Abstract
Objective
To assess the burden of diseases and quality of life (QOL) of patients for a large variety of diseases within general practice.
Design
In a representative nationwide cross-sectional study, a total of 825 general practitioners (GPs) were randomly selected from across France. Independent investigators recruited 8559 patients attending the GPs' practices. Data on QOL (12-Item Short Form questionnaire) and other individual characteristics were documented by the independent investigators for all participants in the waiting room. Medical information was recorded by GPs. Sampling was calibrated to national standards using the CALMAR (CALage sur MARges) weighting procedure. Associations of lower scores (ie, below vs above the first quartile) of physical and mental component scores (physical component summary score (PCS) and mental component summary score (MCS), respectively) with main diseases and patients characteristics were estimated using multivariate logistic regression. Weighted morbidity rates, PCS and MCS were computed for 100 diagnoses using the International Classification of Diseases (9th version).
Results
Overall mental impairment was observed among patients in primary care with an average MCS of 41.5 (SD 8.6), ranging from 33.0 for depressive disorders to 45.3 for patients exhibiting fractures or sprains. Musculoskeletal diseases were found to have the most pronounced effect on impaired physical health (OR=2.31; 95% CI 2.08 to 2.57) with the lowest PCS (45.6 (SD 8.8)) and ranked first (29.0%) among main diagnoses experienced by patients followed by cardiovascular diseases (26.7%) and psychological disorders (22.0%). When combining both prevalence and QOL, musculoskeletal diseases represented the heaviest burden in general practice.
Conclusions
Etude épidémiologique de l'Impact de santé public sur 3 groupes de pathologies (EPI3) is the first study to provide reference figures for burden of disease in general practice across a wide range of morbidities, particularly valuable for health-economics and healthcare-system evaluation.
Article summary
Article focus
The impact of diseases on quality of life (QOL) in general practice has been assessed among selected samples of patients, usually from studies including a limited number of medical practices and/or focusing mainly on chronic conditions.
There is a clear need for more data on QOL of patients in primary care; the aim of the Etude épidémiologique de l'Impact de santé public sur 3 groupes de pathologies (EPI3) survey was to provide reference figures for disease burden in this setting.
Key messages
The EPI3 study was a cross-sectional survey combining unique data from patients and general practitioners (GPs), and allowed provision of reference figures for the vast majority of diseases encountered in primary care for a large number of patients.
The study highlighted the burden of musculoskeletal and psychological disorders, experienced by more than half the patients.
Although social and medical determinants of patients' QOL were somewhat similar than those found in previous studies in primary care, the EPI3 survey showed more pronounced mental impairment in French patients.
Strengths and limitations of this study
No nationwide study on burden of disease combining both prevalence measures and QOL assessment has been conducted to date, addressing such a large variety of diseases in general practice.
On-site selection and recruitment by an independent investigator limited the possibility of selection bias among patients, and the participation of physicians added high specificity to medical data collection.
A study design providing a high specificity in data collection led to a relatively low response rate from GPs. However, stratified recruitment phases and sample sizes from both GPs and patients highly representative of national standards ensured the strong external validity of the results.
Home consultations, which are common among GPs in France, were not surveyed which could have led to an underestimation of the burden of disease.
Introduction
Assessing and measuring the burden of a disease in medical practice is undoubtedly important for the evaluation of medicines and healthcare.1To assess such burden quantitatively, both the prevalence of diseases and their impact on health status and on the quality of life (QOL) of patients need to be taken into account.2
In primary care, the prevalence of morbidities has been shown to be remarkably similar across different industrialised countries.3–5 However, their effect upon QOL is only partially known.6 The impact of the diseases on QOL in general practice has been addressed so far using selected samples of patients,7–13 usually from studies including a limited number of medical practices,8 10 11 13 and/or mainly focused on chronic conditions.7 9–11 To the best of our knowledge, no nationwide study of the burden of disease combining both prevalence measures and QOL assessment for a large variety of diseases is currently available. To compensate for this paucity of information, some studies have evaluated the impact for diseases in primary care calling upon modelling data derived from studies in medical specialties14–16 and/or in hospital settings,17 18 or from general population surveys.19–21 It is not known to what extent these extrapolations are appropriate.
The aim of the Etude épidémiologique de l'Impact de santé public sur 3 groupes de pathologies (EPI3) survey was to provide reference figures for disease burden in primary care. For this purpose, a representative sample of general practitioners (GPs) was assembled through stratified sampling and data from their patients collated during a 1-day survey conducted by independent interviewers in the waiting room of the participating medical practices.
Methods
Study design, settings and population
The EPI3 survey was a nationwide, observational study of a representative sample of GPs and their patients, conducted in France between March 2007 and July 2008. Its aims were to assess the burden of diseases in general practice, considering the physicians' work activity, patients' characteristics, morbidity and prescriptions.
The sample was drawn by applying a two-stage sampling process. First, GPs were randomly selected from the French national directory of physicians and invited to participate, which meant also allowing a trained research assistant to conduct a 1-day survey in the waiting room at the doctor's practice. GPs' sampling was stratified according to the diversity of medicine practices in the country (conventional and complementary medicine such as homeopathy).
The second stage consisted of random 1-day sampling of consultations per participating physician in order to survey all patients attending the practice on a particular day. All adult and accompanied minor patients were eligible for inclusion in the EPI3 survey to the exception of those whose health status or literacy level did not allow responding to a self-administered questionnaire. The research assistant recruited up to a maximum of 15 patients on site (ie, in the waiting room) all eligible patients listed consecutively who consented to participate in the survey. Further, each physician recorded the main reason for consultation, along with the age, gender and type of national health insurance for each patient. The maximum number of patients surveyed per day was set to allow sufficient time for optimal interviews and was followed by patients' examination by the physician.
The EPI3 survey obtained regulatory approval by the national board of physicians (‘Conseil National de l'Ordre des Médecins’) and ethical approval by the French data protection authority (‘Commission Nationale de l'Informatique et des Libertés’). Patients were informed by the participating physician that their responses would be kept confidential, and they were not remunerated for participation. Physicians received compensation fees. The study was sponsored by a pharmaceutical company, whose name was not revealed to investigators or patients.
Data collection
Patients were asked to self-complete a questionnaire covering demographic and social information (age, gender, educational level, employment status and occupation, smoking, alcohol intake, physical activity, height and weight for body mass index calculation), health insurance (regular national insurance, welfare health insurance for low income, with or without supplementary private insurance), number of visits to the participating physician within the last 12 months, or, for the same period, to other GPs or medical specialists, the length and number of hospitalisations and sick leaves.
Participants were asked whether the attending physician was their regular/primary care physician. In the French health insurance system, patients have to choose a regular physician, usually a GP, who plays a gatekeeping role for referral to specialised care. However, referral by regular GPs to other physicians is not compulsory, and patients are allowed to seek care from different physicians and their different reimbursement schemes.
Detailed information on physicians including age, gender, type of contract with national health insurance (regular fees, additional fees and no contract), working days and average duration of consultation were assessed by the research assistants at the time of inclusion.
GPs completed a medical questionnaire on patients including the main reason for consultation and up to five other diagnoses present that day. GPs were requested to record their prescriptions that day for diagnostic tests, drugs and referrals. Diagnoses were coded by a trained archivist using the ninth revision of the International Classification of Diseases using 100-3 digit-categories.22
Health-status measurement
Among adult patients (18 years and over), health-related quality of life was assessed using the 12-Item Short Form questionnaire (SF-12),23 a shortened version of the 36-Item Short Form Health Survey (SF-36) which has been shown to be a reliable outcome measurement tool in primary care.24 The physical and mental component summary scores (PCS and MCS, respectively) were derived from the SF-12 questionnaire. Physical functioning (two questions), role—physical functioning (two questions), bodily pain (one question), general health (one question), vitality (one question), social functioning (one question), role—emotional functioning (two questions) and mental health (two questions) cover the same dimensions as the SF-36. The scores are standardised to population norms (based on a US norm-sample), with the mean score set at 50 (SD 10); lower scores indicate worse health, and higher scores better health. The SF-12 has been validated for use in France, the USA, the UK and many other European countries.21
Statistical analysis
Participating and non-participating patients were compared against the collected variables on gender, age, length of time attending the GPs' practice, type of health insurance and main reason for consultation. A weighting procedure known in demographic studies as the CALMAR (CALage sur MARges) procedure was applied to calibrate the final sample according to participation so that it closely represents the patients attending the practice.25
Participating GPs were compared with the French ‘Institut de recherche et documentation en économie de la santé’ sample.26The physicians' activity-related fractions were also calibrated to the real distribution of the participating physicians across the France. All reported results were obtained after weighting was applied to GPs' patients.
In this study, we reported weighted prevalence, calculated as a percentage reported to the whole population, regardless of whether the diagnosis was isolated or associated with other diagnoses. Weighted PCS and MCS measures of the SF-12 were computed according to the algorithm given by Ware et al for 100 different conditions, which were further grouped into 13 broad diseases categories plus one covering preventive motives of consultation and other medical acts.23 Means and SDs were estimated for the whole adult sample and for each diagnosis. When a three-digit category from the ninth revision of the International Classification of Diseases had <30 patients, the category was grouped with one or several categories under the same heading. When grouping within the same heading was not relevant, categories with <30 patients were grouped in the category ‘other’ within each main category.
Each disease category was calculated as a percentage reported to the whole population of participating patients over the age of 18 years, regardless of whether this diagnosis was isolated or associated with others, in order to provide a complete picture of morbidity cared for in general practice.
Among adults over 18 years, associations of age, gender, education, type of insurance and 13 broad disease categories with lower MCS and PCS scores (defined as below the first quartile with scores of 34 and 39 for MCS and PCS, respectively) were evaluated using multivariate logistic regression. Odds ratios (OR) and 95% CI are presented for each of these factors. In addition, the same analysis was conducted for the two remaining categories, pregnancy follow-up and preventive motives, which were not considered in the multivariate analysis. The association between the number of comorbidities and the two SF-12 composite scores was tested using linear regression. The possibility of a clustering effect at the practice level was tested using Generalised Estimating Equations multivariate models. We used SAS statistical software (V.9.1) for data analysis. The present study was reported following the ‘Strengthening the Reporting of Observational Studies in Epidemiology’ statement for cross-sectional studies.27
Results
The sequential recruitment of physicians was carried out by random stratified sampling from the phone directory for GPs. Among the 3345 GPs initially contacted, 428 (12.8%) agreed to participate in the survey. At the end of recruitment in July 2008, an additional sample of 13 861 GPs were contacted to ensure a representative sample of GPs from all types of primary care practice in France (strictly allopaths, homeopaths and mixed practice). Despite the intrusive nature of the survey, allowing trained research assistants to collect data directly in the waiting room at the medical practice on the very day of consultation, a final sample of 825 participating GPs recruited a total of 11 809 patients eligible for the present study. Of these, 174 were unaccompanied minors, 315 were non-French speakers, 109 had severe psychiatric disorders, 2151 declined participation, 408 were beyond the maximum number possible to be interviewed within the allocated time on site, and 93 had incomplete data and were excluded, allowing a total of 8559 patients for the present analysis.
Physicians
The median age of physicians was 52 years (IQR=33–57) and of these, 24% were women; 54% worked in solo medical practice, 40% worked with other GPs, and 6% collaborated with specialists or other healthcare professionals; 31% of the GPs practised additional medical activities within hospitals, healthcare centres, the health administration or the pharmaceutical industry. Most of the GPs (90%) hold a regular contract with the National Health Insurance organisation, while 9% hold a special contract allowing extra fees; a very small proportion (0.4%) had no contract. The mean daily working time at the practice (excluding home visits) was 9 h (IQR: 2–10.5), and each of the 825 GPs participating in the study recruited on average 8.7 patients (SD 2.2).
Patients
The characteristics of participating (n=8559) and non-participating patients with complete data (n=3157) used to calibrate the sample are presented in table 1. We report here the results based on weighted characteristics. The mean age of the 8559 participating patients was 44.9 years (SD 21.9), and 7133 (83.3%) were adults over 18. At least 44% of patients had a secondary-school degree, 16% were overweight (BMI>30 kg/m2), and more than 61% exercised longer than 31 min per day. Nine out of ten patients were French-born (90%), 9% were covered by a government-funded insurance for low-income people, and 90% had a private supplementary insurance. Among the 8559 patients, 8% had attended the practice for the first time, 12% had attended for 1 year or less, 27% had attended between 1 and 5 years, and 53% had attended for 5 years or more. Over 84% of participants named the recruiting physician as their regular treating physician. About 28% of patients were registered by the national health insurance as having multiple or severe chronic diseases and requiring special health insurance coverage.
Table 1.
Characteristics of non-participating and participating patients: results of the calibrated data (Etude épidémiologique de l'Impact de santé public sur 3 groupes de pathologies (EPI3) survey 2008)
| Non-participants*(n=3157) | Participants (n=8559) | Weighted percentage | |
| Mean (SD) | |||
| Age (years) | 47.7 (24.0) | 43.3 (22.8) | 44.9 (21.9) |
| n (%) | |||
| Female gender | 1701 (53.9) | 5367 (62.7) | 57.9 |
| Length of relationship with the physician | |||
| First time | 265 (8.4) | 782 (9.1) | 7.7 |
| <1 year | 316 (10.0) | 1315 (15.4) | 11.9 |
| 1–5 years | 743 (23.5) | 2380 (27.8) | 27.4 |
| More than 5 years | 1703 (53.9) | 4015 (46.9) | 53.0 |
| Government-funded health insurance | 308 (9.8) | 621 (7.4) | 9.4 |
| Longstanding disease status | 1925 (22.5) | 27.7 | |
| Complementary health insurance | 7839 (91.6) | 90.3 | |
| Index physician declared as treating physician | 6379 (74.5) | 84.3 | |
| Body mass index (kg/m2) | |||
| <25 | 5548 (64.8) | 52.4 | |
| 25–30 | 2045 (23.9) | 31.8 | |
| 30 and over | 966 (11.3) | 15.8 | |
| Tobacco consumption | |||
| Non-smoker | 4303 (50.3) | 47.4 | |
| Past smoker | 1961 (22.9) | 24.4 | |
| Current smoker | 2252 (26.3) | 28.2 | |
| Alcohol consumption | |||
| Never | 2908 (34.2) | 35.2 | |
| Sometimes | 4649 (54.6) | 52.5 | |
| Daily | 957 (11.2) | 12.4 | |
| Physical exercise | |||
| <10 min per day | 2235 (26.1) | 28.3 | |
| 10 min and over | 6199 (72.4) | 71.7 | |
| Nationality | |||
| French-born subjects | 7787 (91.0) | 90.3 | |
| French born abroad | 341 (4.0) | 4.7 | |
| Non-French nationality | 357 (4.2) | 5.0 | |
| Educational attainment | |||
| Secondary-school degree*†, college, university graduation | 4179 (48.8) | 44.0 | |
| Employment status | |||
| Employed | 4544 (53.1) | 50.4 | |
| On unemployment benefits | 378 (4.4) | 4.7 | |
| Homemaker | 647 (7.6) | 6.6 | |
| Retired and other unemployed | 2562 (29.9) | 34.3 | |
| Student | 348 (4.1) | 4.1 | |
Available characteristics used for calibration.
French baccalaureate.
Burden of 100 diseases in primary care
The prevalence of each of the 100 and 13 broad non-exclusive diagnosis categories (a compilation of all five diagnoses recorded by the GPs) is presented in table 2. Altogether, diseases of the musculoskeletal system were the most frequently diagnosed conditions (29%), followed by cardiovascular diseases (26.7%), and sleep, anxiety and depressive disorders (22%). Preventive-care consultations, vaccinations and consultation for administrative purposes accounted for 19% of the total diagnoses. Almost half the patients (49%) exhibited two or more comorbidities.
Table 2.
Morbidity rates and 12-Item Short Form questionnaire mental and physical component scores according to 100 International Classification of Diseases diagnoses (Etude épidémiologique de l'Impact de santé public sur 3 groupes de pathologies (EPI3)-LA-SER-2008, weighted data n=7133)
| Diseases | Morbidity* | PCS | MCS |
| n (%) | Mean (SD) | Mean (SD) | |
| All patients | 45.6 (8.8) | 41.5 (8.6) | |
| Diseases of the musculoskeletal system | 2069 (29.0) | 42.7 (8.4) | 41.6 (8.3) |
| Osteoarthritis | 324 (4.5) | 41.1 (10.7) | 41.7 (11.0) |
| Unspecified joint disorders | 171 (2.4) | 42.5 (9.9) | 42.6 (10.2) |
| Intervertebral and cervical disc disorders | 276 (3.9) | 44.0 (11.4) | 40.6 (10.3) |
| Lumbago | 360 (5.0) | 42.1 (10.1) | 41.6 (9.4) |
| Rotator cuff syndrome of shoulder and allied disorders | 98 (1.4) | 42.5 (10.1) | 42.0 (9.8) |
| Other affections of shoulder region | 121 (1.7) | 42.6 (9.7) | 42.4 (9.1) |
| Enthesopathy of elbow region | 77 (1.1) | 41.8 (9.3) | 43.1 (10.5) |
| Unspecified enthesopathy | 257 (3.6) | 42.3 (10.5) | 42.1 (10.0) |
| Rheumatism, excluding the back | 112 (1.6) | 42.9 (10.1) | 42.3 (9.8) |
| Spondylosis and other inflammatory spondylopathies | 84 (1.2) | 42.1 (9.9) | 43.1 (8.4) |
| Sciatica | 194 (2.7) | 42.3 (10.1) | 41.7 (9.6) |
| Pain in thoracic spine | 51 (0.7) | 42.6 (9.8) | 41.3 (9.2) |
| Osteoporosis | 162 (2.3) | 44.0 (10.7) | 41.9 (10.8) |
| Diseases of connective tissue | 36 (0.5) | 45.5 (9.3) | 40.5 (10.9) |
| Unspecified back disorders | 193 (2.7) | 42.1 (10.2) | 41.3 (9.7) |
| Other unspecified musculoskeletal disorders | 76 (1.1) | 40.3 (11.0) | 41.7 (11.6) |
| Hypertension, cardiovascular and circulatory diseases | 1904 (26.7) | 43.7 (9.0) | 42.5 (8.6) |
| Hypertension | 1111 (15.6) | 43.9 (11.3) | 42.8 (10.7) |
| Acute myocardial infarction | 84 (1.2) | 40.8 (11.4) | 42.0 (9.4) |
| Other symptoms involving cardiovascular system | 53 (0.7) | 46.0 (9.5) | 43.7 (8.2) |
| Angina pectoris | 66 (0.9) | 41.8 (11.8) | 43.7 (10.5) |
| Cardiac dysrhythmias | 139 (1.9) | 42.9 (11.5) | 42.7 (11.1) |
| Diseases of veins and lymphatics | 92 (1.3) | 45.3 (10.5) | 41.3 (10.8) |
| Pulmonary, artery and cerebrovascular diseases | 144 (2.0) | 42.1 (11.7) | 42.7 (10.6) |
| Other ischaemic heart diseases | 96 (1.3) | 41.1 (11.9) | 42.1 (11.1) |
| Other diseases of the circulatory system | 229 (3.2) | 45.3 (10.3) | 41.8 (11.0) |
| Anxiety, depression and sleep disorders | 1569 (22.0) | 44.8 (8.9) | 36.3 (8.5) |
| Psychotic conditions | 68 (1.0) | 44.2 (12.6) | 37.6 (12.3) |
| Anxiety states | 420 (5.9) | 47.8 (10.5) | 35.5 (9.7) |
| Dysrhythmia | 182 (2.6) | 47.2 (10.2) | 35.4 (9.2) |
| Acute and chronic stress | 85 (1.2) | 46.9 (10.6) | 36.1 (9.5) |
| Personality disorders, disturbance of conduct and dependence syndrome | 81 (1.1) | 46.4 (10.9) | 38.8 (11.2) |
| Eating disorders, tics, stuttering and other syndromes | 277 (3.9) | 47.4 (10.4) | 37.1 (10.7) |
| Depressive disorders | 497 (7.0) | 45.3 (11.4) | 33.0 (10.2) |
| Malaise and fatigue | 114 (1.6) | 45.4 (11.1) | 38.7 (9.7) |
| Sleep disorders | 87 (1.2) | 46.1 (10.7) | 38.5 (9.4) |
| Nervousness, cachexia and unspecified psychological distress | 30 (0.4) | 41.9 (13.5) | 39.9 (12.8) |
| Dizziness and giddiness | 59 (0.8) | 46.3 (9.8) | 40.2 (10.4) |
| Other general symptoms | 75 (5.8) | 46.0 (11.1) | 39.2 (10.4) |
| Diseases of the respiratory system | 1419 (19.9) | 46.3 (8.5) | 41.9 (8.4) |
| Acute nasopharyngitis | 306 (4.3) | 48.2 (9.8) | 41.6 (9.7) |
| Acute pharyngitis | 60 (0.8) | 49.3 (10.4) | 42.5 (10.5) |
| Acute tracheitis | 120 (1.7) | 48.4 (10.1) | 41.4 (10.4) |
| Acute bronchitis or other upper-respiratory infections | 66 (0.2) | 41.9 (12.3) | 43.0 (8.0) |
| Chronic nasopharyngitis | 52 (0.3) | 50.0 (8.1) | 42.9 (8.7) |
| Chronic sinusitis and laryngitis | 126 (1.7) | 48.0 (9.9) | 40.3 (8.9) |
| Allergic rhinitis | 124 (1.7) | 47.5 (10.7) | 42.3 (9.6) |
| Pneumonia and flu | 53 (0.6) | 46.8 (10.2) | 41.1 (10.8) |
| Chronic obstructive pulmonary diseases (except asthma) | 160 (2.2) | 44.8 (10.4) | 41.2 (10.0) |
| Asthma | 148 (2.1) | 44.0 (11.5) | 41.4 (10.5) |
| Lung diseases | 64 (0.9) | 41.0 (12.3) | 42.0 (9.5) |
| Other diseases of the respiratory system | 210 (2.9) | 45.1 (11.6) | 42.4 (10.4) |
| Medical exams and preventive motives | 1101 (15.4) | 47.4 (9.2) | 42.4 (10.1) |
| Medical exam: handicap influencing health status | 41 (0.6) | 45.9 (9.4) | 39.4 (12.6) |
| Medical exam: aftercare and specific procedures | 285 (4.0) | 45.9 (11.6) | 41.2 (10.8) |
| Medical exam for health check-up | 286 (4.0) | 46.9 (11.3) | 43.1 (10.8) |
| Laboratories findings | 53 (0.7) | 48.5 (9.8) | 42.9 (10.9) |
| Vaccination | 121 (1.7) | 50.2 (11.0) | 42.6 (10.5) |
| Pregnancy follow-up | 122 (1.7) | 47.4 (11.8) | 41.8 (10.4) |
| Administrative purposes | 380 (5.3) | 47.2 (11.7) | 42.5 (9.9) |
| Diabetes, thyroid gland and other endocrine disorders | 785 (11.0) | 43.9 (8.6) | 41.5 (8.1) |
| Hypothyroidism | 187 (2.6) | 45.3 (11.0) | 39.6 (10.9) |
| Goitre | 45 (0.6) | 46.5 (9.3) | 41.3 (9.8) |
| Diabetes mellitus | 312 (4.4) | 42.9 (11.2) | 42.0 (10.5) |
| Diseases of other endocrine glands | 78 (1.1) | 43.9 (11.9) | 41.0 (10.8) |
| Other thyroid disorders | 52 (0.7) | 45.7 (10.8) | 40.0 (10.1) |
| Obesity and dyslipidaemia | 742 (10.4) | 45.2 (9.1) | 42.0 (8.9) |
| Hypercholesterolaemia | 266 (3.7) | 45.1 (10.9) | 42.6 (10.2) |
| Unspecified disorder of lipoid metabolism | 135 (1.9) | 45.5 (10.5) | 42.8 (9.2) |
| Overweight, obesity and other hyperalimentation | 169 (2.4) | 47.6 (11.4) | 40.2 (10.4) |
| Other hyperlipidaemia | 105 (1.1) | 45.6 (11.3) | 42.4 (10.6) |
| Other symptoms concerning nutrition, metabolism and development | 70 (1.0) | 48.6 (10.1) | 40.3 (10.4) |
| Diseases of the digestive system | 742 (10.4) | 45.9 (8.4) | 39.9 (8.4) |
| Oesophageal diseases | 81 (1.1) | 44.3 (10.3) | 41.5 (9.8) |
| Diseases of stomach | 121 (1.7) | 45.6 (10.0) | 40.1 (9.7) |
| Diseases of intestines and peritoneum | 72 (1.0) | 45.1 (10.1) | 40.2 (9.9) |
| Symptoms involving the abdomen | 161 (2.3) | 46.9 (10.4) | 39.9 (11.0) |
| Non-infectious enteritis and colitis | 105 (1.5) | 47.5 (9.5) | 41.8 (9.9) |
| Diseases of oral cavity, salivary glands and jaws | 39 (0.5) | 49.0 (8.8) | 40.3 (10.0) |
| Appendicitis and hernia | 43 (0.6) | 44.1 (10.6) | 40.0 (10.2) |
| Other diseases of the digestive system | 144 (2.0) | 44.8 (10.8) | 39.7 (9.9) |
| Diseases of the nervous system, head and neck | 449 (6.3) | 43.3 (9.4) | 39.6 (9.1) |
| Disorders of the central nervous system | 95 (1.3) | 41.3 (13.6) | 42.2 (11.2) |
| Migraine | 114 (1.6) | 46.6 (10.2) | 39.7 (10.1) |
| Symptoms involving the head and neck | 96 (1.3) | 46.3 (10.8) | 40.8 (10.2) |
| Diseases of the eye | 54 (0.7) | 51.8 (9.9) | 37.4 (10.6) |
| Diseases of the ear and mastoid processes | 112 (1.6) | 46.9 (10.8) | 41.6 (11.0) |
| Other disorders of the nervous system and sense organs | 145 (2.0) | 44.6 (10.3) | 39.9 (10.6) |
| Diseases of the genitourinary system | 400 (5.6) | 45.5 (9.6) | 41.5 (8.9) |
| Cystitis | 115 (1.6) | 47.9 (11.5) | 40.3 (10.2) |
| Diseases of male genital organs | 85 (1.2) | 46.0 (10.9) | 43.0 (11.0) |
| Diseases of female genital organs | 139 (1.9) | 48.4 (11.3) | 39.8 (10.8) |
| Nephrosis and nephritis | 86 (1.2) | 44.8 (11.6) | 41.7 (10.6) |
| Complications of pregnancy and congenital anomalies | 42 (0.6) | 43.5 (12.8) | 40.4 (10.5) |
| Injury and poisoning | 342 (4.8) | 43.5 (9.2) | 43.5 (8.3) |
| Fractures, sprains and dislocations | 103 (1.4) | 41.9 (10.5) | 45.3 (9.7) |
| Traumas and injuries to organs | 54 (0.8) | 44.3 (12.9) | 44.1 (11.4) |
| Burns and amputations | 62 (0.9) | 44.4 (11.6) | 43.1 (10.4) |
| Intoxications and allergies to toxic drugs | 108 (1.5) | 47.3 (11.6) | 41.5 (10.7) |
| Poisoning, other allergy and side effect of surgery | 55 (0.8) | 44.3 (11.1) | 40.3 (9.3) |
| Cancer and infrequent diseases | 289 (4.1) | 42.0 (9.3) | 40.4 (8.5) |
| Neoplasms | 174 (2.4) | 41.8 (11.6) | 40.7 (10.6) |
| Benign tumours | 54 (0.8) | 44.7 (10.9) | 40.8 (10.7) |
| Blood diseases | 56 (0.8) | 45.3 (11.1) | 41.2 (9.5) |
| Skin and subcutaneous tissue diseases | 243 (3.4) | 48.8 (7.8) | 41.7 (9.6) |
| Infections of skin and subcutaneous tissue | 55 (0.8) | 46.1 (11.0) | 40.4 (11.3) |
| Inflammatory conditions of skin and subcutaneous tissue | 163 (2.3) | 47.3 (9.9) | 42.2 (10.4) |
| Other diseases of skin and subcutaneous tissue | 89 (1.2) | 49.7 (8.5) | 40.1 (11.0) |
| Infectious diseases | 228 (3.2) | 47.1 (7.6) | 40.7 (7.1) |
| Parasitic diseases | 76 (1.1) | 47.1 (11.1) | 42.1 (10.7) |
| Bacterial diseases | 82 (1.1) | 47.0 (10.0) | 39.9 (9.8) |
| Viral diseases (including HIV) | 122 (1.7) | 46.5 (11.6) | 40.5 (10.1) |
| Fever and other physiological disturbances of temperature regulation | 77 (1.1) | 46.3 (10.4) | 40.1 (8.4) |
Each condition category is non-exclusive.
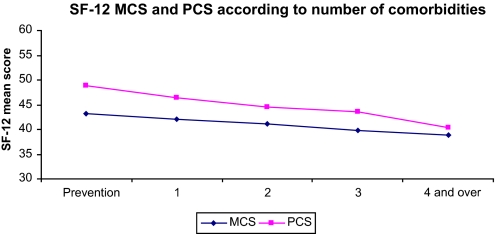
Overall mean scores for PCS and MCS were 45.6 (SD 8.8) and 41.5 (SD 8.6), respectively. Considering PCS, scores ranged from 40.3 (SD 11.0) for one group of unspecified musculoskeletal conditions to 50.2 (SD 11.0) for vaccinations. On the whole, musculoskeletal disorders had the lowest scores with the cancer and other severe diseases category, while skin-related diseases, preventive-care consultation and infectious diseases showed the highest PCS. With regard to MCS, scores ranged from 33.0 (SD 10.2) for depressive disorders to 45.3 for patients with fractures, sprains or dislocation. Overall, the lowest scores were found among patients with mood and sleep disorders, while injury, preventive motives and cardiovascular diseases exhibited the highest scores. Both MCS and PCS decreased significantly with increasing numbers of comorbidities (figure 1). For example, MCS decreased from 43.3 for patients seeking preventive care advice to 38.5 for those with four diagnoses or more (p for trend <0.0001) and PCS from 49.2 to 40.4 (p for trend <0.0001).
Figure 1.
Health-related quality of life: 12-Item Short Form questionnaire (SF-12) mental (MCS) and physical (PCS) component scores per number of comorbidities Etude épidémiologique de l'Impact de santé public sur 3 groupes de pathologies (EPI3)-2008 (weighted data).
Determinants of health-related quality of life
The associations between patient characteristics and 13 broad categories of diseases are presented in table 3. A low PCS was significantly associated with an older age (OR=2.48; 95% CI 2.08 to 2.96 for patients over 75 years as compared with adults between 18 and 44 years). A low PCS was negatively associated with a high educational attainment (OR=0.65; 95% CI 0.59 to 0.72 for secondary-school level or higher in comparison with lower educational level), while low MCS scores were associated with gender (OR=1.62; 95% CI 1.45 to 1.81 for women as compared with men) and younger age. Government-funded health-insurance cover was associated with both poor PCS and MCS (OR=1.38; 95% CI 1.15 to 1.65 and OR=1.42; 95% CI 1.19 to 1.70, respectively).
Table 3.
Health-related quality of life: 12-Item Short Form questionnaire, with Factors and 13 broad diagnoses associated with mental component score and physical component score below the 25th percentile (OR from multivariable logistic regression models adjusted for age, gender, education level, insurance coverage and 13 categories of diseases (aOR) and 95% CI), for Etude épidémiologique de l'Impact de santé public sur 3 groupes de pathologies (EPI3)-LA-SER-2008 (weighted data)
| Low physical component score |
Low mental component score |
|||
| aOR* | 95% CI | aOR* | 95% CI | |
| Age (years) | ||||
| 18–44 | 1 | 1 | 1 | 1 |
| 45–64 | 1.22 | 1.08 to 1.39 | 0.96 | 0.85 to 1.08 |
| 65–74 | 1.47 | 1.25 to 1.73 | 0.65 | 0.55 to 0.78 |
| 75 and over | 2.48 | 2.08 to 2.96 | 0.70 | 0.57 to 0.86 |
| Gender: female versus male | 1.03 | 0.93 to 1.14 | 1.62 | 1.45 to 1.81 |
| Education: secondary school degree versus lower diploma | 0.65 | 0.59 to 0.72 | 1.00 | 0.90 to 1.11 |
| Government-funded insurance (vs regular health insurance) | 1.38 | 1.15 to 1.65 | 1.42 | 1.19 to 1.70 |
| Diseases of the musculoskeletal system | 2.31 | 2.08 to 2.57 | 0.95 | 0.85 to 1.06 |
| Cardiovascular diseases | 1.22 | 1.08 to 1.38 | 0.84 | 0.73 to 0.96 |
| Anxiety, depression and sleep disorders | 0.99 | 0.88 to 1.11 | 3.58 | 3.22 to 3.98 |
| Diseases of the respiratory system | 1.03 | 0.91 to 1.18 | 0.91 | 0.80 to 1.04 |
| Obesity and dyslipidaemia | 0.79 | 0.67 to 0.94 | 0.93 | 0.78 to 1.11 |
| Diabetes, thyroid gland and other endocrine disorders | 1.15 | 0.97 to 1.35 | 1.18 | 0.99 to 1.41 |
| Diseases of the digestive system | 1.01 | 0.86 to 1.19 | 1.15 | 0.89 to 1.38 |
| Diseases of the genitourinary system | 0.95 | 0.76 to 1.19 | 1.11 | 0.89 to 1.38 |
| Diseases of the nervous system, head and neck | 1.24 | 1.02 to 1.51 | 1.07 | 0.88 to 1.31 |
| Skin and subcutaneous tissue diseases | 0.68 | 0.51 to 0.90 | 0.92 | 0.71 to 1.19 |
| Bacterial, viral and parasitic systemic diseases | 1.17 | 0.89 to 1.54 | 1.12 | 0.86 to 1.48 |
| Injury and poisoning | 1.88 | 1.52 to 2.33 | 0.80 | 0.61 to 1.03 |
| Other diseases (cancer and infrequent diseases) | 1.73 | 1.38 to 2.16 | 1.35 | 1.06 to 1.72 |
aOR, adjusted odds ratio.
With regard to the disease categories, musculoskeletal diseases (OR=2.31; 95% CI 2.08 to 2.57), injury and poisoning (OR=1.88; 95% CI 1.52 to 2.33), other diseases including cancer (OR=1.73; 95% CI 1.38 to 2.16), diseases of the nervous system, head and neck (OR=1.24; 95% CI 1.02 to 1.51) and cardiovascular diseases (OR=1.22; 95% CI 1.08 to 1.38) were significant predictors of lower PCS score, whereas the opposite was found for skin and subcutaneous tissue diseases (OR=0.68; 95% CI 0.51 to 0.90) or with obesity and dyslipidaemia (OR=0.79; 95% CI 0.67 to 0.94). Significantly poor MCS were observed in patients suffering from anxiety, depression and sleep disorders (OR=3.58; 95% CI 3.22 to 3.98), and experiencing ‘other diseases’ including cancer (OR=1.35; 95% CI 1.06 to 1.72). Conversely, OR for MCS was significantly decreased for patients with cardiovascular diseases (OR=0.84; 95% CI 0.73 to 0.96). Testing the effect of clustering at the practice level yielded similar results, but to ensure parsimony of the generated models it was decided not to report such effects.
Discussion
The EPI3 survey is, to our knowledge, the first nationwide survey in general practice to provide 100 reference figures for burden of disease assessment, combining both on-site independent recruitment of a large number of patients and additional medical information from GPs. On-site selection and recruitment by an independent investigator limited the possibility of selection bias among patients, and the participation of physicians added high specificity to medical data collection.
There is a clear need for more data on QOL of patients.6 In the UK, the General Practice Research Database assembled a very large sample of 400 surgeries and 2500 individual GPs, providing detailed information on health conditions besides prescriptions, but to our knowledge not on patients' QOL.3 The Dutch national survey of general practice carried out in 1987 and 2001 gives an assessment of quality of care, but only provided by the patients themselves.2
The EPI3 survey found a similar prevalence for both diseases10 28 and comorbidities7 9 10 13 as in several other studies, which indicate a good representativeness of our weighted sample. Musculoskeletal and psychological disorders were experienced by more than half the patients attending physicians during the course of the study and represented a significant case load at GP practices. When both physical- and mental status impairment and prevalence are considered, our study further highlighted the heaviest burden of musculoskeletal disorders.
With regard to physical status, the EPI3 survey showed a similar average PCS score to other primary-care7 8 10 11 or disease-specific14 15 29 surveys using the SF-12 or SF-36 questionnaires. The mean PCS were lower than reference values computed in the French reference sample21 and in the 2003 Household survey (JL Lanoe, unpublished data, 2003). Within practices, older age,30–33 low education attainment and government-funded insurance30 32 33 were associated with lower PCS. When disease categories were considered, musculoskeletal diseases were associated with the lowest PCS,8 34 35 with PCS of similar magnitude to other European surveys including musculoskeletal diseases patients.29
Regarding mental status, although socio-demographic characteristics had a similar impact on MCS, the EPI3 survey showed significantly lower MCS scores than other studies in general practice.7 8 10 11 Additional comorbidities, which were reported for half of the EPI3 survey sample, could not alone explain this difference with other studies: MCS usually scored an average three points lower than those of patients with one morbidity.16 We believe that our findings could be explained instead by a different methodology: in all other studies conducted in general practice,7 8 10 11 mostly including a small number of medical practices,8 10 11 13 physicians may have selected participants. Our study was free from this bias in view of the selection of consecutive eligible patients in the GP's waiting room. In studies in which patients were interviewed for targeted mental disorders15 or when MCS were assessed among patients seeking specialty care,36 37 MCS measures were somewhat similar to ours. In the EPI3 survey, psychological and psychiatric diseases had the greatest negative impact on mental function consistent with other surveys in primary care7 10; it must be appreciated that associated MCS values were more similar to those of another study conducted on patients with specific psychiatric disorders.15 Lower MCS may thus highlight the overall burden of psychological distress and related diseases of patients seen in primary care.
Strengths and limitations of the study
Among the main strengths of our study, the unique combined data from patients and physicians allowed provision of reference figures for the vast majority of diseases encountered in primary care for a large number of patients. Quality-adjusted life years are usually estimated for health economics and mainly derived from QOL measures assessed from EuroQoL standardised instruments (EQ5D).1Interestingly, the conversion of SF-12 values into EQ5D Utility values has been recently documented,38 suggesting that our results could be extended for that purpose as previously reported.39
Aditionally, SF-12 questionnaires have been found to provide reliable QOL measurement across studies,22 24 even among patients with acute conditions.40 Although its validity in older patients is moderate,41 our sample was representative of the general population, thus minimising this possible bias on our results.
Finally, a lack of representativeness was an important limitation in other studies.11 42 The sample size of physicians participating in the EPI3 survey is within the range established for other French surveys (from 100 to 1006).26 Physicians were randomly selected from the national telephone directory, which includes general practitioners currently practising in primary care. This was preferred to professional registries of physicians, which lists all registered GPs, regardless of whether they are currently practising or not.
The weighted geographical distribution of the 825 GPs participating in the survey was similar to the national distribution of GPs in private practice across the 22 French regions surveyed, and the distribution of physicians' individual characteristics regarding age, gender type of contract with national health insurance and type of practice differed only slightly from national statistics26: female participation was slightly lower (23.5% compared with 26% in the IRDES sample), but the distribution between sectors was similar (8.9% vs 8.5% in sectors 1 and 2, respectively).
In terms of the representativeness of the patients, the calibrated sample of the EPI3 survey was compared with other nationwide studies and has demonstrated its efficiency through other criteria that were not used in the calibration.28 For instance, patients registered by health insurance as eligible to the ‘longstanding disease’ programme accounted for 28% in the EPI3 survey, which is very similar to the 27% in national census among GP patients.28
Our study also had several limitations. First, as outlined earlier, requirement to collect very specific data was quite intrusive, leading to a relatively low response rate from the GPs. However, stratified recruitment phases and sample sizes from both GPs and patients highly representative of national standards ensured strong external validity of the results. Second, we did not include an assessment of home consultations, common among GPs in France,27which could have led to an underestimation of burden of disease. Finally, a multiplicative effect of morbidity which has been found to be associated with QOL impairment was not assessed in our study. Some authors suggested using severity scores to complement the information on morbidity12 13and assess the impact of multimorbidity, which have already been tackled here but will be the subject of further development in future research within the EPI3 research project. It was a deliberate choice to provide an instant overview of general practice across France and the burden of a large pattern of diseases on patients' QOL as shown in previous studies which also described an independent effect of diseases on QOL.9–11
Conclusion
The EPI3 survey is the first nationwide study to report reference values for the burden of 100 different diseases in general practice, collected from a large representative sample of patients attending primary care practices. Our findings suggest that mental impairment may be underestimated in general practice. Ongoing development of healthcare policies and clinical guidelines on the treatment of diseases should rely on a direct assessment of QOL and morbidities in GP medical practices.
GPs foster continuous care, sometimes requiring highly specialised therapy to deal with comorbidities and complex situations. The present study shows that the burden of diseases in primary care is high but also can be diverse. The EPI3 survey provides information on the overall burden of diseases in general practice along with the QOL of patients regarding comorbidities as seen in this healthcare setting. This information is of great value to public health and economic assessment of healthcare, at a time when QOL is becoming a prevalent factor for care delivery and the development of clinical practice guidelines.
Supplementary Material
Acknowledgments
We thank D Abed, P Engel and A Fabre, for the statistical analysis, and R Sitta, for his advice on weighting procedures.
Footnotes
To cite: Grimaldi-Bensouda L, Begaud B, Lert F, et al. Benchmarking the burden of 100 diseases: results of a nationwide representative survey within general practices. BMJ Open 2011;1:e000215. doi:10.1136/bmjopen-2011-000215
Funding: Laboratoires Boiron, France, sponsored this independently run study published by the authors. The sponsor had no role in the design, management, data collection, analyses, interpretation, and writing of the manuscript or the decision to publish our findings.
Competing interests: LG-B, BA, MR and LA's institutions received support from Boiron for the submitted work; FR and DG received a consulting fee or honorarium from LA-SER for the submitted work; BB, FL, JM, GD and A-MM have no relationships with Boiron or any other companies that might have an interest in the submitted work in the previous 3 years; LG-B, BA and MR are employees of LA-SER, the company conducting the study; LA is a stockholder in LA-SER; LG-B was the recipient of a research fellowship from INSERM (French National Institute of Health and Medical Research) at the time of the study.
Ethics approval: Ethics approval was provided by French data protection authority (‘Commission Nationale de l'Informatique et des Libertés’).
Contributors: The work presented here was carried out with the involvement of every author. LGB, BB, FL, FR, JM, DG, BA, GD, AMM, MR and LA conceived both the research theme and the methods, analysed the data and interpreted the results. LGB implemented the trial in France, analysed the data, and together with FL, and LA drafted and revised the paper. All members of EPI3-LASER group designed the study. FL and LA analysed the data. All authors have contributed to, read and approved the final manuscript.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
References
- 1.Rawlins MD, Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ 2004;329:224–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadam UT, Croft P, Lewis M. Use of a cross-sectional survey to estimate outcome of health care: the example of anxiety and depression. J Clin Epidemiol 2001;54:1112–19 [DOI] [PubMed] [Google Scholar]
- 3.Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract 2010;60:e128–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerek-Bodden H, Koch H, Brenner G, et al. [Diagnostic spectrum and treatment requirements of general practice clients. Results of the ADT Panel of the Central Institute of National Health Insurance Management](In German). Z Arztl Fortbild Qualitatssich 2000;94:21–30 [PubMed] [Google Scholar]
- 5.Westert GP, Schellevis FG, de Bakker DH, et al. Monitoring health inequalities through general practice: the Second Dutch National survey of General Practice. Eur J Public Health 2005;15:59–65 [DOI] [PubMed] [Google Scholar]
- 6.Rasanen P, Roine E, Sintonen H, et al. Use of quality-adjusted life years for the estimation of effectiveness of health care: a systematic literature review. Int J Technol Assess Health Care 2006;22:235–41 [DOI] [PubMed] [Google Scholar]
- 7.Rijken M, van KM, Dekker J, et al. Comorbidity of chronic diseases: effects of disease pairs on physical and mental functioning. Qual Life Res 2005;14:45–55 [DOI] [PubMed] [Google Scholar]
- 8.Wensing M, Vingerhoets E, Grol R. Functional status, health problems, age and comorbidity in primary care patients. Qual Life Res 2001;10:141–8 [DOI] [PubMed] [Google Scholar]
- 9.Lam CL, Lauder IJ. The impact of chronic diseases on the health-related quality of life (HRQOL) of Chinese patients in primary care. Fam Pract 2000;17:159–66 [DOI] [PubMed] [Google Scholar]
- 10.Wang HM, Beyer M, Gensichen J, et al. Health-related quality of life among general practice patients with differing chronic diseases in Germany: cross sectional survey. BMC Public Health 2008;8:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayasinghe UW, Proudfoot J, Barton CA, et al. Quality of life of Australian chronically-ill adults: patient and practice characteristics matter. Health Qual Life Outcomes 2009;7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadam UT, Schellevis FG, van der Windt DA, et al. Morbidity severity classifying routine consultations from English and Dutch general practice indicated physical health status. J Clin Epidemiol 2008;61:386–93 [DOI] [PubMed] [Google Scholar]
- 13.Fortin M, Dubois MF, Hudon C, et al. Multimorbidity and quality of life: a closer look. Health Qual Life Outcomes 2007;5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson JA, Maddigan SL. Performance of the RAND-12 and SF-12 summary scores in type 2 diabetes. Qual Life Res 2004;13:449–56 [DOI] [PubMed] [Google Scholar]
- 15.Sanderson K, Andrews G. Prevalence and severity of mental health-related disability and relationship to diagnosis. Psychiatr Serv 2002;53:80–6 [DOI] [PubMed] [Google Scholar]
- 16.Hopman WM, Harrison MB, Coo H, et al. Associations between chronic disease, age and physical and mental health status. Chronic Dis Can 2009;29:108–16 [PubMed] [Google Scholar]
- 17.Parkerson GR, Jr, Michener JL, Yarnall KS, et al. Duke Case-Mix System (DUMIX) for ambulatory health care. J Clin Epidemiol 1997;50:1385–94 [DOI] [PubMed] [Google Scholar]
- 18.Harrison MB, Browne GB, Roberts J, et al. Quality of life of individuals with heart failure: a randomized trial of the effectiveness of two models of hospital-to-home transition. Med Care 2002;40:271–82 [DOI] [PubMed] [Google Scholar]
- 19.Kind P, Dolan P, Gudex C, et al. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konig HH, Heider D, Lehnert T, et al. ; ESEMeD/MHEDEA 2000 investigators Health status of the advanced elderly in six European countries: results from a representative survey using EQ-5D and SF-12. Health Qual Life Outcomes 2010;8:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandek B, Ware JE, Aaronson NK, et al. ; Cross-validation of item selection and scoring for the SF-12 Health survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998;51:1171–8 [DOI] [PubMed] [Google Scholar]
- 22.Organisation mondiale de la Santé Manuel de la classification statistique internationale des maladies, traumatismes et causes de décès, fondé sur les recommandations de la Conférence pour la 9e révision, 1975. Geneva: Organisation mondiale de la Santé, 1977 [Google Scholar]
- 23.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33 [DOI] [PubMed] [Google Scholar]
- 24.Garratt AM, Ruta DA, Abdalla MI, et al. The SF36 health survey questionnaire: an outcome measure suitable for routine use within the NHS? BMJ 1993;306:1440–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deville JC, Särndal CE. Calibration estimators in survey sampling. J Am Stat Assoc 1992;87:376–82 [Google Scholar]
- 26.Institut de recherche et documentation en économie de la santé (IRDES) Démographie des médecins 2008. http://www.irdes.fr/EspaceEnseignement/ChiffresGraphiques/Cadrage/DemographieProfSante/DemoMedecins.htm (accessed 2 Jun 2011). [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labarthe G. Les consultations et visites des médecins généralistes Un essai de typologie. Études et Résultats. DREES. No. 315, June 2004 [Google Scholar]
- 29.Loza E, Jover JA, Rodriguez L, et al. ; EPISER Study Group Multimorbidity: prevalence, effect on quality of life and daily functioning, and variation of this effect when one condition is a rheumatic disease. Semin Arthritis Rheum 2009;38:312–19 [DOI] [PubMed] [Google Scholar]
- 30.Burdine JN, Felix MR, Abel AL, et al. The SF-12 as a population health measure: an exploratory examination of potential for application. Health Serv Res 2000;35:885–904 [PMC free article] [PubMed] [Google Scholar]
- 31.Fleishman JA, Lawrence WF. Demographic variation in SF-12 scores: true differences or differential item functioning? Med Care 2003;41(7 Suppl):III75–86 [DOI] [PubMed] [Google Scholar]
- 32.Fone D, Dunstan F, Lloyd K, et al. Does social cohesion modify the association between area income deprivation and mental health? A multilevel analysis. Int J Epidemiol 2007;36:338–45 [DOI] [PubMed] [Google Scholar]
- 33.Wainwright NW, Surtees PG. Places, people, and their physical and mental functional health. J Epidemiol Community Health 2004;58:333–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadam UT, Croft PR; North Staffordshire GP Consortium Group Clinical multimorbidity and physical function in older adults: a record and health status linkage study in general practice. Fam Pract 2007;24:412–19 [DOI] [PubMed] [Google Scholar]
- 35.Antonopoulou MD, Alegakis AK, Hadjipavlou AG, et al. Studying the association between musculoskeletal disorders, quality of life and mental health. A primary care pilot study in rural Crete, Greece. BMC Musculoskelet Disord 2009;10:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaynes BN, Rush AJ, Trivedi MH, et al. Major depression symptoms in primary care and psychiatric care settings: a cross-sectional analysis. Ann Fam Med 2007;5:126–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGorm K, Burton C, Weller D, et al. Patients repeatedly referred to secondary care with symptoms unexplained by organic disease: prevalence, characteristics and referral pattern. Fam Pract 2010;27:479–86 [DOI] [PubMed] [Google Scholar]
- 38.Le QA, Doctor JN. Probabilistic mapping of descriptive health status responses onto health state utilities using Bayesian networks: an empirical analysis converting SF-12 into EQ-5D utility index in a national US sample. Med Care 2011;49:451–60 [DOI] [PubMed] [Google Scholar]
- 39.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851–9 [DOI] [PubMed] [Google Scholar]
- 40.Failde I, Medina P, Ramirez C, et al. Construct and criterion validity of the SF-12 health questionnaire in patients with acute myocardial infarction and unstable angina. J Eval Clin Pract 2010;16:569–73 [DOI] [PubMed] [Google Scholar]
- 41.Jakobsson U, Westergren A, Lindskov S, et al. Construct validity of the SF-12 in three different samples. J Eval Clin Pract. Published Online First: 6 January 2011. doi:10.1111/j.1365-2753.2010.01623.x [DOI] [PubMed] [Google Scholar]
- 42.Sprangers MA, de Regt EB, Andries F, et al. Which chronic conditions are associated with better or poorer quality of life? J Clin Epidemiol 2000;53:895–907 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.