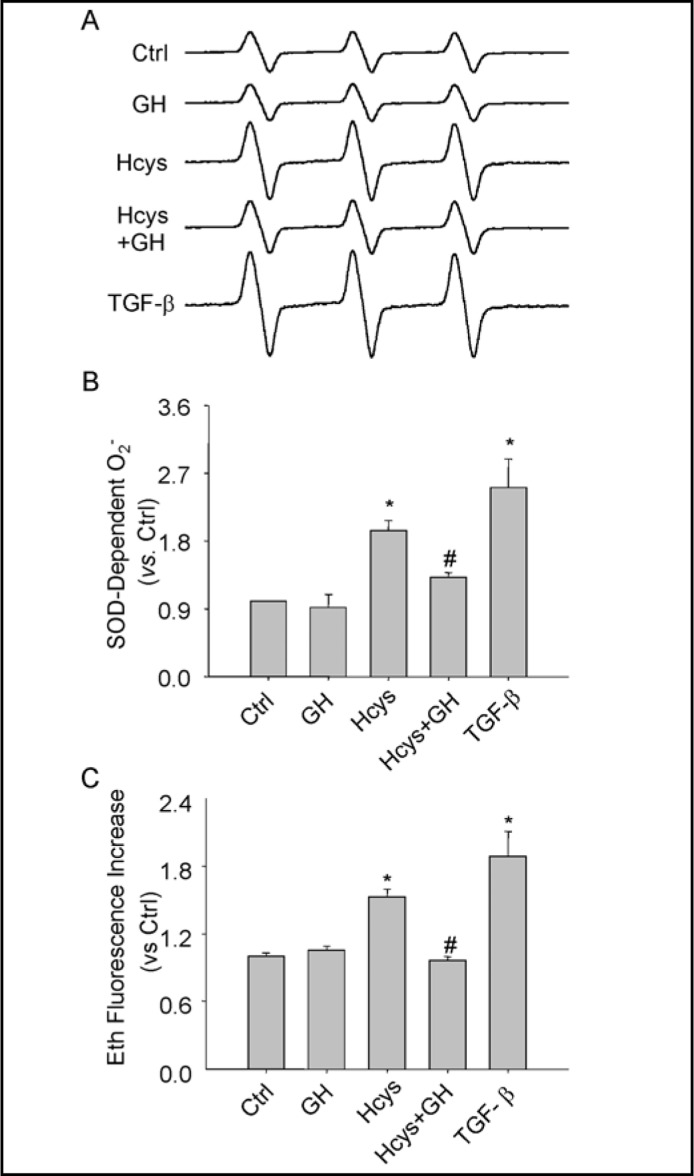
Redox signaling via NADPH oxidase has been reported to be importantly involved in progressive glomerular injury or glomerulosclerosis associated with hHcys [10, 11, 20]. To explore potential mechanisms underlying the action of GH on Hcys-induced EMT of podocytes, we examined the NADPH oxidase-dependent O2.- production of podocytes treated by Hcys with or without GH for 24 hours. Fig. 3A shows typical ESR spectrographs, which depict increased amplitude of the spectrum of trapped NADPH-derived O2.- in podocytes treated with L-Hcys in comparison with control podocytes. The action of L-Hcys to increase O2.- production was relatively weaker compared to that obtained in podocytes treated with TGF-β, which doubled this O2.- production compared to control cells (Fig. 3B). After podocytes were co-treated with GH, L-Hcys-induced O2.- production was significantly reduced (Fig. 3B, Hcys+GH). To further demonstrate the action of GH on L-Hcys-induced NADPH oxidase activity, a fluorescence spectrometric assay was also used to determine the activity of NADPH oxidase in podocytes (Fig. 3C). Similarly, L-Hcys was found to significantly increase NADPH oxidase activity in the homogenates of podocytes, which was similar like TGF-β. GH treatment completely blocked Hcys-induced increase in NADPH oxidase activity. However, the expression of NADPH oxidase subunits was not altered by Hcys and GH treatments as observed by real time RT-PCR and Western blot (data not shown).
Fig. 3.
Inhibition by GH of Hcys-induced NADPH oxidase activation. A. Representative ESR spectra traces for SOD-dependent O2.- producton in podocytes. B. Summarized data show O2.- production in podocytes under different conditions, which were normalized to control podocytes (n=5). ∗ P<0.05 vs. Ctrl; # P<0.05 vs. Hcys. C. Summarized DHE data show NADPH oxidase activity in podocytes under different conditions, which were normalized to control podocytes (n=5). ∗ P<0.05 vs. Ctrl; # P<0.05 vs. Hcys.
Effect of GH on Hcys-induced enhancement of podocyte-monolayer permeability and VEGF secretion
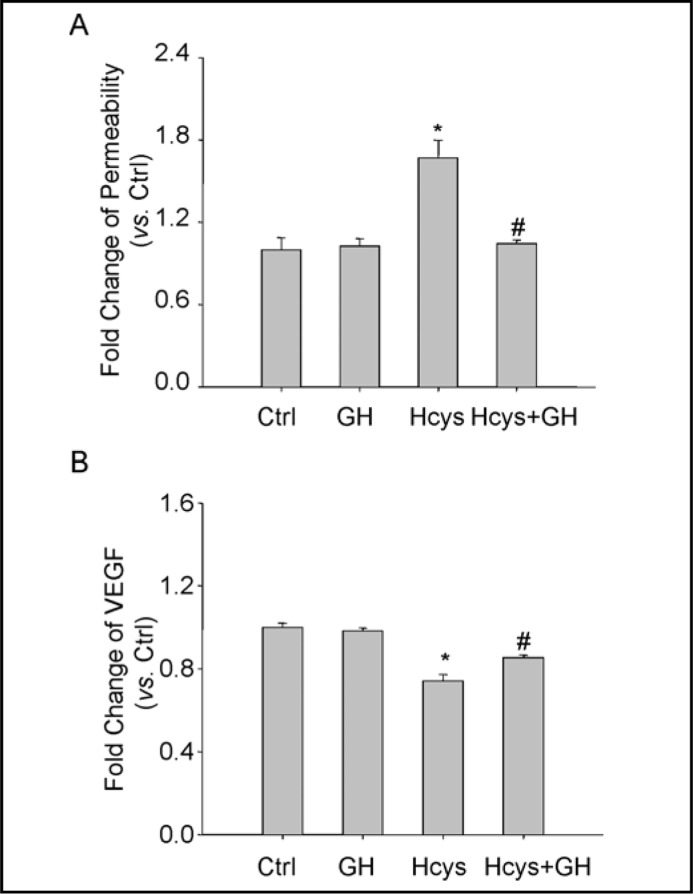
To determine the functional significance of the GH action, we examined its influence on L-Hcys-induced changes in barrier function of podocyte monolayers. As shown in Fig. 4A, dextran flux significantly increased in podocytes treated with Hcys. This L-Hcys-induced increase in permeability of podocytes was markedly reduced in the presence of GH.
Fig. 4.
Effects of GH on Hcys-induced enhancement of podocyte monolayer permeability and VEGF-A secretion in podocytes. A. Effects of GH on Hcys-induced enhancement of podocyte monolayer permeability after 48 h (n=4-5). B. VEGF secretion in the supernatant of podocytes detected by ELISA after treatment with Hcys alone or with GH for 24 h (n=6). ∗ P<0.05 vs. Ctrl; # P<0.05 vs. Hcys.
To further confirm the effect of GH on Hcys-induced functional abnormality of podocytes, we determined the production of VEGF-A in the cultured medium of podocytes after treatment of these cells with L-Hcys and L-Hcys plus GH. It was found that VEGF-A secretion were significantly reduced by the treatment of podocytes with L-Hcys for 24 h. In the presence of GH, however, the Hcys-induced decrease in VEGF-A secretion was almost completely restored (Fig. 4B).
Reversal of Hcys-induced podocin decrease by GH
Hcys has been reported to lead to podocyte injury as shown by decreased expression of podocin, a slit diaphragm molecule, at the mRNA and protein levels [23]. We determined whether GH also reverses Hcys-induced decreases in podocin production. By double-immuno-staining analysis, co-localization of podocin and epithelial markers, P-cadherin and ZO-1, were found to be significantly decreased when podocytes were treated with Hcys for 24 h. TGF-β as positive control also had similar effects on expression of these molecules. Such co-localization of podocin and epithelial markers was recovered in the presence of GH. Compared to control, GH alone had no significant effect on the expression of podocin and epithelial markers in podocytes (Fig. 5A). The summarized data were shown in Fig 5B.
Fig. 5.
Podocin expression linking to changes in EMT markers of podocytes. A. Images showing double-immunostained podocytes for P-cadherin or ZO-1 (Alex555, red color) with podocytes marker, podocin (Alex488, green color). B. Summarized data showing colocalization coefficient of P-cadherin or ZO-1 with podocin (n=5). C. Images showing double-immunostained podocytes for α-SMA or FSP-1 (Alex555, red color) with podocin (Alex488, green color). D. Summarized data showing colocalization coefficient of α-SMA or FSP-1 with podocin (n=5), ∗P<0.05 vs. ctrl; # P<0.05 vs. Hcys.
Different from P-cadherin and ZO-1, colocalization of podocin with mesenchymal markers FSP-1 or α-SMA was very weak under control condition. Hcys markedly increased the expression of both mesenchymal markers as shown in red fluorescence, but reduced podocin levels, revealing an inverse relationship between podocin and FSP-1 or α-SMA. In the presence of GH, Hcys-increased local production of FSP-1 and α-SMA and the decreased podocin expression in podocytes was inhibited (Fig. 5C and 5D).
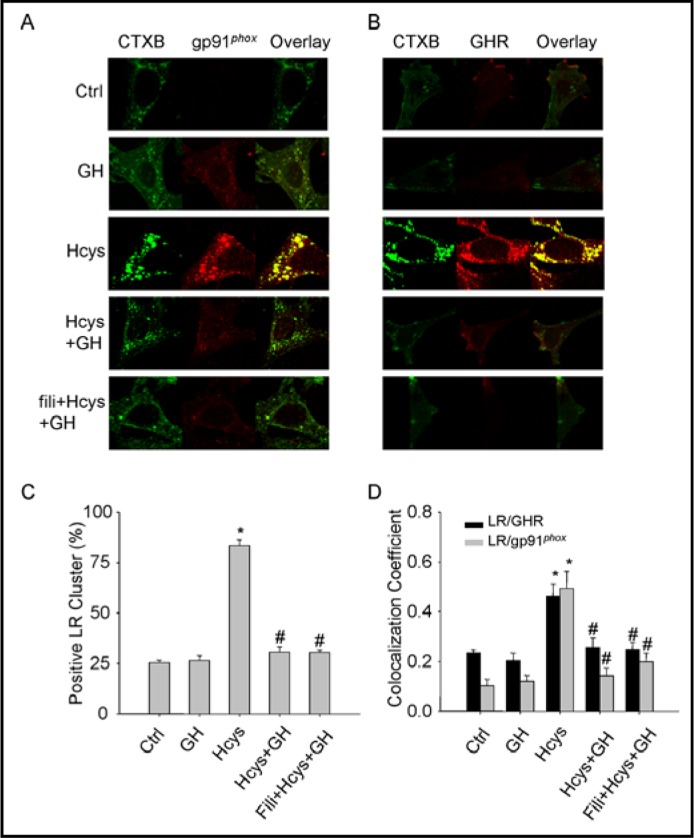
Clustering of gp91phox and GH receptor (GHR) with membrane raft platforms in podocytes upon Hcys stimulation
These experiments were designed to explore the mechanisms mediating the action of GH on Hcys-induced podocyte EMT and injury. In previous studies, we have demonstrated that Hcys-induced podocyte injury was associated with the formation of a membrane raft redox signaling platforms, which involves molecular trafficking and aggregation of NADPH oxidase subunits in membrane raft clusters on the podocyte membrane and consequent assembling and activation of NADPH oxidase [23]. In the present study, we hypothesized that GH exerts its protective action on podocytes from Hcys-induced injury by inhibition of membrane raft redox signaling platform formation. To test this hypothesis, podocytes were stained with Alexa488-labeled cholera toxin B (CTXB, a membrane raft marker) and colocalized with GHR or NADPH oxidase subunit, gp91phox. Fig. 6A and 6B present typical confocal microscopic images depicting membrane raft patches or platforms with GHR or gp91phox. Membrane rafts or related proteins were evenly spread throughout the cell membrane under control condition as indicated by weak diffuse green FITC fluorescence of CTXB in control podocytes. There was no significant increase in membrane raft clustering after treatment with GH alone. Upon stimulation of Hcys, however, membrane raft clusters were found to be increased as displayed by large and intense green fluorescence patches. However, treatment of podocytes with GH led to disruption of membrane raft clusters and abrogated patching and clustering of FITC-CTXB induced by Hcys. Pretreatment of podocytes with filipin, a membrane raft disruptor alone or together with GH also blocked Hcys-induced the formation of membrane raft platforms. The results showing these effects of L-Hcys and GH on the formation of membrane raft platforms were summarized in Fig. 6C, where L-Hcys was demonstrated to increase the formation of membrane raft platforms by more than 3 fold as shown by positive cells with detectable green CTXB patches (from 25% to 83%).
Fig. 6.
Effects of GH on Hcys-induced membrane rafts clustering and associated aggregation of gp91Phox or GHR in podocytes. A. Representative confocal fluorescent microscopic images for membrane raft clusters (Alexa488-CTXB) and gp91phox (Texas Red conjugated antibody) on the podocytes membrane after different treatments. B. Representative confocal fluorescent microscopic images for membrane raft clusters (Alexa488-CTXB) and GHR (Texas Red conjugated antibody) on the podocytes membrane after different treatments. C. Summarized data depicting the percentage of positive cells with membrane raft clusters (fluorescent patches or dots) under different treatments (n=5). D. Summarized colocalization coefficient data of membrane raft clusters with gp91phox or GHR after different treatments (n=5). ∗ P<0.05 vs. Ctrl; # P<0.05 vs. Hcys.
It was also found that Hcys induced aggregation of gp91phox, a major NADPH oxidase subunit in membrane raft platforms of podocytes as shown by colocalization of Alexa Fluo 488 labeled CTXB and anti-gp91Phox plus Alexa 555 labeled antibody, as shown in yellow spot or patches in overlaid images of Fig. 6A. Similarly, L-Hcys stimulated clustering of GHR in membrane raft clusters (Fig. 6B). However, when podocytes were pretreated with GH, Hcys-induced clustering of both gp91phox and GHR into membrane raft clusters were almost completely blocked, which was similar to the effect of the membrane raft disruptor, filipin. All these colocalization experiments were summarized in Fig. 6D, showing that membrane raft platforms clustered with GHR and gp91phox in response to L-Hcys stimulation as shown by significantly increased colocalization coefficient and that this effect of L-Hcys was completely abrogated by GH treatment and membrane raft disruptor.
Antagonistic effect of GH on Hcys-induced O2.- production associated with NADPH oxidase-dependent redox signaling
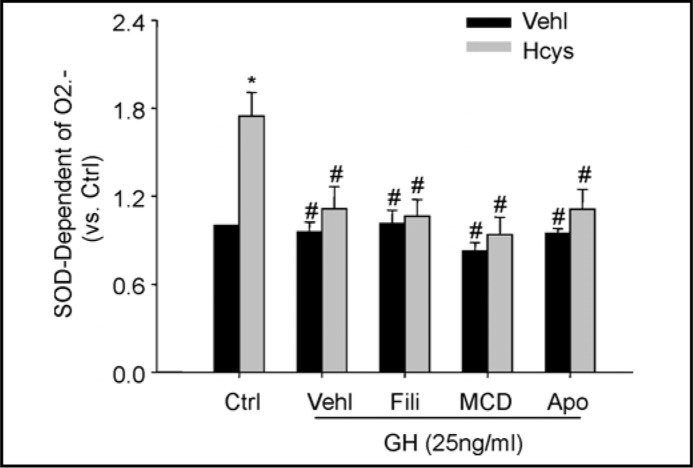
We analyzed the effects of GH on Hcys-induced O2.- production derived from membrane raft-associated NADPH oxidase in podocytes in the absence or presence of different inhibitors related to the formation of membrane raft platforms and NADPH oxidase activity. After pretreated with apocynin (100 μM, Sigma, St. Louis, MO, USA), MCD (1 mM, Sigma, St. Louis, MO, USA) or filipin (1 μg/ml) for 30 min, podocytes were treated with
L-Hcys (40 μM) or vehicle with or without GH for 30 min. As shown in Fig. 7, ESR analysis showed that Hcys-induced O2.- production was dramatically reduced by GH. This effect of GH on Hcys-induced production of O2.- was not further enhanced by membrane raft disruptors, filipin and MCD, suggesting that GH may share the same mechanism as filipin to inhibit membrane raft clustering and thereby redox signaling. In the presence of GH, similarly, pretreatment of podocytes with NADPH oxidase inhibitor, apocynin, had no further effects on Hcys-induced production of O2.-, further suggesting that antagonistic effect of GH on Hcys-induced O2.- production relates to inhibition of membrane raft clustering and NADPH oxidase activation (Fig. 7).
Fig. 7.
Antagonistic effects of GH on Hcys-induced O2.- production associated with membrane ran clusters. Summarized data showing the fold changes in SOD-dependent O2.- production in podocytes upon stimulation of Hcys under different treatments with or without GH. Ctrl: control; GH: growth hormone; Fili: flipin; MCD: methyl-β-cyclodextrin; Apo: apocynin (n=5-6). ∗ P < 0.05 vs. Ctrl; # P<0.05 vs. Hcys.
Discussion
The major goal of the present study was to determine whether Hcys-induced podocyte EMT and functional disorder is regulated or changed by GH and to explore the mechanisms mediating the effect of GH in association with the formation of GHR/ membrane raft clusters and redox signaling. We demonstrated that a low dose of GH (25 ng/mL) had no significant effect on EMT process and associated podocyte function. However, treatment of podocytes with GH prevented Hcys-induced podocyte injury, which related to its inhibitory action on Hcys-induced enhancement of EMT. This protective action of GH on EMT and consequent functional injury in podocytes was found to be due to blockade of the formation of membrane raft platforms with GHR and NADPH oxidase subunits and consequent O2.- production.
Although there were reports that excessive endogenous production of GH as a peptide hormone secreted by the pituitary gland may be an injurious factor in pathogenesis of some diseases or pathological process such as diabetes mellitus and progressive glomerulosclerosis [24, 25], this hormone is often used as a therapeutic agent for different medical conditions, some of which were approved by the U.S. Food and Drug Administration, such as cachexia [26], Turner syndrome [27], chronic renal failure [28], Prader-Willi syndrome [29], and idiopathic short stature (ISS) [30]. In addition to these and other therapeutic uses for improvement of growth in children or adults, GH has also been reported to be used for healing of large burns [31]or in obesity [32], Crohn's disease [33], chronic fatigue syndrome, aging [34] and various degenerative diseases such as Alzheimer's disease [35], multiple sclerosis [36], atherosclerosis [37] and chronic heart failure [38]. Although GH was used in patients with chronic renal failure, the goal of such therapy is mainly for improvement of slowed growth in children or young adults under related pathological conditions [39]. It remains unknown whether the application of GH during chronic renal failure could improve renal function and slow the progression of end-stage renal disease. In this regard, GH has been reported to possess significant cardiac protective effect in uremia rats [28, 40]. Given accumulating evidence that GH has strong antioxidant actions [12, 13, 41-43], it is possible that its therapeutic application may improve renal damage or glomerular sclerosis associated with local oxidative stress. The present study attempted to examine whether GH exerts its protective action from podocyte injury and consequent glomerular damage during hHcys, which is a well-known pathogenic factor resulting in end-stage renal disease through enhanced local oxidative stress [44, 45]. Indeed, GH-releasing peptide ghrelin has recently been reported to have protective effect on the endothelium from Hcys-induced injury, which is associated with increases in endothelial nitric oxide synthase expression and reduction of oxidative stress [14]. In the first series of experiments, we demonstrated that increased Hcys concentrations in the medium significantly induced EMT in podocytes, which was comparable to a well-established EMT inducer, TGF-β [46, 47]. This Hcys-enhanced EMT was almost completely blocked by co-treatment of GH. To our knowledge, these results represent the first experimental evidence that GH abrogates the EMT associated with increased Hcys stimulation in podocytes. Since podocytes injury is an important early mechanism in the development of glomerular sclerosis or end-stage renal disease [48] and EMT is critically involved in the initiation or development of glomerular sclerosis [46], the abrogating action of GH on podocyte EMT suggests that is may be used to block the progression of glomerular injury during hHcys. We also examined whether the effect of GH is associated with Hcys-enhanced oxidative stress in podocytes. It was found that GH blocked Hcys-induced NADPH oxidase activation that was reported to be a critical early mechanism initiating or promoting Hcys-induced podocyte injury and glomerulosclerosis [23]. However, GH had no effect on the expression of NADPH oxidase subunits. Although there is no report about the protective action of GH on local oxidative stress in glomeruli, many studies have demonstrated that GH indeed has beneficial effect on oxidative stress-associated injury in other tissues or organs such as heart, vessels and lymphocytes [12, 43, 49].
To address the functional significance of GH-induced suppression of EMT in podocytes, we determined the role of GH in Hcys-induced enhancement of podocyte monolayer permeability. There is substantial evidence that glomerular oxidative stress contributes to increases in epithelial monolayer permeability under different pathological conditions such as diabetes, nephritis, hypertension and hHcys [50, 51]. This increased cell permeability importantly participates in the development of glomerular injury and sclerosis [52, 53]. The present study demonstrated that Hcys-induced increase in the podocyte permeability was markedly attenuated by treatment of these cells with GH. Moreover, Hcys-induced decrease in the production of VEGF-A, as a glomerular permeability factor, was also reversed by GH treatment of podocytes. These results suggest that Hcys may lead to an abnormality of EMT and thereby reduce podocyte function and glomerular barrier integrity and that GH treatment prevents such pathological actions of Hcys and protects glomeruli from increased permeability. Given the evidence discussed above that GH also inhibited NADPH oxidase activity and reduced the production of O2.-, the suppression of local oxidative stress during application of GH may be attributed to its beneficial effect in preserving podocyte function during Hcys stimulation. In this regard, previous studies in our laboratory and by others demonstrated that Hcys-decreased production of VEGF-A and corresponding dysfunction of podocytes was restored by inhibition of NADPH oxidase activity or silencing of the gene coding this enzyme [11].
The next question we addressed in the present study is how GH acts to activate NADPH oxidase and thereby promote EMT in podocytes. It has been reported that GH-mediated biological responses are mainly associated with its binding to GHR and consequent activation of the tyrosine kinase, Janus kinase 2 (JAK2). These biological responses include cellular proliferation, differentiation and migration, prevention of apoptosis, cytoskeletal reorganization and regulation of metabolic pathways [54]. In addition, other signaling mechanisms related to signal transducers and activators of transcription (Stats), the mitogen activated protein kinase (MAPK) pathway, and the phosphatidylinositol 3’-kinase (PI3K) pathway were also reported to be involved in the action of GH in a variety of cells or tissues [54, 55]. However, there is no report that delineates the mechanism mediating the antioxidant action of GH. In our previous studies, the formation of membrane raft platforms in the plasma membrane of podocytes was found to be critically involved in Hcys-induced activation of NADPH oxidase due to aggregation, recruitment and assembling of NADPH oxidase subunits to form a redox platform [23]. Based on those studies, we hypothesized that the effect of GH to inhibit Hcys-induced NADPH oxidase activation in podocytes may be due to its action on the formation of membrane raft platforms. Indeed, there are some reports that GHRs are highly lipid raft-concentrated [56] and membrane raft targeting of GHR resides in subdomain 2 of glycosylated extracellular domain, a region relatively uninvolved in GH binding [55, 57]. In the present study, we provided three lines of evidence demonstrating that GH activates NADPH oxidase and thereby blocks EMT of podocytes through inhibition of membrane raft platforms formation and consequent redox signaling. First, we found by confocal microscopy that both GHR and gp91phox were coupled to membrane raft platforms on podocytes upon Hcys stimulation. However, this Hcys-induced formation of membrane raft platforms and related molecular aggregation were substantially blocked by GH treatment, suggesting that the assembling of NADPH oxidase subunits possibly occurs in membrane raft clusters. Second, the effects of GH on the formation of membrane raft platforms and associated aggregation of GHRs or NADPH oxidase subunits were similar to filipin, a compound that binds to cholesterol and disrupts membrane rafts. Third, ESR analysis demonstrated that NADPH oxidase-derived O2.- production upon Hcys stimulation was blocked by GH. However, in the presence of membrane raft disruptors, filipin and MCD, or NADPH oxidase inhibitor apocynin, GH had no further effects on Hcys-induced O2.- production. From these results, we propose that GH acts on its receptor to activate the formation of membrane raft platforms and thereby result in NADPH oxidase assembling and activation. O2.- as NADPH oxidase product initiates redox regulation leading to EMT in podocytes.
In summary, the present study demonstrated a novel regulatory role or therapeutic action of GH on Hcys-induced EMT of podocytes. This action of GH is associated with inhibition of membrane raft clustering and consequent inhibition of NADPH oxidase activation and O2.- production induced by Hcys stimulation.
Acknowledgements
This study was supported by grants DK54927, HL75316 and HL57244 from National Institutes of Health. No competing financial interests exist.
References
- 1.Moshal KS, Singh M, Sen U, Rosenberger DS, Henderson B, Tyagi N, Zhang H, Tyagi SC. Homocysteine-mediated activation and mitochondrial translocation of calpain regulates mmp-9 in mvec. Am J Physiol Heart Circ Physiol. 2006;291:H2825–2835. doi: 10.1152/ajpheart.00377.2006. [DOI] [PubMed] [Google Scholar]
- 2.Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N, Porcellini E, Licastro F. Homocysteine and folate as risk factors for dementia and alzheimer disease. Am J Clin Nutr. 2005;82:636–643. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- 3.Sato Y, Honda Y, Iwamoto J, Kanoko T, Satoh K. Effect of folate and mecobalamin on hip fractures in patients with stroke: A randomized controlled trial. JAMA. 2005;293:1082–1088. doi: 10.1001/jama.293.9.1082. [DOI] [PubMed] [Google Scholar]
- 4.Triantafyllou N, Evangelopoulos ME, Kimiskidis VK, Kararizou E, Boufidou F, Fountoulakis KN, Siamouli M, Nikolaou C, Sfagos C, Vlaikidis N, Vassilopoulos D. Increased plasma homocysteine levels in patients with multiple sclerosis and depression. Ann Gen Psychiatry. 2008;7:17. doi: 10.1186/1744-859X-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi F, Zhang AY, Li N, Muh RW, Fillet M, Renert AF, Li PL. Inhibition of ceramide-redox signaling pathway blocks glomerular injury in hyperhomocysteinemic rats. Kidney Int. 2006;70:88–96. doi: 10.1038/sj.ki.5001517. [DOI] [PubMed] [Google Scholar]
- 6.Yi F, dos Santos EA, Xia M, Chen QZ, Li PL, Li N. Podocyte injury and glomerulosclerosis in hyperhomocy-steinemic rats. Am J Nephrol. 2007;27:262–268. doi: 10.1159/000101471. [DOI] [PubMed] [Google Scholar]
- 7.Ingram AJ, Krepinsky JC, James L, Austin RC, Tang D, Salapatek AM, Thai K, Scholey JW. Activation of mesangial cell mapk in response to homocysteine. Kidney Int. 2004;66:733–745. doi: 10.1111/j.1523-1755.2004.00795.x. [DOI] [PubMed] [Google Scholar]
- 8.Leeuwis JW, Nguyen TQ, Dendooven A, Kok RJ, Goldschmeding R. Targeting podocyte-associated diseases. Adv Drug Deliv Rev. 2010;62:1325–1336. doi: 10.1016/j.addr.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–1401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 10.Sen U, Basu P, Abe OA, Givvimani S, Tyagi N, Metreveli N, Shah KS, Passmore JC, Tyagi SC. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am J Physiol Renal Physiol. 2009;297:F410–419. doi: 10.1152/ajprenal.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C, Hu JJ, Xia M, Boini KM, Brimson CA, Laperle LA, Li PL. Protection of podocytes from hyperhomocysteinemia-induced injury by deletion of the gp91Phox gene. Free Radic Biol Med. 2010;48:1109–1117. doi: 10.1016/j.freeradbiomed.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ungvari Z, Gautam T, Koncz P, Henthorn JC, Pinto JT, Ballabh P, Yan H, Mitschelen M, Farley J, Sonntag WE, Csiszar A. Vasoprotective effects of life span-extending peripubertal gh replacement in lewis dwarf rats. J Gerontol A Biol Sci Med Sci. 2010;65:1145–1156. doi: 10.1093/gerona/glq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived gh/igf-deficient ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–1894. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedayati N, Annambhotla S, Jiang J, Wang X, Chai H, Lin PH, Yao Q, Chen C. Growth hormone-releasing peptide ghrelin inhibits homocysteine-induced endothelial dysfunction in porcine coronary arteries and human endothelial cells. J Vasc Surg. 2009;49:199–207. doi: 10.1016/j.jvs.2008.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Titterington JS, Sukhanov S, Higashi Y, Vaughn C, Bowers C, Delafontaine P. Growth hormone-releasing peptide-2 suppresses vascular oxidative stress in apoe-/- mice but does not reduce atherosclerosis. Endocrinology. 2009;150:5478–5487. doi: 10.1210/en.2009-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato Y, Iwase M, Ichihara S, Kanazawa H, Hashimoto K, Noda A, Nagata K, Koike Y, Yokota M. Beneficial effects of growth hormone-releasing peptide on myocardial oxidative stress and left ventricular dysfunction in dilated cardiomyopathic hamsters. Circ J. 2010;74:163–170. doi: 10.1253/circj.cj-09-0378. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Hu JJ, Xia M, Boini KM, Brimson C, Li PL. Redox signaling via lipid raft clustering in homocysteineinduced injury of podocytes. Biochim Biophys Acta. 2010;1803:482–491. doi: 10.1016/j.bbamcr.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang DX, Zou AP, Li PL. Ceramideinduced activation of nadph oxidase and endothelial dysfunction in small coronary arteries. Am J Physiol Heart Circ Physiol. 2003;284:H605–612. doi: 10.1152/ajpheart.00697.2002. [DOI] [PubMed] [Google Scholar]
- 19.Yi F, Zhang AY, Janscha JL, Li PL, Zou AP. Homocysteine activates nadh/nadph oxidase through ceramide-stimulated rac gtpase activity in rat mesangial cells. Kidney Int. 2004;66:1977–1987. doi: 10.1111/j.1523-1755.2004.00968.x. [DOI] [PubMed] [Google Scholar]
- 20.Yi F, Xia M, Li N, Zhang C, Tang L, Li PL. Contribution of guanine nucleotide exchange factor vav2 to hyperhomocysteinemic glomerulosclerosis in rats. Hypertension. 2009;53:90–96. doi: 10.1161/HYPERTENSIONAHA.108.115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi F, Jin S, Zhang F, Xia M, Bao JX, Hu J, Poklis JL, Li PL. Formation of lipid raft redox signalling platforms in glomerular endothelial cells: An early event of homocysteine-induced glomerular injury. J Cell Mol Med. 2009;13:3303–3314. doi: 10.1111/j.1582-4934.2009.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia SJ, Jin S, Zhang F, Yi F, Dewey WL, Li PL. Formation and function of ceramide-enriched membrane platforms with cd38 during m1-receptor stimulation in bovine coronary arterial myocytes. Am J Physiol Heart Circ Physiol. 2008;295:H1743–1752. doi: 10.1152/ajpheart.00617.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, Hu JJ, Xia M, Boini KM, Brimson C, Li PL. Redox signaling via lipid raft clustering in homocysteineinduced injury of podocytes. Biochim Biophys Acta. 2010;1803:482–491. doi: 10.1016/j.bbamcr.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doi SQ, Jacot TA, Sellitti DF, Hirszel P, Hirata MH, Striker GE, Striker LJ. Growth hormone increases inducible nitric oxide synthase expression in mesangial cells. J Am Soc Nephrol. 2000;11:1419–1425. doi: 10.1681/ASN.V1181419. [DOI] [PubMed] [Google Scholar]
- 25.Kumar PA, Kotlyarevska K, Dejkhmaron P, Reddy GR, Lu C, Bhojani MS, Menon RK. Growth hormone (gh)-dependent expression of a natural antisense transcript induces zinc finger e-box-binding homeobox 2 (zeb2) in the glomerular podocyte: A novel action of gh with implications for the pathogenesis of diabetic nephropathy. J Biol Chem. 2010;285:31148–31156. doi: 10.1074/jbc.M110.132332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pisters PW, Pearlstone DB. Protein and amino acid metabolism in cancer cachexia: Investigative techniques and therapeutic interventions. Crit Rev Clin Lab Sci. 1993;30:223–272. doi: 10.3109/10408369309084669. [DOI] [PubMed] [Google Scholar]
- 27.Raiti S, Moore WV, Van Vliet G, Kaplan SL. Growth-stimulating effects of human growth hormone therapy in patients with turner syndrome. J Pediatr. 1986;109:944–949. doi: 10.1016/s0022-3476(86)80273-6. [DOI] [PubMed] [Google Scholar]
- 28.Rabkin R, Awwad I, Chen Y, Ashley EA, Sun D, Sood S, Clusin W, Heidenreich P, Piecha G, Gross ML. Low-dose growth hormone is cardioprotective in uremia. J Am Soc Nephrol. 2008;19:1774–1783. doi: 10.1681/ASN.2007121386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sipila I, Sintonen H, Hietanen H, Apajasalo M, Alanne S, Viita AM, Leinonen E. Long-term effects of growth hormone therapy on patients with prader-willi syndrome. Acta Paediatr. 2010;99:1712–1718. doi: 10.1111/j.1651-2227.2010.01904.x. [DOI] [PubMed] [Google Scholar]
- 30.Krysiak R, Gdula-Dymek A, Bednarska-Czerwinska A, Okopien B. Growth hormone therapy in children and adults. Pharmacol Rep. 2007;59:500–516. [PubMed] [Google Scholar]
- 31.Ramirez RJ, Wolf SE, Barrow RE, Herndon DN. Growth hormone treatment in pediatric burns: A safe therapeutic approach. Ann Surg. 1998;228:439–448. doi: 10.1097/00000658-199810000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seiva FR, Berbert CM, Souza GA, Rocha KK, Ebaid GM, Burneiko RC, Novelli EL. Energy expenditure, lipid profile, oxidative stress, and cardiac energy metabolism after growth hormone treatment in obese young rats. Horm Metab Res. 2010;42:496–501. doi: 10.1055/s-0030-1249651. [DOI] [PubMed] [Google Scholar]
- 33.Ellegard L, Bosaeus I, Nordgren S, Bengtsson BA. Low-dose recombinant human growth hormone increases body weight and lean body mass in patients with short bowel syndrome. Ann Surg. 1997;225:88–96. doi: 10.1097/00000658-199701000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrini S, Laviola L, Carreira MC, Cignarelli A, Natalicchio A, Giorgino F. The gh/igfl axis and signaling pathways in the muscle and bone: Mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol. 2010;205:201–210. doi: 10.1677/JOE-09-0431. [DOI] [PubMed] [Google Scholar]
- 35.Ling FA, Hui DZ, Ji SM. Protective effect of recombinant human somatotropin on amyloid beta-peptide induced learning and memory deficits in mice. Growth Horm IGF Res. 2007;17:336–341. doi: 10.1016/j.ghir.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Poljakovic Z, Zurak N, Brinar V, Korsic M, Basic S, Hajnsek S. Growth hormone and insulin growth factor-i levels in plasma and cerebrospinal fluid of patients with multiple sclerosis. Clin Neurol Neurosurg. 2006;108:255–258. doi: 10.1016/j.clineuro.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 37.Boschetti M, Goglia U, Teti C, Esposito D, Giusti M, Minuto F, Ferone D. Replacement therapy and cardiovascular diseases. J Endocrinol Invest. 2008;31:85–90. [PubMed] [Google Scholar]
- 38.Le Corvoisier P, Hittinger L, Chanson P, Montagne O, Macquin-Mavier I, Maison P. Cardiac effects of growth hormone treatment in chronic heart failure: A meta-analysis. J Clin Endocrinol Metab. 2007;92:180–185. doi: 10.1210/jc.2006-1313. [DOI] [PubMed] [Google Scholar]
- 39.Santos F, Moreno ML, Neto A, Ariceta G, Vara J, Alonso A, Bueno A, Afonso AC, Correia AJ, Muley R, Barrios V, Gomez C, Argente J. Improvement in growth after 1 year of growth hormone therapy in well-nourished infants with growth retardation secondary to chronic renal failure: Results of a multicenter, controlled, randomized, open clinical trial. Clin J Am Soc Nephrol. 2010;5:1190–1197. doi: 10.2215/CJN.07791109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krieg RJ, Veldhuis JD, Thornhill BA, Chevalier RL, Gil G. Growth hormone (gh) secretion, gh-dependent gene expression, and sexually dimorphic body growth in young rats with chronic renal failure. Endocrine. 2008;33:323–330. doi: 10.1007/s12020-008-9094-6. [DOI] [PubMed] [Google Scholar]
- 41.Kireev RA, Tresguerres AF, Vara E, Ariznavarreta C, Tresguerres JA. Effect of chronic treatments with gh, melatonin, estrogens, and phytoestrogens on oxidative stress parameters in liver from aged female rats. Biogerontology. 2007;8:469–482. doi: 10.1007/s10522-007-9089-3. [DOI] [PubMed] [Google Scholar]
- 42.Baeza I, Fdez-Tresguerres J, Ariznavarreta C, De la Fuente M. Effects of growth hormone, melatonin, oestrogens and phytoestrogens on the oxidized glutathione (gssg)/reduced glutathione (gsh) ratio and lipid peroxidation in aged ovariectomized rats. Biogerontology. 2010;11:687–701. doi: 10.1007/s10522-010-9282-7. [DOI] [PubMed] [Google Scholar]
- 43.Seiva FR, Ebaid GM, Castro AV, Okoshi K, Nascimento A, Rocha KK, Padovani CR, Cicogna AC, Novelli EL. Growth hormone and heart failure: Oxidative stress and energetic metabolism in rats. Growth Horm IGF Res. 2008;18:275–283. doi: 10.1016/j.ghir.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Yi F, Li PL. Mechanisms of homocysteine-induced glomerular injury and sclerosis. Am J Nephrol. 2008;28:254–264. doi: 10.1159/000110876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang SY, Siow YL, Au-Yeung KK, House J, O K. Folic acid supplementation inhibits nadph oxidase-mediated superoxide anion production in the kidney. Am J Physiol Renal Physiol. 2011;300F:189–198. doi: 10.1152/ajprenal.00272.2010. [DOI] [PubMed] [Google Scholar]
- 46.Kang YS, Li Y, Dai C, Kiss LP, Wu C, Liu Y. Inhibition of integrin-linked kinase blocks podocyte epithelial-mesenchymal transition and ameliorates proteinuria. Kidney Int. 2010;78:363–373. doi: 10.1038/ki.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol. 2008;172:299–308. doi: 10.2353/ajpath.2008.070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003;7:255–259. doi: 10.1007/s10157-003-0259-6. [DOI] [PubMed] [Google Scholar]
- 49.Arnold RE, Weigent DA. The inhibition of superoxide production in el4 lymphoma cells overexpressing growth hormone. Immunopharmacol Immunotoxicol. 2003;25:159–177. doi: 10.1081/iph-120020467. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez JE, DiGeronimo RJ, Arthur DE, King JM. Remodeling of the tight junction during recovery from exposure to hydrogen peroxide in kidney epithelial cells. Free Radic Biol Med. 2009;47:1561–1569. doi: 10.1016/j.freeradbiomed.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meyer TN, Schwesinger C, Ye J, Denker BM, Nigam SK. Reassembly of the tight junction after oxidative stress depends on tyrosine kinase activity. J Biol Chem. 2001;276:22048–22055. doi: 10.1074/jbc.M011477200. [DOI] [PubMed] [Google Scholar]
- 52.Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM. Endothelin-1 increases glomerular permeability and inflammation independent of blood pressure in the rat. Hypertension. 2010;56:942–949. doi: 10.1161/HYPERTENSIONAHA.110.156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boini KM, Zhang C, Xia M, Han WQ, Brimson C, Poklis JL, Li PL. Visfatininduced lipid raft redox signaling platforms and dysfunction in glomerular endothelial cells. Biochim Biophys Acta. 2010;1801:1294–304. doi: 10.1016/j.bbalip.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lanning NJ, Carter-Su C. Recent advances in growth hormone signaling. Rev Endocr Metab Disord. 2006;7:225–235. doi: 10.1007/s11154-007-9025-5. [DOI] [PubMed] [Google Scholar]
- 55.Birzniece V, Sata A, Ho KK. Growth hormone receptor modulators. Rev Endocr Metab Disord. 2009;10:145–156. doi: 10.1007/s11154-008-9089-x. [DOI] [PubMed] [Google Scholar]
- 56.Yang N, Huang Y, Jiang J, Frank SJ. Caveolar and lipid raft localization of the growth hormone receptor and its signaling elements: Impact on growth hormone signaling. J Biol Chem. 2004;279:20898–20905. doi: 10.1074/jbc.M400625200. [DOI] [PubMed] [Google Scholar]
- 57.Yang N, Jiang J, Deng L, Waters MJ, Wang X, Frank SJ. Growth hormone receptor targeting to lipid rafts requires extracellular subdomain 2. Biochem Biophys Res Commun. 2010;391:414–418. doi: 10.1016/j.bbrc.2009.11.072. [DOI] [PubMed] [Google Scholar]