Abstract
Objective
To evaluate effectiveness of physiotherapy management in patients experiencing whiplash associated disorder II, on clinically relevant outcomes in the short and longer term.
Design
Systematic review and meta-analysis. Two reviewers independently searched information sources, assessed studies for inclusion, evaluated risk of bias and extracted data. A third reviewer mediated disagreement. Assessment of risk of bias was tabulated across included trials. Quantitative synthesis was conducted on comparable outcomes across trials with similar interventions. Meta-analyses compared effect sizes, with random effects as primary analyses.
Data sources
Predefined terms were employed to search electronic databases. Additional studies were identified from key journals, reference lists, authors and experts.
Eligibility criteria for selecting studies
Randomised controlled trials (RCTs) published in English before 31 December 2010 evaluating physiotherapy management of patients (>16 years), experiencing whiplash associated disorder II. Any physiotherapy intervention was included, when compared with other types of management, placebo/sham, or no intervention. Measurements reported on ≥1 outcome from the domains within the international classification of function, disability and health, were included.
Results
21 RCTs (2126 participants, 9 countries) were included. Interventions were categorised as active physiotherapy or a specific physiotherapy intervention. 20/21 trials were evaluated as high risk of bias and one as unclear. 1395 participants were incorporated in the meta-analyses on 12 trials. In evaluating short term outcome in the acute/sub-acute stage, there was some evidence that active physiotherapy intervention reduces pain and improves range of movement, and that a specific physiotherapy intervention may reduce pain. However, moderate/considerable heterogeneity suggested that treatments may differ in nature or effect in different trial patients. Differences between participants, interventions and trial designs limited potential meta-analyses.
Conclusions
Inconclusive evidence exists for the effectiveness of physiotherapy management for whiplash associated disorder II. There is potential benefit for improving range of movement and pain short term through active physiotherapy, and for improving pain through a specific physiotherapy intervention.
Article summary
Article focus
Physiotherapy intervention is recommended in whiplash associated disorder II, although the most beneficial intervention and the effectiveness of physiotherapy management are unclear.
Systematic reviews have not focused on whiplash associated disorder II, which represents approximately 93% of patients presenting for management post-whiplash injury.
The objective of this systematic review was to evaluate the effectiveness of physiotherapy management in patients experiencing whiplash associated disorder II, on clinically relevant outcomes in the short and longer term.
Key messages
This systematic review demonstrates inconclusive very low/low quality evidence for the effectiveness of physiotherapy management for whiplash associated disorder II.
There is potential benefit for improving pain and range of movement short term through active physiotherapy and for improving pain through specific physiotherapy interventions.
This potential benefit merits further consideration in a properly powered clinical trial with attention to ensure low risk of bias.
Strengths and limitations of this study
The strengths of this review are its focus to physiotherapy intervention and the most common whiplash associated disorder II classification requiring physiotherapy intervention.
A limitation is that differences between participants, interventions and trial designs limited potential meta-analyses.
Surprisingly, no chronic interventions were comparable for analysis, considering the high number of patients experiencing chronicity with whiplash associated disorder.
Introduction
Road traffic accidents are the primary cause of whiplash, a soft tissue injury to the neck following an acceleration–deceleration mechanism of injury.1 The cumulative incidence of patients seeking healthcare post-whiplash from a road traffic accident has increased during the last 30 years to recent estimates of >3/1000 inhabitants in North America and Western Europe2 and 1.0–3.2/1000 inhabitants in Sweden.3 In the UK, insurance statistics indicate that 300 000 patients present per annum with whiplash associated disorders.4 Whiplash associated disorders are the resulting clinical presentations following the injury and can range in severity, clinical symptoms and physical findings.1 Many patients with whiplash associated disorders experience persistent pain and disability, with reports suggesting that 40–60% of those injured have chronic symptoms.5–8 The annual economic cost associated with management of whiplash associated disorders and associated time off work is estimated as $3.9 billion in the USA,9 and €10 billion in Europe.10
Patients experiencing whiplash associated disorders may be regarded as a distinct group within the broader non-specific neck pain population,1 2 7 11–13 although following review of trial data (n=4 trials), recent evidence questions this distinction for a primary care population and has identified a need for further research.14 Whiplash associated disorders can be categorised as grades 0–IV,1 where a higher grade indicates increased severity. The classification system is widely used in clinical practice15 and guidelines.16 Patients with whiplash associated disorder II who experience neck pain accompanied by stiffness or tenderness, and musculoskeletal signs, for example a reduced range of available movement, form the major group of patients (93.4%)15 who might benefit from conservative management, commonly involving physiotherapy intervention. A recent best evidence synthesis3 recommended a focus of research to the most common whiplash associated disorder I and II classifications, excluding classification III and above (ie, patients with neurological signs and fracture and/or dislocation) and classification 0 (no complaint at the neck, and no physical signs).1 However, a classification of whiplash associated disorder I is less commonly seen by physiotherapists as there are no accompanying physical findings (neck pain, stiffness or tenderness but with no physical findings) and patients are known to recover within 6 months post-injury.15
Evidence of the effectiveness of physiotherapy intervention for the treatment of whiplash associated disorder II is scarce. Existing systematic reviews instead tend to focus on a range of whiplash associated disorder classifications and a broad range of conservative intervention strategies such as educational videos, include studies of non-traumatic neck pain, and lack rigorous assessment of the risk of bias of included studies. The most robust evidence, a Cochrane review,17 on the management of whiplash associated disorder I/II patients does not specifically assess physiotherapy. No review has included trials published post-2006. The effectiveness of physiotherapy for the whiplash associated disorder II population is therefore unclear.
The objective of this systematic review was to investigate the short and longer term effectiveness of physiotherapy outpatient management of patients presenting with whiplash associated disorder II, in terms of function, disability and health,18 in patients aged >16 years.
Materials and methods
A systematic review was conducted according to a predefined protocol based on the method guidelines of the Back Review Group of the Cochrane Collaboration19 and the Cochrane handbook.20 It is reported in line with the PRISMA statement.21
Eligibility criteria
Studies
Randomised controlled trials (RCTs) evaluating the effectiveness of physiotherapy outpatient management of patients experiencing whiplash associated disorder II were included. Studies not written in English were excluded rather than restricting the inclusion of studies, thereby providing information of potential bias.22 No restrictions were placed on publication date.
Participants
Patients aged >16 years who had experienced a whiplash injury, classified as whiplash associated disorder II, were included. Acute and chronic presentations were included and analysed separately. Mixed populations of different classifications of whiplash associated disorder were included if patients presenting with whiplash associated disorder II formed part of the population.
Interventions
Any physiotherapy outpatient management intervention was included.
Outcome measures
Measures addressing domains within the international classification of function, disability and health,18 in the short term (approximately 3 months post-injury/intervention) and/or longer term (approximately 12 months) were included.
Information sources
Each of the following databases was searched using sensitive topic based search strategies to the end of December 2010:
The Cochrane Library: Controlled Trials Register, Health Technology Assessment Database, NHS Economic Evaluation Database.
CINAHL, EMBASE, MEDLINE, PEDro, ZETOC databases.
Selected internet sites and indexes: Turning Research into Practice, Health Services/Technology Assessment, PUBMED.
National Research Register, Current Controlled Trials website (York).
Cochrane Back Review Group.
Cochrane Cervical Overview Group.
Hand searches in key journals, for example Spine, Manual Therapy, Physiotherapy, Physical Therapy, Australian Journal of Physiotherapy.
Science Citation Index and Social Science Citation Index.
Unpublished research22: British National Bibliography for Report literature, Dissertation Abstracts, Index to Scientific and Technical Proceedings, National Technical Information Service, System for Information on Grey Literature.
Personal citations for key authors in the field.
The searches used predefined terms. Box 1 provides two examples of the searches utilised.
Box 1. Examples of search strategies.
Medline (Ovid) 1948–31 December 2010
1. Acute whiplash or cervical spine disorder or cervical spine injury.mp
2. Manual therapy or manipulation or massage.mp
3. Clinical trial or randomised controlled trial or RCT.mp
4. 1 and 2
5. 3 and 4
6. WAD II or whiplash associated disorders or whiplash injury or whiplash patients or whiplash syndrome.mp
7. 2 and 6
8. 3 and 7
9. Conservative approach or conservative intervention or conservative management or conservative therapy.mp
10. Physical approach or physical intervention or physical management or physical therapy.mp
11. Exercise or active range of motion exercise$ or strengthening exercise$ or stretching exercise$ or therapeutic exercise$ or endurance training or home exercise$ or proprioception exercise$
12. Transcutaneous electrical nerve stimulation or TENS or thermotherapy or electrical stimulation or heat or electrotherapy.mp
13. Pain management program$.mp
14. Patient education or educational or self management program$.mp
15. Posture or (postural and balance) or traction.mp
16. 1 and 9
17. 3 and 16
18. 6 and 9
19. 3 and 18
20. 1 and 10
21. 3 and 20
22. 6 and 10
23. 3 and 22
24. 1 and 11
25. 3 and 24
26. 6 and 11
27. 3 and 26
28. 1 and 12
29. 3 and 28
30. 6 and 12
31. 3 and 30
Embase (Ovid) 1947–31 December 2010
1. Acute whiplash or cervical spine disorder or cervical spine injury.mp
2. Manual therapy or manipulation or massage.mp
3. Clinical trial or randomised controlled trial or RCT.mp
4. 1 and 2
5. 3 and 4
6. WAD II or whiplash associated disorders or whiplash injury or whiplash patients or whiplash syndrome.mp
7. 2 and 6
8. 3 and 7
9. Conservative approach or conservative intervention or conservative management or conservative therapy.mp
10. Physical approach or physical intervention or physical management or physical therapy.mp
11. Exercise or active range of motion exercise$ or strengthening exercise$ or stretching exercise$ or therapeutic exercise$ or endurance training or home exercise$ or proprioception exercise$
12. Transcutaneous electrical nerve stimulation or TENS or thermotherapy or electrical stimulation or heat or electrotherapy.mp
13. Pain management program$.mp
14. Patient education or educational or self management program$.mp
15. Posture or (postural and balance) or traction.mp
16. 1 and 9
17. 3 and 16
18. 6 and 9
19. 3 and 18
20. 1 and 10
21. 3 and 20
22. 6 and 10
23. 3 and 22
24. 1 and 11
25. 3 and 24
26. 6 and 11
27. 3 and 26
28. 1 and 12
29. 3 and 28
30. 6 and 12
31. 3 and 30
Study selection
Two subject experts independently searched information sources (GE/NH), and independently assessed identified studies for inclusion by grading each criterion (table 1) as eligible/not eligible/might be eligible.19 A study was potentially relevant and its full text was obtained, when it could not be unequivocally excluded on the basis of its title and abstract22 following discussion between the two independent reviewers. In a situation of disagreement or when abstracts contained insufficient information, the full text was obtained. A study was included in the review when both reviewers independently assessed it as satisfying the inclusion criteria from the full text. If agreement was not obtained, a third reviewer (AR, subject and methodological expert) mediated following discussion.19
Table 1.
Criteria for inclusion and exclusion of studies in the review
| Criteria | |
| Inclusion criteria | |
| Study design | RCT |
| Population | |
| Age | 16 years or older |
| Subjects | Human; outpatients |
| Condition | Post-whiplash injury |
| Experiencing whiplash associated disorder II | |
| Intervention | Conservative physiotherapy outpatient management |
| Comparison group(s) | At least one comparison group: placebo/other intervention/no intervention |
| Outcome | Measurement of at least one of the following outcomes: disability; functional status; physical impairment; impact on social and occupational levels of fitness; pain; quality of life; patient satisfaction |
| Measurement of short term outcome (approx 3 months post-surgery) and/or long term outcomes (≥1 year post-surgery) | |
| Time frame | All studies conducted from 1979 onwards |
| Exclusion criteria | |
| Study design | Initial search: studies stated as RCTs but do not have a comparison group or random allocation to groups |
| Participant characteristics | Multiple pathology |
| Whiplash associated disorder not classified according to severity to provide clarity of whiplash associated disorder II population | |
| Intervention | None |
| Outcome | None |
| Language | Full article not written in English |
RCT, randomised controlled trial.
Risk of bias was independently assessed by the same reviewers for each included study. Risk of bias, and homogeneity of participants, interventions and outcomes were key considerations informing the potential for including trials in meta-analyses, in line with Cochrane.20 The third reviewer again mediated.20 Agreement between reviewers was evaluated using Cohen's κ.23 All processes and tools were piloted.
Data collection process
Two reviewers (AR/CW) independently extracted the data20 24 using a standardised form. A third independent reviewer (NH) checked for consistency and clarity.
Data items
Data extracted for each trial included: design, participants and indication, whiplash associated disorder categorisation, interventions, study setting, outcome measures, timing of assessments, power calculations, loss to follow-up, intention to treat analyses and main results. Key outcome measures were predefined as valid tools to measure pain, disability, function, physical impairment, social impact and patient satisfaction, reflecting domains from the International Classification of Functioning, Disability and Health.18 Based on recommendations, a maximum of two primary outcomes were considered acceptable,25 when more than one primary outcome was reported and alpha spend was not considered.
Risk of bias in individual studies
The Cochrane ‘risk of bias’ assessment tool was used to appraise the internal validity of each included trial.21 26 In contrast to the majority of quality scales used in health research,21 27 28 the Cochrane tool is informed by empirical research.26 Each component of bias was reported independently and considered with regard to each key outcome measure.26 29 The component including ‘blinding’ the treating therapist has been acknowledged as generally impossible26 and this formed part of the appraisal by the reviewers as the Cochrane tool also permits evaluation of the likely influence of any lack of blinding. The rigour of the risk of bias assessment was ensured through strict application of the defined criteria to inform conclusions, making explicit the trials of high risk of bias or poor reporting.30
Summary measures
Quantitative synthesis was conducted in line with the protocol on comparable key outcomes across trials evaluating similar interventions (nature of intervention, and timing of assessments at approximately 3 months and/or 12 months post-injury or intervention). Results were reported in the context of overall risk of bias. Comparable outcomes were defined as tools developed to measure the same underlying domain. Two subject experts and two methodological experts identified the combinations of studies and outcomes on which to conduct meta-analyses.
Using RevMan,31 meta-analyses compared standardised differences in means using DerSimonian–Laird random effects32 for the principal analyses to allow for systematic differences in effects estimated across the included trials.22 32 For summary statistics, 95% CIs were reported. Standardised mean differences were selected to make comparisons across studies that used different tools to measure the same outcome,22 or reported a mixture of final value scores and change from baseline scores.33 Hedges–Olkin fixed effects34 were used as the supportive analyses.
Planned methods of analysis
Data were requested from all authors, except for those with no comparability of outcome measures to other trials.35 36 Data defined by whiplash associated disorder classification was also requested from all authors of trials that reported combined whiplash associated disorder classifications. Analyses were conducted on final summary statistics when reported or the raw data where supplied. When necessary, standard deviations were estimated from reported CIs or percentiles.33 In line with the use of random effects as primary analyses,32 change scores were used for studies when no other data were forthcoming. Heterogeneity in treatment effects was evaluated through computation of I2.
Risk of bias across studies
A summary assessment for risk of bias was tabulated across studies, and consensus agreed concerning the overall potential risk of bias. It was not helpful to attempt to assess potential publication bias visually using Funnel plots22 as less than 10 trials were included in meta-analyses.37
Additional analyses
No post-hoc supportive analyses were conducted owing to the inconsistency of outcome measures across the trials.
Results
Study selection
Included trials were grouped according to the whiplash associated disorder classification1 into five categories:
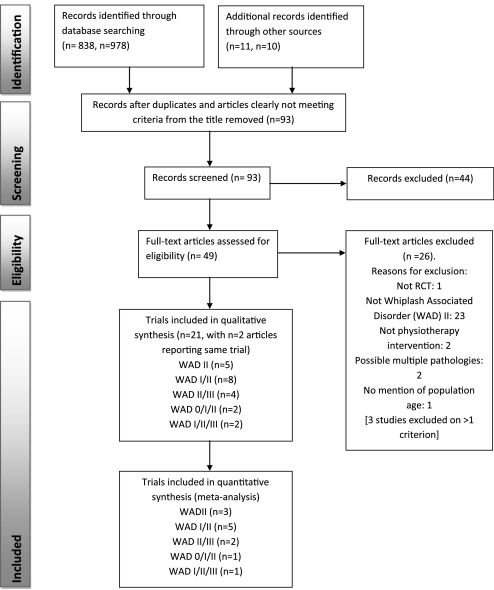
Whiplash associated disorder II: five articles and five trials,36 38–41 from four countries were included.
Whiplash associated disorders I/II: eight articles and eight trials,42–49 from six countries were included.
Whiplash associated disorders II/III: four articles and four trials,35 50–52 from three countries were included.
Whiplash associated disorders 0/I/II: three articles and two trials,53–55 from two countries were included.
Whiplash associated disorders I/II/III: three articles and two trials,56–58 from one country were included.
Most retrieved trials were published in English with only two in other languages. One relevant unpublished study was found (Managing Injuries of the Neck Trial, accessible at http://www.hta.ac.uk/1399, due to be published 2011). Figure 1 presents the numbers of studies at each stage of selection. Complete inter-reviewer agreement was achieved on study inclusion across all categories following discussion.
Figure 1.
Study selection flow diagram (from Moher et al21). WAD, whiplash associated disorder.
Study characteristics
Descriptive data for the 21 included trials are summarised in online table 1.
Methods
Eighteen trials randomised participants across two groups, one trial across three groups, and two trials across four groups. Eight trials compared a specific physiotherapy intervention, for example manipulation, to no management, sham or placebo. Thirteen trials compared an active physiotherapy intervention to standard care; the active approaches were characterised by additional interventions, a multimodal intervention or a progressive intervention. Duration of interventions ranged from one treatment session to 12 months. The number of assessments varied from 1 to 4, occurring immediately post-treatment to 3 years.
Participants
The 21 trials randomised 2126 participants. Age varied from 16 to 70 years. A total of 271/2126 participants were randomised in trials focused to whiplash associated disorder II.i Of the authors who responded, no authors were able to provide data for their included whiplash associated disorder classifications separately. In the eight whiplash associated disorder I/II category trials, 934 participants were randomised but no distinction of whiplash associated disorder II participants was possible. In the four whiplash associated disorder II/III category trials, 333/409 (81.5%, two trials) participants were classified as whiplash associated disorder II; in a further 111 participants (two trials), no distinction of whiplash associated disorder II participants was possible. In the two whiplash associated disorder O/I/II category trials, 302 participants were randomised with no distinction of whiplash associated disorder II participants possible. In the two whiplash associated disorder I/II/III category trials, 49/66 (74%, 1 trial) participants were classified as whiplash associated disorder II; in a further 33 participants (1 trial), no distinction of whiplash associated disorder II participants was possible. A total of 1395 participants were randomised in the 12 trials included in the meta-analyses.
Interventions
Eight trials were conducted at single centres that included physiotherapy clinics or outpatient departments. Both a clinic and home setting were used in one trial. The setting was unclear in 12 trials. One trial investigated a group intervention. Interventions could be grouped according to whether they were a specific physiotherapy intervention or an active intervention comprising different components. Timing of interventions included acute/sub-acute (13 trials) and chronic stages (8 trials), ranging from 2 days to 15 years post-injury.
Primary outcomes
Only six (28.5%) trials specified primary outcomes a priori that included: Neck Pain and Disability Index, Nociceptive Flexion Reflex, Neck Disability Index, Pain Visual Analogue Scale (VAS), Pain VAS and Work Activities VAS, and Pain VAS and Disability VAS. One trial46 specified three primary outcome measures with no adjustment for alpha spend and was therefore evaluated as unacceptable in specifying primary outcomes.25
Secondary and additional outcomes
Most trials reported some assessment of pain (general or specific to the neck) (15 trials), and range of movement (ROM) (13 trials). Nine trials reported assessment of disability. A wide range of other outcomes included: work status, SF36, Tampa, patient satisfaction, muscle stability, posture and kinaesthetic sensibility. Two trials reported outcomes that were not consistent with any other trial, for example temperature pain threshold36 and the tandem standing balance test.35
Risk of bias within studies
‘Almost perfect’59 93% inter-reviewer agreement was achieved on risk of bias assessment prior to discussion (Cohen's κ=0.90, p<0.0005) and 100% agreement was reached following discussion. Only two trial protocols were available.60 61 Of the 21 included trials, 20 were evaluated as high risk of bias and one as unclear risk of bias (table 2). The very high proportion of trials identified as high risk of bias should affect the interpretation of results.26
Table 2.
Summary assessment of the overall risk of bias for each trial
| Study (authors, year, country) | Components of risk of bias |
Summary risk of bias | Comments, high risk components | ||||||
| 1 | 2 | 3 | 4 | 5a | 5b | 6 | |||
| WAD II | |||||||||
| Aigner et al38 (2006) | U | U | U | U | U | U | H |
|
|
| Dehner et al39 (2009) | L | L | U | U | U | N/A | H |
|
|
| Gonzalez-Inglesias et al40 (2009) | L | L | L | L | U | N/A | H |
|
|
| Jull et al41 (2007) | L | L | L | L | U | N/A | L |
|
|
| Sterling et al36 (2010) | L | U | L | L | U | N/A | H |
|
|
| WAD I/II | |||||||||
| Ask et al42 (2009) | U | L | L | L | U | U | H |
|
|
| Bonk et al43 (2000) | U | U | H | L | U | N/A | H |
|
|
| Pato et al44 (2010) | U | U | L | L | U | N/A | H |
|
|
| Scholten-Peeters et al45 (2006) [Scholten-Peeters et al60 (2003) trial protocol] | L | L | L | L | L | L | H |
|
|
| Stewart et al46 (2007), [Stewart et al61 (2003) trial protocol] | L | L | L | L | L | N/A | H |
|
|
| Thuile and Walzl47 (2002) | U | U | U | U | U | N/A | H |
|
|
| Vassiliou et al48 (2006) | L | L | L | H | U | N/A | H |
|
|
| Vikne et al49 (2007) | U | L | L | H | U | U | H |
|
|
| WAD II/III | |||||||||
| Armstrong et al50 (2005) | U | U | U | L | U | N/A | H |
|
|
| Fernandez-de-las-Penas51 (2004a) | L | U | U | U | U | N/A | H |
|
|
| Fernandez-de-las-Penas52 (2004b) | L | U | U | U | U | N/A | H |
|
|
| Hansson et al35 (2006) | L | L | L | H | U | N/A | H |
|
|
| WAD 0/I/II | |||||||||
| Rosenfeld et al54 (2003), [Rosenfeld et al (2006) reporting same trial] | U | L | L | H | U | U | H |
|
|
| Schnabel et al55 (2004) | H | U | U | H | U | N/A | H |
|
|
| WAD I/II/III | |||||||||
| Soderlund et al56 (2000) | U | U | U | L | U | N/A | H |
|
|
| Soderlund and Lindberg57 (2001), [Soderlund and Lindberg58 (2007) reporting same trial] | U | U | L | L | U | N/A | H |
|
|
Components of risk of bias: 1, sequence generation; 2, allocation concealment; 3, blinding of participants, personnel and outcome assessors; 4, incomplete outcome data; 5a, short term selective outcome reporting; 5b, long term selective outcome reporting; 6, other potential threats to validity.
Levels of risk of bias: H, high risk of bias; U, unclear risk of bias; L, low risk of bias. N/A, not applicable, no investigation of long term outcome.
Risk of bias across studies
Only trials evaluated as high risk of bias were available for meta-analysis. Although reasons for the high risk components provided concern for potential bias, results from meta-analyses evaluated critically within this context enabled an overview of the evidence to be presented, strength of effect to be presented, and tentative conclusions to be proposed to advance research.
Results of individual studies and synthesis of results
Comparability of interventions, timing of assessments and outcome measures were considered to determine appropriate quantitative syntheses of trials.22 In exploring the compatibility of outcomes for management in the acute/sub-acute and chronic stages, no possible quantitative syntheses within the five categories of whiplash associated disorders were possible. No further information regarding whiplash associated disorder classification was provided by authors to assist potential comparisons regarding whiplash associated disorder II. In comparing across categories, no comparison was possible for intervention in the chronic stage or long term. The following meta-analyses were conducted in the acute/sub-acute stage in the short term:
Active intervention versus standard intervention for: pain, 4–12 weeks (n=6 trials); ROM flexion/extension (flex/ext), 12 weeks (n=3 trials); ROM rotation (Rot), 12 weeks (n=4); ROM side flexion (SF), 12 weeks (n=3); total ROM, 4–12 weeks (n=3)ii; disability, 6–12 weeks (n=5).
Specific intervention versus control post-intervention for: pain (n=4 trials)iii; ROM flex/ext, ROM Rot, and ROM SF (n=3 trials).iv
Active versus standard intervention short term
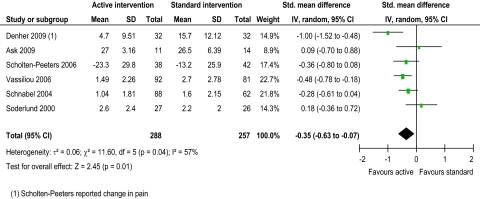
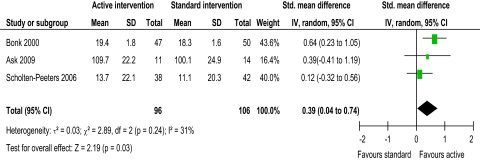
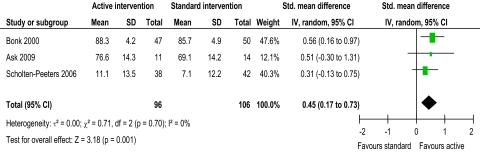
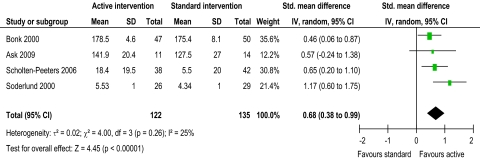
Evidence from two trials39 48 suggested that intervention might reduce pain, with active intervention being beneficial compared to standard intervention (figure 2). This was not supported by four trials.42 45 55 56 The pooled random effects (−0.35, 95% CI −0.63 to −0.07) did support evidence of an effect short term. Evidence from one trial43 suggested that intervention might improve ROM flex/ext and ROM SF, with active intervention being beneficial compared to standard intervention (figures 3 and 4). This was not supported by two trials.42 45 The pooled random effects (ROM flex/ext: 0.39, 95% CI 0.04 to 0.74; ROM SF: 0.45, 95% CI 0.17 to 0.73) did support evidence of an effect short term. Evidence from three trials43 45 56 suggested that intervention might improve ROM Rot, with active intervention being beneficial compared to standard intervention (figure 5). This was not supported by one trial.42 The pooled random effects (0.68, 95% CI 0.38 to 0.99) did support evidence of an effect short term.
Figure 2.
Pain short-term.
Figure 3.
ROM (range of movement) flexion/extension short-term.
Figure 4.
ROM (range of movement) right side flexion/left side flexion short-term.
Figure 5.
ROM (range of movement) rotation right/left short-term.
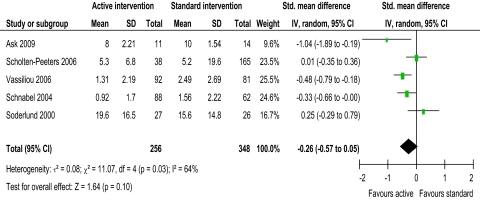
Overall, there was no evidence of short term benefit of active over standard intervention on total ROM (pooled random effects 0.28, 95% CI −0.03 to 0.59) or disability (figure 6: −0.26, 95% CI −0.57 to 0.05).
Figure 6.
Disability short-term.
Specific physiotherapy intervention versus control
Evidence from four trials40 47 51 52 suggested that intervention might reduce pain short term, with specific physiotherapy intervention being beneficial compared to control. The pooled random effects (−2.11, 95% CI −3.85 to −0.36) did support evidence of an effect short term. Overall, there was no evidence of short term benefit of specific physiotherapy intervention over control on ROM flex/ext (pooled random effects 0.83, 95% CI −3.79 to 5.44), ROM Rot (pooled random effects −1.02, 95% CI −3.73 to 1.68) or ROM SF (pooled random effects −1.21, 95% CI −3.11 to 0.69).
Discussion
Summary of evidence
Evidence was assessed from 21 RCTs (2126 participants) conducted across nine countries. Only one trial investigated a group intervention. Interventions were grouped into active versus standard intervention, and specific physiotherapy intervention versus control. No meta-analyses were possible exclusively on a whiplash associated disorder II population, as most trials included combined classifications of whiplash associated disorders in their populations. Disappointingly, as many trials were recent, 20/21 trials were assessed as high risk of bias, and one as unclear risk. All 12 trials (1395 participants from six countries) included in the meta-analyses were assessed as high risk. Comparable outcomes across trials included pain, ROM flex/ext, ROM Rot, ROM SF, total ROM and disability in the short term. There was no evidence beyond individual results of benefit in the longer term as no meta-analyses were possible. The one trial that evaluated as unclear risk of bias was, therefore, not included in any meta-analyses.41
In evaluating short term outcome in the acute/sub-acute stage, there was some evidence that active physiotherapy intervention reduces pain. This was supported by statistically significant differences in two trials.39 48 Although the finding is interesting, further trials are required since one trial possessed one high risk component of bias, and the other possessed two. Only one trial43 suggested that active physiotherapy intervention changes ROM (flex/ext and SF); three trials43 45 56 suggested a change in ROM Rot. There was evidence from the meta-analyses to support this. Again, risk of bias was high for all trials, with two high risk components for one trial43 and one high risk component for the two other trials. There was no evidence that active physiotherapy intervention affects disability.
In evaluating short term outcome in the acute/sub-acute stage, there was some evidence that specific physiotherapy intervention reduces pain. This was supported by statistically significant differences found in four trials40 47 51 52 using interventions of Kinesio taping, magnetic therapy and manipulation. Although the finding is interesting, further trials are required because all trials possessed one high risk component of bias and two trials had an additional four unclear risks. Only one individual trial47 suggested that specific physiotherapy intervention (magnetic therapy) changes ROM (flex/ext or Rot or SF) in the short term. There was no evidence from the meta-analyses to support this.
Limitations
The strengths of this review are its focus to physiotherapy intervention and the most common whiplash associated disorder II classification requiring physiotherapy intervention. Heterogeneity in treatment effects can be explained by variation in the quality of administration of interventions. Differences were evident in the outcome measures, assessment points, and classification of whiplash associated disorder participants, where many trials combined whiplash associated disorder classifications even though interventions in practice would vary between classifications.15 16 Differences in components of the physiotherapy interventions were also evident, with some variation explained by diversity in practice across countries. The differences limited the possible comparisons in the meta-analyses. Surprisingly, no chronic interventions were comparable for analysis, considering the high number of patients experiencing chronicity with whiplash associated disorder.7 8 Also surprisingly, work status was not possible for analysis considering the economic implications of whiplash associated disorder.9 10
Moderate heterogeneity (I2=57%) was present in the evidence for active intervention for pain,33 identifying significant difference in treatment effects between trials. However, heterogeneity might not be important for ROM flex/ext, Rot and SF (I2=31%, 25% and 0%, respectively). Substantial heterogeneity (I2=64%) was present in the evidence for active intervention for disability, perhaps explaining the lack of evidence of an effect. Considerable heterogeneity33 was present in the evidence for specific physiotherapy intervention for pain, ROM flex/ext, Rot, and SF (I2=98.1%, 99.0%, 98.1% and 96.6%, respectively), perhaps explaining the lack of evidence of an effect for all ROM evaluations. This anticipated heterogeneity was accounted for by using the random effects model.
Using GRADE62 (the Grading of Recommendations Assessment, Development and Evaluation system), the quality of the body of evidence for physiotherapy rehabilitation in the management of whiplash associated disorder II, based on the 12 trials included in the meta-analyses, is ‘very low’ for pain, ROM flex/ext and SF (active vs standard intervention), and ‘low’ for ROM Rot (active vs standard intervention) and pain (specific intervention vs control) in the short term. These estimates are interpreted as ‘little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect’ (very low) and ‘confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect’ (low).62 Downgrading of quality was due to high risk of bias, and issues of imprecision and inconsistency.62
The limitations in the context of the high risk of bias and number of trials available necessitate urgent attention to focus a future high quality and properly powered trial to evaluate a whiplash associated disorder II population. The very low/low quality of trials is consistent with earlier findings for physiotherapy management post-lumbar discectomy.30 63 There is limited scope at present for good quality meta-analyses in physiotherapy with rigorous and well reported trial inclusion. Physiotherapy trials need to avoid risk of bias. Planning for quality is important, particularly for issues that present known problems for physiotherapy trials, for example loss to follow-up. Consensus for minimum core sets of outcome measures for specific populations is also required.
Conclusions
This systematic review has identified inconclusive very low/low quality evidence for the effectiveness of physiotherapy management for whiplash associated disorder II. Inclusion of large numbers of participants in the poorly designed trials published to date is unethical. Best practice for physiotherapy management, therefore, remains unclear. This lack of clarity might explain the variability of interventions across the trials that made comparability of interventions difficult. There is potential benefit for improving pain and ROM flex/ext, Rot and SF short term through active physiotherapy, and for improving pain through specific physiotherapy interventions. This potential benefit merits further consideration in a properly powered clinical trial with attention to ensure low risk of bias.
Supplementary Material
Footnotes
To cite: Rushton A, Wright C, Heneghan N, et al. Physiotherapy rehabilitation for whiplash associated disorder II: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 2011;1:e000265. doi:10.1136/bmjopen-2011-000265
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None.
Contributors: AR and GE are senior lecturers in Physiotherapy and NH is a lecturer. MC and CW are both senior lecturers. NF is Professor of Clinical Epidemiology and Biostatistics. AR, MC, CW and NF have longstanding professional interests in the quality and reporting of randomised controlled trials in medicine and physiotherapy. AR, NH and GE have a professional focus to musculoskeletal physiotherapy. AR and CW were responsible for the conception of the study. All authors have contributed to the systematic review and have been involved in developing the content of the article. AR wrote the first draft of the paper and developed it initially with CW. AR has worked with all authors reworking content into subsequent drafts. All authors gave final approval of the version to be published. AR is the guarantor.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
In Aigner et al38, three subject experts agreed that the Kramer grade II evaluated as equivalent to the WADII classification.
Included Thuile and Walzl47 although timing of intervention and assessment was unclear from trial.
Aigner et al38 n=5 loss to follow up but not clear from which group.
References
- 1.Spitzer WO, Skovron ML, Salmi LR, et al. Scientific monograph of the Quebec Task Force on Whiplash Associated Disorders: redefining ‘whiplash’ and its management. Spine (Phila Pa 1976) 1995;20(8 Suppl):1S–73S [PubMed] [Google Scholar]
- 2.Holm LW, Carroll LJ, Cassidy JD, et al. The burden and determinants of neck pain in Whiplash associated disorders after traffic collisions, results of the Bone and Joint Decade 2000-2010 Task Force on Neck pain and its Associated Disorders. Spine (Phila Pa 1976) 2008;33(4 Suppl):S52–9 [DOI] [PubMed] [Google Scholar]
- 3.Jansen GB, Edlund C, Grane P, et al. ; The Swedish Society of Medicine and the Whiplash Commission Medical Task Force Whiplash injuries: diagnosis and early management. Eur Spine J 2008;17(Suppl 3):S359–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton K. Treatment guidelines: is there a need? Proceedings of Whiplash Conference 2003, Bath, England, 6–8th May. Bristol: Lyons Davidson Solicitors, 2003 [Google Scholar]
- 5.Barnsley L, Lord S, Bogduk N. Whiplash injury: clinical review. Pain 1994;58:283–307 [DOI] [PubMed] [Google Scholar]
- 6.Scholten-Peeters GG, Verhagen AP, Bekkering GE, et al. Prognostic factors of whiplash-associated disorders: a systematic review of prospective cohort studies. Pain 2003;104:303–22 [DOI] [PubMed] [Google Scholar]
- 7.Carroll LJ, Hurwitz EL, Cote P, et al. Research priorities and methodological implications. The Bone and Joint Decade 2000-2010 Task Force on Neck Pain and its Associated Disorders. Spine (Phila Pa 1976) 2008;33(4 Suppl):S214–20 [DOI] [PubMed] [Google Scholar]
- 8.Kampner SJ, Rebbeck TJ, Maher CG, et al. Course and prognostic factors of whiplash: a systematic review and analysis. Pain 2008;138:617–29 [DOI] [PubMed] [Google Scholar]
- 9.Eck JC, Hodges SD, Humphreys SC. Whiplash: a review of a commonly misunderstood injury. Am J Med 2001;110:651–6 [DOI] [PubMed] [Google Scholar]
- 10.Galasko CSB, Murray P, Stephenson W. Incidence of whiplash-associated disorder. BC Med J 2002;44:237–40 [Google Scholar]
- 11.Field S, Treleaven J, Jull G. Standing balance: a comparison between idiopathic and whiplash-induced neck pain. Man Ther 2008;13:183–91 [DOI] [PubMed] [Google Scholar]
- 12.Chien A, Sterling M. Sensory hypoaesthesia is a feature of chronic whiplash but not chronic idiopathic neck pain. Man Ther 2010;15:48–53 [DOI] [PubMed] [Google Scholar]
- 13.Woodhouse A, Liljebäck P, Vasseljen O. Reduced head steadiness in whiplash compared with non-traumatic neck pain. J Rehabil Med 2010;42:35–41 [DOI] [PubMed] [Google Scholar]
- 14.Verhagen AP, Lewis M, Schellingerhout JM, et al. Do whiplash patients differ from other patients with non-specific neck pain regarding pain, function or prognosis? Man Ther 2011;16:452–62 [DOI] [PubMed] [Google Scholar]
- 15.Sterling M. A proposed new classification system for whiplash associated disorders—implications for assessment and management. Man Ther 2004;9:60–70 [DOI] [PubMed] [Google Scholar]
- 16.Moore A, Jackson A, Jordan J, et al. Clinical Guidelines for the Physiotherapy Management of Whiplash Associated Disorder (WAD). London: Chartered Society of Physiotherapy, 2005 [Google Scholar]
- 17.Verhagen AP, Scholten-Peeters GG, van Wijngaarden S, et al. Conservative treatments for whiplash. Cochrane Database Syst Rev 2007;(2):CD003338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization International Classification of Functioning, Disability and Health: ICF. Geneva, Switzerland: World Health Organization, 2001 [Google Scholar]
- 19.Furlan AD, Pennick V, Bombardier C, et al. ; Editorial Board of the Cochrane Collaboration Back Review Group 2009 updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine 2009;34:1929–41 [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org/ (accessed Mar 2011). [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centre for Reviews and Dissemination (CRD) Systematic Reviews: CRD's Guidance for Undertaking Reviews in Healthcare. 3rd edn York: CRD University of York, York Publishing Services Ltd, 2009 [Google Scholar]
- 23.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960;20:37–46 [Google Scholar]
- 24.Higgins JPT, Deeks JJ, eds. Chapter 7: selecting studies and collecting data. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org (accessed Mar 2011). [Google Scholar]
- 25.Machin D, Fayers PM. Randomized Clinical Trials: Design, Practice and Reporting. West Sussex: Wiley-Blackwell, 2010 [Google Scholar]
- 26.Higgins JPT, Altman DG, Sterne JAC, eds. Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org (accessed Mar 2011). [Google Scholar]
- 27.Jüni P, Witschi A, Bloch R, et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282:1054–60 [DOI] [PubMed] [Google Scholar]
- 28.Katrak P, Bialocerkowski AE, Massy-Westropp N, et al. A systematic review of the content of critical appraisal tools. BMC Med Res Methodol 2004;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olivio SA, Macedo LG, Gadotti IC, et al. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther 2008;88:156–75 [DOI] [PubMed] [Google Scholar]
- 30.Rushton A, Calvert M, Wright C, et al. Physiotherapy trials for the 21st century—time to raise the bar? J Roy Soc Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green S, Higgins JPT, eds. Chapter 2: preparing a Cochrane review. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org (accessed Mar 2011). [Google Scholar]
- 32.DerSimonian R, Laird N. Meta-analyses in clinical trials. Control Clin Trials 1986;7:177–88 [DOI] [PubMed] [Google Scholar]
- 33.Deeks JJ, Higgins JPT, Altman DG, eds. Chapter 9: analyzing data and undertaking meta-analyses. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org (accessed Mar 2011). [Google Scholar]
- 34.Hedges LV, Olkin I. Statistical Methods for Meta-Analyses. San Diego: Academic Press Inc., 1985 [Google Scholar]
- 35.Ekvall Hansson E, Månsson NO, Ringsberg KA, et al. Dizziness among patients with whiplash-associated disorder: a randomised controlled trial. J Rehabil Med 2006;38:387–90 [DOI] [PubMed] [Google Scholar]
- 36.Sterling M, Pedler A, Chan C, et al. Cervical lateral glide increases nociceptive flexion reflex threshold but not pressure or thermal pain thresholds in chronic whiplash associated disorders: a pilot randomised controlled trial. Man Ther 2010;15:149–53 [DOI] [PubMed] [Google Scholar]
- 37.Sterne JAC, Egger M, Moher D; on behalf of the Cochrane Bias Methods Group Chapter 10: addressing reporting biases. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org (accessed Mar 2011). [Google Scholar]
- 38.Aigner N, Fialka C, Radda C, et al. Adjuvant laser acupuncture in the treatment of whiplash injuries: a prospective, randomized placebo-controlled trial. Wein Klin Wochenschr 2006;118:95–9 [DOI] [PubMed] [Google Scholar]
- 39.Dehner C, Elbel M, Strobel P, et al. Grade II whiplash injuries to the neck: what is the benefit for patients treated by different physical therapy modalities? Patient Saf Surg 2009;3:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez-Inglesias J, Fernandez-de-las-Penas C, Cleland J, et al. Short-term effects of cervical kinesio taping on pain and cervical range of motion in patients with acute whiplash injury: a randomized clinical trial. J Orthop Sports Phys Ther 2009;39:515–21 [DOI] [PubMed] [Google Scholar]
- 41.Jull G, Sterling M, Kenardy J, et al. Does the presence of sensory hypersensitivity influence outcomes of physical rehabilitation for chronic whiplash? A preliminary RCT. Pain 2007;129:28–34 [DOI] [PubMed] [Google Scholar]
- 42.Ask T, Strand LI, Skouen JS. The effect of two exercise regimes; motor control versus endurance/strength training for patients with whiplash-associated disorders: a randomized controlled pilot study. Clin Rehabil 2009;23:812–23 [DOI] [PubMed] [Google Scholar]
- 43.Bonk AD, Ferrari R, Giebel GD, et al. Prospective, randomized, controlled study of activity versus collar, and the natural history for whiplash injury, in Germany. J Muscoskel Pain 2000;8:123–32 [Google Scholar]
- 44.Pato U, Di Stefano G, Fravi N, et al. Comparison of randomized treatments for late whiplash. Neurology 2010;74:1223–30 [DOI] [PubMed] [Google Scholar]
- 45.Scholten-Peeters GG, Neeleman-van der Steen CW, van der Windt DA, et al. Education by general practitioners or education with exercises by physiotherapists for patients with whiplash-associated disorders? A randomized clinical trial. Spine (Phila Pa 1976) 2006;31:723–31 [DOI] [PubMed] [Google Scholar]
- 46.Stewart MJ, Maher CG, Refshauge KM, et al. Randomized controlled trial of exercise for chronic whiplash-associated disorders. Pain 2007;128:59–68 [DOI] [PubMed] [Google Scholar]
- 47.Thuile C, Walzl M. Evaluation of electromagnetic fields in the treatment of pain in patients with lumbar radiculopathy or the whiplash syndrome. NeuroRehabilitation 2002;17:63–7 [PubMed] [Google Scholar]
- 48.Vassiliou T, Kaluza G, Putzke C, et al. Physical therapy and active exercises—an adequate treatment for prevention of late whiplash syndrome? Randomized controlled trial in 200 patients. Pain 2006;124:69–76 [DOI] [PubMed] [Google Scholar]
- 49.Vikne J, Oedegaard A, Laerum E, et al. A randomized study of new sling exercise treatment vs traditional physiotherapy for patients with chronic whiplash-associated disorders with unsettled compensation claims. J Rehabil Med 2007;39:252–9 [DOI] [PubMed] [Google Scholar]
- 50.Armstrong BS, McNair PJ, Williams M. Head and neck position sense in whiplash patients and healthy individuals and the effect of the cranio-cervical flexion action. Clin Biomech (Bristol, Avon) 2005;20:675–84 [DOI] [PubMed] [Google Scholar]
- 51.Fernandez-de-las-Penas C, Fernandez-Carnero J, Fernandez AP, et al. Dorsal manipulation in whiplash injury treatment: a randomised controlled trial. J Whiplash & Relat Disord 2004a;3:55–72 [Google Scholar]
- 52.Fernandez-de-las-Penas C, Fernandez-Carnero J, Palomeque del Cerro L, et al. Manipulative treatment vs conventional physiotherapy treatment in whiplash injury: a randomised controlled trial. J Whiplash & Relat Disord 2004b;3:73–90 [Google Scholar]
- 53.Rosenfeld M, Seferiadis A, Carlsson J, et al. Active intervention in patients with whiplash associated disorders improves long term prognosis. Spine 2003;28:2491–8 [DOI] [PubMed] [Google Scholar]
- 54.Rosenfeld M, Seferiadis A, Gunnarsson R. Active involvement and intervention in patients exposed to whiplash trauma in automobile crashes reduces costs. Spine 2006;31:1799–804 [DOI] [PubMed] [Google Scholar]
- 55.Schnabel M, Ferrari R, Vassiliou T, et al. Randomised, controlled outcome study of active mobilisation compared with collar therapy for whiplash injury. Emerg Med J 2004;21:306–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soderlund A, Olerud C, Lindberg P. Acute whiplash-associated disorders (WAD): the effects of early mobilization and prognostic factors in long-term symptomatology. Clin Rehabil 2000;14:457–67 [DOI] [PubMed] [Google Scholar]
- 57.Soderlund A, Lindberg P. Cognitive behavioural components in physiotherapy management of chronic whiplash associated disorders (WAD)—a randomised group study. Physiother Theor Pract 2001;17:229–38 [PubMed] [Google Scholar]
- 58.Soderlund A, Lindberg P. Cognitive behavioural components in physiotherapy management of chronic whiplash associated disorders (WAD)—a randomised group study. G Ital Med Lav Ergon 2007;29(1 Suppl A):A5–11 [PubMed] [Google Scholar]
- 59.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74 [PubMed] [Google Scholar]
- 60.Scholten-Peeters GG, Verhagen AP, Neeleman-van der Steen CW, et al. Randomized clinical trial of conservative treatment with whiplash-associated disorders: considerations for the design and dynamic treatment protocol. J Manipulative Physiol Ther 2003;26:412–20 [DOI] [PubMed] [Google Scholar]
- 61.Stewart MJ, Maher CG, Refshauge KM, et al. Advice or exercise for chronic whiplash-associated disorders? Design of a randomized controlled trial. BMC Musculoskelet Disord 2003;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6 [DOI] [PubMed] [Google Scholar]
- 63.Rushton A, Wright C, Goodwin P, et al. Physiotherapy rehabilitation post first lumbar discectomy: a systematic review and meta-analysis of Randomised Controlled Trials. Spine (Phila Pa 1976) 2011;36:E961–72 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.