Abstract
The aurea (au) and yellow-green-2 (yg-2) mutants of tomato (Solanum lycopersicum L.) are unable to synthesize the linear tetrapyrrole chromophore of phytochrome, resulting in plants with a yellow-green phenotype. To understand the basis of this phenotype, we investigated the consequences of the au and yg-2 mutations on tetrapyrrole metabolism. Dark-grown seedlings of both mutants have reduced levels of protochlorophyllide (Pchlide) due to an inhibition of Pchlide synthesis. Feeding experiments with the tetrapyrrole precursor 5-aminolevulinic acid (ALA) demonstrate that the pathway between ALA and Pchlide is intact in au and yg-2 and suggest that the reduction in Pchlide is a result of the inhibition of ALA synthesis. This inhibition was independent of any deficiency in seed phytochrome, and experiments using an iron chelator to block heme synthesis demonstrated that both mutations inhibited the degradation of the physiologically active heme pool, suggesting that the reduction in Pchlide synthesis is a consequence of feedback inhibition by heme. We discuss the significance of these results in understanding the chlorophyll-deficient phenotype of the au and yg-2 mutants.
The use of mutants with impaired responses to light has been instrumental in developing our current understanding of photoperception and signal transduction in higher plants (von Arnim and Deng, 1996; Fankhauser and Chory, 1997). The phytochrome chromophore-deficient mutants have been particularly useful in defining the role played by phytochrome during photomorphogenesis, because all members of the phytochrome photoreceptor family appear to use the same chromophore, resulting in plants that have reduced activity of all phytochrome species (Terry, 1997). The phytochrome chromophore 3(E)-PΦB is synthesized in the plastid from the heme branch of the tetrapyrrole-biosynthetic pathway (Fig. 1; Terry et al., 1993, 1995; Weller et al., 1996). Biochemical analyses of chromophore biosynthesis have identified mutants in this pathway from a number of species.
Figure 1.
The tetrapyrrole-biosynthesis pathway.
In pea two mutants have been identified, pcd1 and pcd2, that are unable to convert heme to biliverdin IXα and biliverdin IXα to 3(Z)-PΦB, respectively (Weller et al., 1996, 1997). There are also two chromophore-deficient mutants in tomato (Solanum lycopersicum L.). The aurea (au) mutant is specifically deficient in PΦB synthase activity (Fig. 1; Terry and Kendrick, 1996), whereas the phenotypically indistinguishable yellow green-2 (yg-2) mutant is blocked in the preceding step in the pathway and cannot synthesize biliverdin IXα from heme (Terry and Kendrick, 1996). The hy1 and hy2 mutants of Arabidopsis (Parks and Quail, 1991) and the pew mutants of Nicotiana plumbaginifolia (Kraepiel et al., 1994) are also deficient in chromophore synthesis.
The au mutant is one of the most exhaustively characterized photomorphogenic mutants and until recently was thought to be specifically deficient in phyA despite considerable physiological data to the contrary. The phenotype of au is typical of chromophore-deficient mutants (Terry, 1997). White light-grown seedlings are elongated and have impaired chloroplast development and reduced levels of chlorophyll and anthocyanin, resulting in a pale, yellow-green phenotype (Koornneef et al., 1985). This pleiotropic phenotype is the consequence of the loss of multiple phytochromes. Analyses of light-dependent inhibition of hypocotyl elongation and anthocyanin synthesis have demonstrated that au seedlings are deficient in both phyB1 and phyA activities (Koornneef et al., 1985; van Tuinen et al., 1995a, 1995b; Kerckhoffs et al., 1997).
These physiological data were recently supported by experiments showing that the absence of spectrophotometrically detectable phytochrome in vivo corresponds to the loss of at least three holophytochrome species in etiolated au seedlings (Kerckhoffs, 1996). In mature au plants, however, the situation is very different. Leaves from 4- to 6-week-old au plants grown in the greenhouse contained 60% to 70% of wild-type phytochrome levels (López-Juez et al., 1990), whereas the amount of photoactive phytochrome detected by an antibody raised to pea phyB was the same in both au and wild-type plants (Sharma et al., 1993). This increase in holophytochrome levels relative to wild-type plants results, not surprisingly, in the recovery of many phytochrome responses, and mature au plants exhibit relatively normal shade avoidance (Whitelam and Smith, 1991) and end-of-day far-red responses (López-Juez et al., 1990). The recovery of phytochrome responses during development seen in the au tomato mutant appears to be characteristic of all chromophore-deficient mutants, although the basis for this phenomenon is not yet established.
Although mature au plants are less phytochrome deficient than au seedlings, they retain their dramatic yellow-gold coloring that gave the au mutant its name. This can be seen clearly in Figure 2. Tissue at the base of each au leaflet is almost white and becomes gradually darker until by the leaflet edges it is pale green. There is also a distinctive mosaic effect caused by the veins of the au leaflets being pale green and the cells in between remaining yellow. Chlorophyll levels in mature au plants vary between 33% and 61% of wild type depending on the light and temperature conditions in which they are grown (Koornneef et al., 1985; López-Juez et al., 1990; Becker et al., 1992). However, this reduction in chlorophyll levels is not the consequence of reduced photoinduction of CAB genes (encoding chlorophyll a/b-binding proteins), because CAB expression levels are normal in au plants of this age (Becker et al., 1992). Moreover, the characteristic leaf coloring as a result of the au mutation is still apparent in the high pigment-1 (hp-1) background (Fig. 2). The hp-1 mutation is believed to affect light signal transduction, since light responses are exaggerated in this mutant (Peters et al., 1992), and it might therefore be expected to compensate for the effect of phytochrome deficiency on chlorophyll synthesis.
Figure 2.
Leaf phenotype of au and hp-1 mutants. Leaves are from 6-week-old wild-type (left), au (second from left), au,hp-1 (second from right), and hp-1 (right) plants grown in the greenhouse.
There are other features of the au phenotype that are inconsistent with our current understanding of phytochrome function. Dark-grown au seedlings have reduced CAB expression in the dark compared with wild-type seedlings (Sharrock et al., 1988; Ken-Dror and Horwitz, 1990). Ken-Dror and Horwitz (1990) also showed that levels of phototransformable Pchlide were reduced in au under these conditions and noted the difficulty of explaining these results in terms of a phytochrome deficiency. In addition, etioplasts from hypocotyl cells of dark-grown au seedlings do not develop normally, appearing as smaller proplastids lacking a prolamellar body (Neuhaus et al., 1993). Is it possible that these observations are related to an additional consequence of inhibiting PΦB synthesis?
One obvious effect of such an inhibition would be to change the flux through the tetrapyrrole-biosynthetic pathway. Chlorophyll and heme are both synthesized in the plastid from ALA and share a common pathway between ALA and Proto IX (Fig. 1; Beale and Weinstein, 1991; von Wettstein et al., 1995). In the dark chlorophyll synthesis proceeds only as far as Pchlide because the enzyme POR has an absolute requirement for light. The rate-limiting step for chlorophyll or Pchlide synthesis is the formation of ALA from glutamate via the C5 pathway (Beale and Weinstein, 1991), and experiments in which heme synthesis has been blocked using chelators of iron (Duggan and Gassman, 1974; Chereskin and Castelfranco, 1982; Beale and Weinstein, 1991) or herbicides (Masuda et al., 1990) have demonstrated that ALA synthesis (and therefore chlorophyll synthesis) is under the control of heme. We examined tetrapyrrole synthesis in the au and yg-2 mutants to test the hypothesis that the inhibition of heme degradation leads to a reduction in chlorophyll synthesis via feedback inhibition.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The tomato (Solanum lycopersicum L.) genotypes used in this study are given in Table I. All seeds were treated with 1% (v/v) bleach, washed thoroughly, and sown on 0.6% (w/v) agar containing 0.46 g/L of Murashige-Skoog salts (Life Technologies) in tissue-culture containers (Flow Laboratories, McLean, VA). Seedlings were grown in the dark for 5 d (unless otherwise stated) at 25°C. The au and yg-2 mutants were sown 12 h prior to wild-type seeds to synchronize germination.
Table I.
Mutant genotypes used in this study
| Mutant | Genetic Background | Classification | Ref. |
|---|---|---|---|
| au | ACa | chr−b | Terry and Kendrick (1996)c |
| auls | Condine Red | chr− | van Tuinen et al. (1996)c |
| autl | VF145 | chr− | van Tuinen et al. (1996)c |
| auw | MMd | chr− | Koornneef et al. (1985)c |
| au6 | AC | chr− | van Tuinen et al. (1996)c |
| yg-2 | Unknown | chr− | Terry and Kendrick (1996)c |
| yg-2aud | AC | chr− | van Tuinen et al. (1996)c |
| auw,yg-2aud | MM/AC | chr− | van Tuinen et al. (1996) |
| fri1 | MM | phyA−e | van Tuinen et al. (1995a) |
| tri1 | Breeding line GT | phyB1−f | van Tuinen et al. (1995b) |
| fri1,tri1 | MM/GT | phyA− and phyB− | Kerckhoffs et al. (1997) |
| hp-1 | AC | resp+g | Adamse et al. (1989)c |
| au,hp-1 | AC | chr− and resp+ | Adamse et al. (1989) |
AC, Ailsa Craig.
chr−, Chromophore deficient.
The full history of this mutant allele is given in this reference.
MM, Moneymaker.
phyA−, phyA deficient.
phyB1−, phyB1 deficient.
resp+, Response amplification.
Porphyrin Extraction and Quantitation
Cotyledons and hypocotyl hooks (0.5 g) were harvested under a dim-green safelight, and porphyrins were extracted based on the method of Rebeiz et al. (1975). Tissue was homogenized in 2.5 mL of cold acetone: 0.1 m NH4OH (90/10, v/v) and transferred to a centrifuge tube with an additional 1 mL of solvent. Samples were centrifuged at 30,000g for 10 min. The pellet was then reextracted in 1.5 mL of solvent and centrifuged again. The supernatants were combined and washed successively with an equal volume and a one-third volume of hexane prior to spectrophotometric analysis. For determination of Pchlide ester the hexane washes were pooled and analyzed directly.
Absorption spectroscopy of porphyrin samples was performed using a spectrophotometer (model U-3410, Hitachi, Tokyo, Japan). Pchlide concentrations were determined using a molar absorption coefficient of 31,100 m−1 cm−1 at 626 nm (Kahn, 1983). For samples containing significant quantities of Proto IX (i.e. ALA-feeding experiments), the concentration of Proto IX was determined using a molar absorption coefficient of 16,200 m−1 cm−1 at 503 nm (Gough, 1972), and the concentration of Pchlide was calculated by subtracting the contribution of Proto IX (molar absorption coefficient of 5,500 m−1 cm−1 at 628 nm; Gough, 1972) to the absorption peak of Pchlide at 628 nm. Fluorescence spectroscopy was performed (model F-3010, Hitachi), and fluorescence was measured using excitation wavelengths of 410 nm for Proto IX and Mg-protoporphyrin and 440 nm for Pchlide and Pchlide ester.
In Vivo Feeding Experiments
For ALA feeding experiments, seedlings were cut under water and placed in a beaker containing 15 mm Hepes/NaOH buffer, pH 7.0, with 5 mm MgCl2 for 20 h at 25°C. ALA was added to a final concentration of 10 mm. Following incubation, porphyrins were extracted as described above except that 0.25 g of tissue was used with the same volumes of solvent. The iron chelator 2′2′-bipyridyl (10 mm final concentration diluted from a 1 m stock in ethanol) was fed in 15 mm Hepes/NaOH buffer, pH 7.4, containing 10 mm glutamate under identical conditions. Tissue (0.25 g) was extracted as above, but using one-half volume of solvent.
RESULTS
au and yg-2 Mutants Have Reduced Synthesis of Pchlide in the Dark
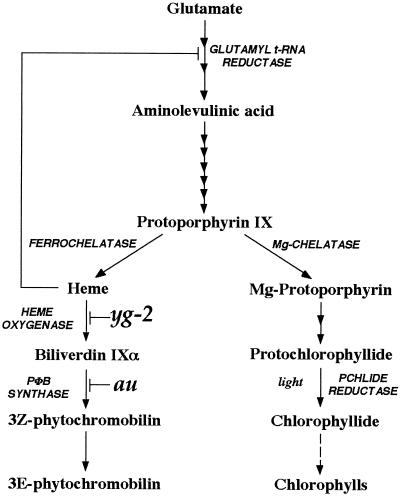
Pchlide levels in wild-type, au, and yg-2 seedlings were determined by absorption spectroscopy. Figure 3A shows representative absorption spectra of hexane-washed acetone extracts from 5-d-old dark-grown seedlings. Both au and yg-2 had reduced levels of Pchlide compared with wild type, although the absorption spectra were otherwise identical and consistent with previously published spectra from other species (Gough, 1972). Quantitation of the amount of Pchlide using absorption spectra from replicate experiments demonstrated that Pchlide levels were reduced in the mutants whether compared on a seedling or fresh weight basis (Fig. 3, B and C). From these data it can be calculated that au and yg-2 contain 68% and 84% of wild-type Pchlide per seedling, respectively. Analysis of Pchlide by fluorescence spectroscopy following excitation at 440 nm (Rebeiz et al., 1975) gave qualitatively identical results (Fig. 3A, inset, and data not shown).
Figure 3.
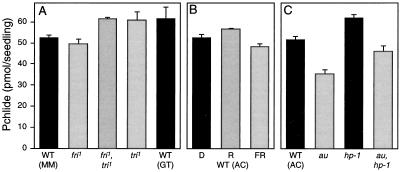
Analysis of Pchlide levels in au and yg-2. A, Room-temperature absorption and fluorescence (inset, excitation at 440 nm) spectra of hexane-washed acetone extracts from 5-d-old, dark-grown wild-type, au, and yg-2 seedlings. B and C, Quantitation of Pchlide from multiple absorption spectra in different alleles of au and yg-2 expressed per seedling (B) or on a fresh weight basis (C). The wild-type (WT) value represents the mean ± se of four different backgrounds (Ailsa Craig, Moneymaker, breeding line GT, and VF145 [Table I]; n ≥ 3 for each). All other values are means ± se (n ≥ 3).
To confirm that the reduced Pchlide was a direct consequence of the au and yg-2 mutations, Pchlide was also measured in additional alleles of both au and yg-2 (Fig. 3, B and C). A reduction in Pchlide was observed in all of the au alleles tested and in the only other known yg-2 allele, yg-2aud. Three of the four additional au alleles and yg-2aud all contained less Pchlide per seedling than the original au and yg-2 alleles. The auw,yg-2aud double mutant was also examined and was found to contain lower levels of Pchlide than either single mutant, indicating an additive phenotype for this effect (Fig. 3, B and C). To confirm that the reduction in Pchlide levels was not a consequence of slower growth of the mutants, we also measured the hypocotyl length of au, yg-2, and wild-type seedlings grown in the dark for 5 d. Under these conditions there was no difference in the hypocotyl length between wild-type and mutant seedlings (data not shown).
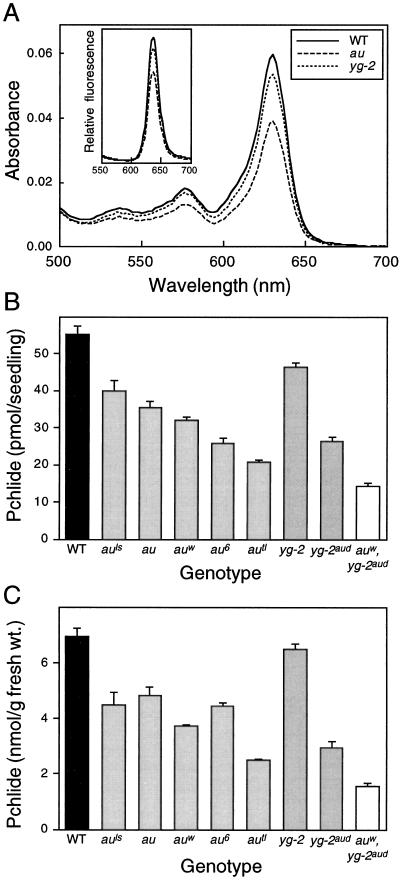
There are two possible explanations for the reduction in the amount of Pchlide: either the rate of synthesis of Pchlide is reduced or the Pchlide synthesized is being further metabolized to give a product that is not detectable under these assay conditions. The most likely candidate for the latter is Pchlide ester, which would be removed during the hexane wash. We therefore determined the relative amounts of Pchlide ester in wild-type and mutant seedlings by fluorescence spectroscopy. Table II shows that the levels of Pchlide ester were more reduced than Pchlide in au and yg-2, indicating that an increased synthesis of Pchlide ester was not the reason for the lower levels of Pchlide. We next compared the rate of accumulation of Pchlide in the wild type and in the mutants by measuring Pchlide levels during seedling growth. Figure 4 shows that the rate of Pchlide accumulation was slower in both the au and yg-2 mutants than in wild-type seedlings. Since Pchlide does not appear to be further metabolized, these results show that the reduced Pchlide levels in au and yg-2 are the consequence of a reduced rate of Pchlide synthesis.
Table II.
Analysis of Pchlide ester in au and yg-2 mutants
| Genotype | Pchlide Ester Fluorescencea | |
|---|---|---|
| g−1 fresh wt | seedling−1 | |
| Wild type | 49.5 ± 4.0 | 0.36 ± 0.03 |
| au | 26.4 ± 0.8 | 0.19 ± 0.00 |
| yg-2 | 29.7 ± 1.8 | 0.24 ± 0.02 |
Pchlide ester was quantified by fluorescence spectroscopy in 5-d-old dark-grown tomato seedlings. Values are means ± se (n = 3).
Fluorescence is given as relative units.
Figure 4.
Time course of Pchlide accumulation in au and yg-2 mutants. Pchlide was measured at various times in dark-grown wild-type (WT), au, and yg-2 seedlings. Values are means ± se (n ≥ 3).
Synthesis of Pchlide from ALA Is Not Affected by the au and yg-2 Mutations
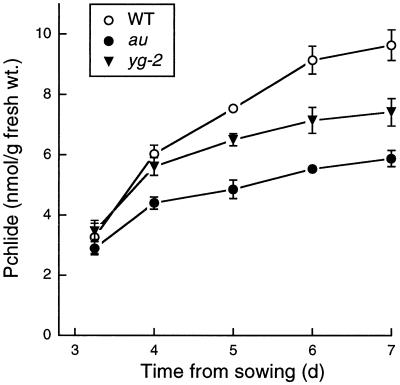
The inhibition of Pchlide synthesis in au and yg-2 is likely to be the result of a reduction in ALA synthesis, since Pchlide synthesis is controlled at this point in the pathway (Beale and Weinstein, 1991). However, it is also possible that the mutations lead to inhibition of an intermediate step(s) in the biosynthetic pathway between ALA and Pchlide. We tested this hypothesis by feeding ALA to wild-type, au, and yg-2 seedlings and then examining the accumulation of porphyrins. Figure 5 shows that wild-type, au, and yg-2 seedlings accumulated between 5- and 12-fold more Pchlide (determined by absorption spectroscopy) after being fed ALA. This accumulation of Pchlide was also detected by fluorescence spectroscopy following excitation at 440 nm (Fig. 5A, inset). Pchlide levels in seedlings fed buffer alone were identical to those measured previously for intact seedlings, with lower levels in au and yg-2 than in wild-type plants (data not shown).
Figure 5.
Analysis of porphyrin synthesis in au and yg-2 mutants following incubation in porphyrin precursors. A, Room-temperature absorption and fluorescence (inset, excitation at 440 nm) spectra of hexane-washed acetone extracts from dark-grown wild-type (WT), au, and yg-2 seedlings incubated in the dark for 20 h in 10 mm ALA. B, Quantitation of Pchlide and Proto IX following incubation in 10 mm ALA or 10 mm glutamate (glu). Values are means ± se (n ≥ 3). The data for no addition (no add.) are from intact seedlings and are the same as those shown in Figure 3 (au and yg-2 only).
Seedlings fed the ALA precursor glutamate contained slightly more Pchlide than intact seedlings in all cases, but the inhibition of Pchlide synthesis in au and yg-2 was still maintained (Fig. 5B). The small effect of feeding glutamate would be expected, since ALA synthesis is rate limiting for Pchlide synthesis. It is clear from these data that the au and yg-2 mutations do not affect the Pchlide-synthesis pathway after ALA, since Pchlide accumulates to high levels in the mutants. Indeed, more Pchlide accumulated in au seedlings fed ALA than in wild-type seedlings (Fig. 5B). The accumulation of excess Pchlide in au and yg-2 seedlings following ALA feeding confirms that the reduced accumulation of Pchlide in dark-grown seedlings (Figs. 3 and 4) was the result of a reduced rate of Pchlide synthesis and is also consistent with the inhibition of Pchlide synthesis being the consequence of a reduced rate of ALA synthesis.
In addition to Pchlide, other pigments were also detected following ALA feeding. Small amounts of Mg-protoporphyrin were measured as a fluorescence peak at 595 nm following excitation at 410 nm. Mg-protoporphyrin was present in wild-type, au, and yg-2 seedlings fed ALA but was not present in buffer controls (data not shown). The most noticeable feature of the ALA-fed seedlings was that both au and yg-2 accumulated large quantities of Proto IX in addition to excess Pchlide (Fig. 5). Since there was no corresponding inhibition of Pchlide synthesis, the most likely explanation is that the accumulated Proto IX represents a Proto IX pool that is no longer available for conversion to Pchlide because it is spatially separated from the Pchlide-synthesis pathway.
Inhibition of Pchlide Synthesis Is Independent of Phytochrome Deficiency
The synthesis of ALA is known to be regulated by both light, which activates ALA synthesis through phytochrome (Kasemir, 1983; Huang et al., 1989), and heme, which is an inhibitor of glutamyl-tRNA reductase (Pontoppidan and Kannangara, 1994; Fig. 1). It is important to distinguish between these two possibilities in order to understand the phenotype of both the au and yg-2 mutants. The experiments described to date were deliberately performed using dark-grown seedlings to eliminate the effect of phytochrome on ALA synthesis. However, seeds of both au and yg-2 are likely to contain reduced levels of Pfr because the parent plant is still phytochrome deficient at the mature stage of development; Indeed, auw is impaired in phytochrome-mediated dark germination (Koornneef et al., 1985). Although a deficiency in seed Pfr has not been reported to have any additional phenotypic effects (e.g. on hypocotyl length), it is possible that the reduction in Pchlide in au and yg-2 is due to lower levels of Pfr in seeds of these mutants.
We tested this possibility with a series of three experiments (Fig. 6). First, we measured Pchlide levels in other phytochrome-deficient mutants. Figure 6A shows that the phyA-deficient fri mutant (van Tuinen et al., 1995a), the phyB1-deficient tri mutant (van Tuinen et al., 1995b), and the fri,tri double mutant (Kerckhoffs et al., 1997) did not have reduced levels of Pchlide in the dark. Next we tested whether phytochromes other than phyA and phyB1 could affect Pchlide levels by giving saturating red and far-red light pulses to wild-type seedlings 24 h after imbibition. These light treatments were designed to convert the majority of the phytochrome present in seeds to either the active Pfr form (red) or the inactive Pr form (far red). As can be seen in Figure 6B, these light treatments had very little effect on the subsequent synthesis of Pchlide. Although a small response may exist, the absence of part of this response could not account for the much larger inhibition of Pchlide synthesis in the au seedlings. The third approach was to examine Pchlide levels in the hp-1 mutant background. The hp-1 mutation leads to an amplification of light responses (Peters et al., 1992) and might therefore be expected to amplify any difference between wild-type and au seedlings if the reduced level of Pchlide in au was a consequence of reduced seed Pfr.
Figure 6.
Analysis of Pchlide levels in a range of photomorphogenic mutants following brief light treatments. A and C, Pchlide was measured in 5-d-old, dark-grown wild-type (WT) and phyA (fri), phyB1 (tri), au, hp-1, and double-mutant seedlings. MM, GT, and AC represent different genetic backgrounds (Table I). B, Pchlide was measure in 5-d-old wild-type (WT) seedlings treated with 5 min of red (R; 17 μmol m−2 s−1) or 15 min of far red (FR; 12 μmol m−2 s−1) light 24 h after imbibition. Values are means ± se (n ≥ 3).
Figure 6C shows that the hp-1 background leads to a small increase (20%) in Pchlide compared with wild-type seedlings. A small increase is also seen in the au,hp-1 double mutant when compared with au alone. Therefore, if the increase in hp-1 represents the amplification of the action of seed Pfr, it is evident that au contains sufficient Pfr to saturate this response. It should also be noted that the au,hp-1 double mutant contains less Pchlide than wild-type seedlings. Taken together, these results clearly indicate that the reduced Pchlide levels in au and yg-2 are not a consequence of a deficiency in seed Pfr. Similar arguments can be used to discount a stimulatory role of Pr on Pchlide synthesis in the dark. Both the fri and tri mutant alleles contain barely detectable amounts of phytochrome protein (van Tuinen et al., 1995a, 1995b), and light treatments resulting in differing concentrations of Pr had little effect in wild-type seedlings (Fig. 6B). In addition, experiments in which harvest times were varied excluded the possibility that the difference in Pchlide levels between mutant and wild-type seedlings was the result of a light-independent circadian regulation (data not shown).
au and yg-2 Mutations Affect the Physiologically Active Heme Pool
Since the inhibition of Pchlide synthesis in au and yg-2 is independent of reduced phytochrome levels, it is likely that it results from the effects of heme accumulation following lesions in the phytochrome chromophore (heme-degradation) pathway. To test this hypothesis it was necessary to determine whether the au and yg-2 mutations affect the physiologically active free heme pool. It is not currently possible to measure this heme pool directly; therefore, we used an alternative strategy of manipulating the levels of free heme by incubating wild-type and mutant seedlings in the iron chelator 2′2′-bipyridyl. Chelation of free iron will reduce the synthesis of heme from Proto IX, and it has been shown in numerous studies that such a treatment leads to an increase in the synthesis of porphyrins because of the release of the feedback inhibition by heme on ALA synthesis (Duggan and Gassman, 1974; Chereskin and Castelfranco, 1982; Beale and Weinstein, 1991). The proposed mechanism for this action is that, when the synthesis of new heme is inhibited, the regulatory heme pool continues to be rapidly degraded (Castelfranco and Jones, 1975).
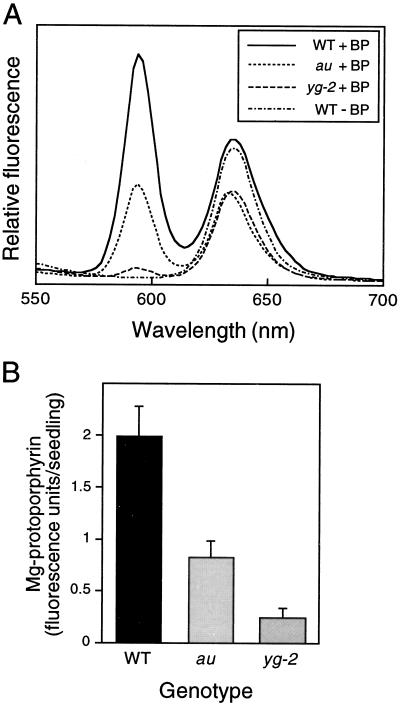
We reasoned that, because the mutants were impaired in the degradation of plastidic heme, the free heme pool would remain for longer and porphyrin synthesis would not be stimulated. The chelator treatment would therefore have the effect of amplifying the difference in porphyrin synthesis between wild-type and mutant seedlings. The results of this experiment are shown in Figure 7. In wild-type seedlings the major product following 2′2′-bipyridyl feeding was Mg-protoporphyrin (including Mg-protoporphyrin methyl ester, which was indistinguishable in this assay), identified by its sharp fluorescent peak at 595 nm following excitation at 410 nm (Fig. 7A). Fluorescence spectra after excitation at 440 nm indicated that there was no increase in Pchlide during this period (data not shown). These results are consistent with previous observations that iron chelators inhibit the conversion of Mg-protoporphyrin methyl ester to Pchlide in addition to ferrochelatase (Duggan and Gassman, 1974). Mg-protoporphyrin was not detectable in control incubations; therefore, it represents new porphyrin synthesis during the incubation period. The peak in Figure 7A at approximately 635 nm represents a mixture of Pchlide with some Proto IX and was similar in treated and untreated wild-type seedlings.
Figure 7.
Analysis of Mg-protoporphyrin synthesis in au and yg-2 mutants following incubation in the iron chelator 2′2′-bipyridyl (BP). A, Room-temperature fluorescence spectra (excitation at 410 nm) of hexane-washed acetone extracts from dark-grown wild-type (WT), au, and yg-2 seedlings incubated in the dark for 20 h in 10 mm 2′2′-bipyridyl. B, Quantitation of Mg-protoporphyrin from multiple fluorescence spectra. Values are means ± se (n = 3).
Feeding 2′2′-bipyridyl to au and yg-2 seedlings led to much smaller increases in Mg-protoporphyrin (Fig. 7A). This was particularly apparent in yg-2, in which the Mg-protoporphyrin levels were just 10% of wild-type levels, whereas au contained 35% of that of control seedlings (Fig. 7B). Fluorescence spectra after excitation at 440 nm indicated that Pchlide levels were unaffected by 2′2′-bipyridyl treatment in au seedlings, although there was a small reduction in Pchlide in yg-2 seedlings (data not shown). This is also apparent in Figure 7A, where the peak at 635 nm in yg-2 is similar to that in au and is considerably reduced in comparison with wild-type. Our results indicate that when heme synthesis is blocked the release of porphyrin synthesis is much greater in wild-type seedlings than in the mutants and, therefore, that the au and yg-2 mutations affect the regulatory heme pool by blocking normal heme turnover in plastids.
DISCUSSION
Feedback Inhibition of Chlorophyll Synthesis
The data presented here clearly demonstrate that the au and yg-2 mutations lead to a decrease in Pchlide synthesis in the dark. There are two possible explanations for this: either inhibition of Pchlide synthesis results from the absence of holophytochrome in au and yg-2 mutants or lesions in the chromophore-biosynthesis pathway cause a decrease in Pchlide synthesis that is independent of phytochrome deficiency. The poor dark germination of au and yg-2 (Koornneef et al., 1985) indicates that seeds of these mutants have reduced levels of active phytochrome, and it is therefore possible that a lack of seed phytochrome (either as Pfr or Pr) could lead to a reduction in Pchlide synthesis. However, from the data shown in Figure 6 we can exclude such an effect. The fri,tri double mutant, which lacks detectable PHYA and PHYB1, accumulates Pchlide normally, and light treatments designed to convert seed phytochrome predominantly to Pr or Pfr were also ineffectual. We are not able to completely rule out the possibility that Pr synthesized during seedling growth is necessary for normal Pchlide accumulation, but this effect would clearly not require the presence of phyA or phyB1. Therefore, the inhibition of Pchlide synthesis is the direct result of lesions in chromophore biosynthesis.
One possible explanation for this is that Pchlide accumulation is inhibited in a nonspecific manner as a result of pleiotropic effects on plastid structure caused by the absence or mistargeting of the chromophore-biosynthesis enzymes themselves or by the accumulation or loss of molecules that might affect plastid development (e.g. heme or PΦB). Although etioplasts from hypocotyl cells of the au mutant have been shown to have structural defects (Neuhaus et al., 1993), there is good evidence that the effects of the mutations on the tetrapyrrole-biosynthesis pathway are actually quite specific. Isolated plastids from au and yg-2 can synthesize Proto IX from ALA at rates equal to wild-type plastids, and neither mutation affects chromophore biosynthesis other than in the step it specifically blocks (Terry and Kendrick, 1996). These results are supported by the finding that feeding ALA results in a substantial accumulation of Pchlide in both wild-type and mutant seedlings (Fig. 5), indicating that all of the enzymes between ALA and Pchlide are functioning normally. This experiment is important because it demonstrates that the reduction in Pchlide is not the result of a reduced number of plastids and that the total capacity for tetrapyrrole synthesis is uncompromised in au and yg-2 seedlings.
The simplest explanation for the reduction in Pchlide synthesis is therefore that the au and yg-2 mutations directly alter the regulation of tetrapyrrole biosynthesis. The most likely mechanism for such an effect is that an increase in the physiologically active free heme pool directly inhibits ALA synthesis, which leads to a reduction in Pchlide accumulation (Fig. 1). This explanation is supported by data showing that inhibition of Pchlide synthesis takes place prior to the formation of ALA (Fig. 5) and that both the au and yg-2 mutations affect the degradation of physiologically active heme (Fig. 7). It is also entirely consistent with previously published data demonstrating that ALA synthesis is rate limiting for chlorophyll synthesis and that heme is a feedback regulator of this step (Beale and Weinstein, 1991). From our data we cannot exclude the possibility that, in addition to feedback inhibition by heme, PΦB has a stimulatory effect on Pchlide synthesis, but there is no evidence in the literature to support such a role.
The mechanism by which heme inhibits ALA synthesis is not entirely understood. Three enzymes are required for the synthesis of ALA from glutamate (von Wettstein et al., 1995; Kumar et al., 1996). The second of the three enzymes, glutamyl-tRNA reductase, which converts glutamate activated by ligation to tRNAGlu to glutamate 1-semialdehyde, is believed to be the rate-limiting step in ALA synthesis and is therefore a likely target for heme inhibition. In vitro experiments with purified and recombinant glutamyl-tRNA reductase have shown that heme is a potent inhibitor of this enzyme (Pontoppidan and Kannangara, 1994; Vothknecht et al., 1996). Whether heme also has a regulatory role in the transcription or translation of the genes encoding this or other ALA-synthesizing enzymes has yet to be determined. However, since feeding heme to plant tissues is exceedingly problematic, phytochrome chromophore-deficient mutants may prove to be invaluable tools with which to address such questions. There is no compelling evidence for the regulation of ALA synthesis by other products of the tetrapyrrole-biosynthetic pathway, except that Pchlide appears to limit its own synthesis in the dark (Beale and Weinstein, 1991). Again, the mechanism is not completely understood but is thought to be related to the formation of the ternary POR-Pchlide-NADPH complex. The data presented here indicate that, although Pchlide may be important in preventing its own excess accumulation, it cannot override the regulatory effect of heme under conditions in which Pchlide synthesis is limiting.
Consequences of Feedback Inhibition of Chlorophyll Synthesis in the Dark
The data presented here provide an explanation for the observation that etiolated auw seedlings contain less photoactive Pchlide than wild-type seedlings (Ken-Dror and Horwitz, 1990). However, the reduction in photoactive Pchlide (20% of wild type) was greater than the reduction of Pchlide pigment seen for auw in this study (60% of wild type) and there may be additional reasons for the lower level of photoactive Pchlide. For example, Pchlide is required for the import of POR (Reinbothe et al., 1995), the major protein component of the prolamellar body (Ikeuchi and Murakami, 1983). A reduced rate of Pchlide synthesis might therefore be expected to result in reduced levels of POR accumulation, which would further inhibit the formation of the photoactive POR-Pchlide complex. Analysis of POR in au and yg-2 seedlings has indeed shown that POR protein levels are reduced in both cotyledons and hypocotyls of au seedlings (M.J. Terry, unpublished results), and this may also contribute to the reduction in photoactive Pchlide in auw.
The synthesis of tetrapyrroles is closely linked to plastid development, and it has long been established that mutations leading to defects in chlorophyll biosynthesis have a profound effect on the structure of plastids (von Wettstein et al., 1971; Mascia and Robertson, 1978). The formation of the prolamellar body correlates with levels of the photoactive POR-Pchlide complex (Sperling et al., 1998); therefore, it might be expected that dark-grown au and yg-2 seedlings have poorly developed etioplasts. There has been no analysis to date of etioplast structure in dark-grown cotyledons of these mutants, but hypocotyl cells of dark-grown auw seedlings contain only small proplastids that lack a prolamellar body (Neuhaus et al., 1993). Moreover, preliminary analysis of Pchlide in dark-grown au hypocotyls indicates that, compared with wild type, they have even lower levels than cotyledons (M.J. Terry, unpublished results), consistent with the hypothesis that feedback inhibition of chlorophyll synthesis affects normal etioplast development in these mutants.
The results described in this paper also provide a possible explanation for the observation that CAB gene expression is reduced in dark-grown au seedlings (Sharrock et al., 1988; Ken-Dror and Horwitz, 1990). This phenomenon has also been observed in the hy1 mutant of Arabidopsis (López-Juez et al., 1996), which also has reduced levels of Pchlide in the dark (C.E. Raitt and M.J. Terry, unpublished results), and it is possible that these two observations are related. CAB gene expression is known to be regulated by a signal from the plastid (Taylor, 1989), and reduced Pchlide synthesis may affect the expression of this gene either directly or through effects on etioplast development.
There may be some additional reasons for the phenotype of etiolated au seedlings, such as an effect of heme accumulation or a loss of PΦB. Heme is a potent regulator of gene expression in other organisms (Sassa and Nagai, 1996) and may also play a significant role in plant development. It is also possible that PΦB (or its precursor, biliverdin IXα) can act as a signaling molecule, although there is currently no evidence to support such a role. Alternatively, although phytochrome (Pr or Pfr) did not have a direct effect on Pchlide accumulation in these mutants, it is possible that it has an independent role in plastid development in etiolated seedlings.
Consequences of Feedback Inhibition of Chlorophyll Synthesis in the Light
It is difficult to estimate the contribution of feedback inhibition of chlorophyll synthesis on the light-grown phenotype of au and yg-2 mutants. The difficulty arises because phytochrome is crucial for normal plastid development and has been shown to regulate the synthesis of both chlorophyll (Kasemir, 1983) and chlorophyll a/b-binding proteins (Batschauer et al., 1994). It is therefore hard to separate the role of phytochrome in these processes from any contribution that feedback inhibition may have to the phenotype of light-grown au leaves. However, light-grown auw plants contain substantial amounts of phytochrome (López-Juez et al., 1990; Sharma et al., 1993), resulting in many phytochrome-mediated responses being relatively normal at this developmental stage (López-Juez et al., 1990; Whitelam and Smith, 1991), including the expression of CAB mRNA (Becker et al., 1992). This last observation in particular suggests that the au phenotype is not the result of abnormal photoregulation of CAB genes. Indeed, pigment synthesis rather than CAB gene expression appears to be the most important factor in determining chlorophyll levels. Experiments using antisense expression have demonstrated that reducing CAB mRNA to almost undetectable levels results in plants with no visible phenotype (Flachmann and Kühlbrandt, 1995); in contrast, a 50% reduction in glutamate semialdehyde aminotransferase activity leads to plants containing less than 30% of wild-type chlorophyll (Höfgen et al., 1994). It is therefore probable that the phenotype of light-grown au plants is a consequence of a reduced rate of chlorophyll pigment synthesis arising from a combination of reduced phytochrome activation and feedback inhibition.
The hypothesis that feedback inhibition of chlorophyll synthesis contributes to the au phenotype will require rigorous testing but is supported by a number of observations. The first is that the au,hp-1 double mutant retains a relatively severe pale phenotype (Fig. 2), even though many phytochrome responses are amplified by the hp-1 mutation (Peters et al., 1992). Since mature au plants contain considerable amounts of phytochrome, we might expect that the hp-1 mutation would be able to fully rescue the leaf-color phenotype of mature au plants. Further support comes from the comparison of au with other phytochrome-deficient tomato mutants. The phyA-deficient fri mutant (van Tuinen et al., 1995a), the phyB1-deficient tri mutant (van Tuinen et al., 1995b), and the fri,tri double mutant do not exhibit the pale coloring that is characteristic of au and yg-2. This suggests that, if the phenotype of au and yg-2 is primarily the result of a phytochrome deficiency, it is not mediated by phyA or phyB1 but by the absence of one or more of the additional phytochrome species in tomato, possibly in combination with reduced levels of phyA. A mutant plant deficient in all phytochrome apoproteins will be required before we can confidently estimate the extent to which phytochrome deficiency contributes to the golden phenotype of au.
Both au and yg-2 exhibit a marked susceptibility to bleaching under high-light and low-temperature conditions. This phenotypic trait can be accounted for by the explanations given above, because plants with a low steady-state rate of chlorophyll synthesis are more likely to be severely chlorophyll deficient under these conditions. However, there are two additional explanations for this bleaching phenotype. The first is that mutant plants might accumulate pigments that cause photooxidative damage. Proto IX, which was present in au and yg-2 after ALA feeding in the dark (Fig. 5), would be a prime candidate for mediating such an effect. Alternatively, results of experiments with transgenic Arabidopsis plants expressing mammalian biliverdin reductase have led to the suggestion that phytochrome has an important role in regulating light tolerance (Lagarias et al., 1997). Biliverdin reductase can metabolize both biliverdin IXα and PΦB to inactive rubins, and the resulting phytochrome-chromophore-deficient plants are particularly sensitive to high fluence rates of light. Since feedback inhibition does not contribute to the phenotype of these plants, it is possible that the loss of phytochrome is the primary cause of the chlorophyll deficiency by preventing normal regulation of photosytem stoichiometry. Further work on the characterization of phytochrome-chromophore-deficient mutants is required to resolve these issues. This will be particularly important if this class of mutants are to be used effectively as phytochrome-deficient controls in the study of plant growth and development.
Abbreviations:
- ALA
5-aminolevulinic acid
- Pchlide
protochlorophyllide
- phyA
phytochrome A holoprotein
- phyB1
phytochrome B1 holoprotein
- PHYA
phytochrome A apoprotein
- PHYB1
phytochrome B1 apoprotein
- POR
NADPH:protochlorophyllide oxidoreductase
- Proto IX
protoporphyrin IX
- PΦB
phytochromobilin
LITERATURE CITED
- Adamse P, Peters JL, Jaspers PAPM, van Tuinen A, Koornneef M, Kendrick RE. Photocontrol of anthocyanin synthesis in tomato seedlings: a genetic approach. Photochem Photobiol. 1989;50:107–111. [Google Scholar]
- Batschauer A, Gilmartin PM, Nagy F, Schäfer E. The molecular biology of photoregulated genes. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants, Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 559–600. [Google Scholar]
- Beale SI, Weinstein JD. Biochemistry and regulation of photosynthetic pigment formation in plants and algae. In: Jordan PM, editor. Biosynthesis of Tetrapyrroles. Amsterdam, The Netherlands: Elsevier; 1991. pp. 155–235. [Google Scholar]
- Becker TW, Foyer C, Caboche M. Light-regulated expression of the nitrate-reductase and nitrite-reductase genes in tomato and in the phytochrome-deficient aurea mutant of tomato. Planta. 1992;188:39–47. doi: 10.1007/BF00198937. [DOI] [PubMed] [Google Scholar]
- Castelfranco PA, Jones OTG. Protoheme turnover and chlorophyll synthesis in greening barley tissue. Plant Physiol. 1975;55:485–490. doi: 10.1104/pp.55.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chereskin BM, Castelfranco PA. Effects of iron and oxygen on chlorophyll biosynthesis. II. Observations on the biosynthetic pathway in isolated etiochloroplasts. Plant Physiol. 1982;68:112–116. doi: 10.1104/pp.69.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan J, Gassman M. Induction of porphyrin synthesis in etiolated bean leaves by chelators of iron. Plant Physiol. 1974;53:206–215. doi: 10.1104/pp.53.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Chory J. Light control of plant development. Annu Rev Cell Dev Biol. 1997;13:203–229. doi: 10.1146/annurev.cellbio.13.1.203. [DOI] [PubMed] [Google Scholar]
- Flachmann R, Kühlbrandt W. Accumulation of plant antenna complexes is regulated by post-transcriptional mechanisms in tobacco. Plant Cell. 1995;7:149–160. doi: 10.1105/tpc.7.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough S. Defective synthesis of porphyrins in barley plastids caused by mutation in nuclear genes. Biochim Biophys Acta. 1972;286:36–54. doi: 10.1016/0304-4165(72)90086-4. [DOI] [PubMed] [Google Scholar]
- Höfgen R, Axelsen KB, Kannangara CG, Schüttke I, Pohlenz H-D, Willmitzer L, Grimm B, von Wettstein D. A visible marker for antisense mRNA expression in plants: inhibition of chlorophyll synthesis with a glutamate-1-semialdehyde aminotransferase antisense gene. Proc Natl Acad Sci USA. 1994;91:1726–1730. doi: 10.1073/pnas.91.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Bonner BA, Castelfranco PA. Regulation of 5-aminolevulinic acid (ALA) synthesis in developing chloroplasts. II. Regulation of ALA-synthesizing capacity by phytochrome. Plant Physiol. 1989;90:1003–1008. doi: 10.1104/pp.90.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M, Murakami S. Separation and characterization of prolamellar bodies and prothylakoids from squash etioplasts. Plant Cell Physiol. 1983;24:71–80. [Google Scholar]
- Kahn A. Spectrophotometric quantitation of protochlorophyll(ide): specific absorption and molar extinction coefficients reconsidered. Physiol Plant. 1983;59:99–102. [Google Scholar]
- Kasemir H. Light control of chlorophyll accumulation in higher plants. In: Shropshire W, Mohr H, editors. Encyclopedia of Plant Physiology, New Series, Vol 16B. Berlin: Springer-Verlag; 1983. pp. 662–686. [Google Scholar]
- Ken-Dror S, Horwitz BA. Altered phytochrome regulation of greening in an aurea mutant of tomato. Plant Physiol. 1990;92:1004–1008. doi: 10.1104/pp.92.4.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerckhoffs LHJ (1996) Physiological functions of phytochromes in tomato: a study using photomorphogenic mutants. PhD thesis. Wageningen Agricultural University, The Netherlands
- Kerckhoffs LHJ, Schreuder MEL, van Tuinen A, Koornneef M, Kendrick RE. Phytochrome control of anthocyanin biosynthesis in tomato seedlings: analysis using photomorphogenic mutants. Photochem Photobiol. 1997;65:374–381. [Google Scholar]
- Koornneef M, Cone JW, Dekens RG, O'Herne-Robers EG, Spruit CJP, Kendrick RE. Photomorphogenic responses of long-hypocotyl mutants of tomato. J Plant Physiol. 1985;120:153–165. [Google Scholar]
- Kraepiel Y, Jullien M, Cordonnier-Pratt M-M, Pratt L. Identification of two loci involved in phytochrome expression in Nicotiana plumbaginifolia and lethality of the corresponding double mutant. Mol Gen Genet. 1994;242:559–565. doi: 10.1007/BF00285279. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Schaub U, Söll D, Ujwal ML. Glutamyl-transfer RNA: at the crossroad between chlorophyll and protein biosynthesis. Trends Plant Sci. 1996;1:371–376. [Google Scholar]
- Lagarias DM, Crepeau MW, Maines MD, Lagarias JC. Regulation of photomorphogenesis by expression of mammalian biliverdin reductase in transgenic Arabidopsis plants. Plant Cell. 1997;9:675–688. doi: 10.1105/tpc.9.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Juez E, Nagatani A, Buurmeijer WF, Peters JL, Furuya M, Kendrick RE, Wesselius JC. Response of light-grown wild type and au-mutant tomato plants to end-of-day far-red light. J Photochem Photobiol B Biol. 1990;4:391–405. [Google Scholar]
- López-Juez E, Streatfield S, Chory J (1996) Light signals and autoregulated chloroplast development. In WR Briggs, RL Heath, EM Tobin, eds, Regulation of Plant Growth and Development. American Society of Plant Physiologists, Rockville, MD, pp 144–152
- Mascia PN, Robertson DS. Studies of chloroplast development in four maize mutants defective in chlorophyll biosynthesis. Planta. 1978;143:207–211. doi: 10.1007/BF00387791. [DOI] [PubMed] [Google Scholar]
- Masuda T, Kouji H, Matsunaka S. Diphenyl ether herbicide-decreased heme contents stimulate 5-aminolevulinic acid synthesis. Pest Biochem Physiol. 1990;36:106–114. [Google Scholar]
- Neuhaus G, Bowler C, Kern R, Chua N-H. Calcium/calmodulin-dependent and -independent phytochrome signal transduction pathways. Cell. 1993;73:937–952. doi: 10.1016/0092-8674(93)90272-r. [DOI] [PubMed] [Google Scholar]
- Parks BM, Quail PH. Phytochrome-deficient hy1 and hy2 long hypocotyl mutants of Arabidopsis are defective in phytochrome chromophore biosynthesis. Plant Cell. 1991;3:1177–1186. doi: 10.1105/tpc.3.11.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JL, Schreuder MEL, Verduin SJW, Kendrick RE. Physiological characterization of a high pigment mutant of tomato. Photochem Photobiol. 1992;56:75–82. [Google Scholar]
- Pontoppidan B, Kannangara CG. Purification and partial characterisation of barley glutamyl-tRNAGlu reductase, the enzyme that directs glutamate to chlorophyll biosynthesis. Eur J Biochem. 1994;225:529–537. doi: 10.1111/j.1432-1033.1994.00529.x. [DOI] [PubMed] [Google Scholar]
- Rebeiz CA, Mattheis JR, Smith BB, Rebeiz CC, Dayton DF. Chloroplast biogenesis. Biosynthesis and accumulation of protochlorophyll by isolated etioplasts and developing chloroplasts. Arch Biochem Biophys. 1975;171:549–567. doi: 10.1016/0003-9861(75)90065-x. [DOI] [PubMed] [Google Scholar]
- Reinbothe S, Runge S, Reinbothe C, van Cleve B, Apel K. Substrate-dependent transport of the NADPH:protochlorophyllide oxidoreductase into isolated plastids. Plant Cell. 1995;7:161–172. doi: 10.1105/tpc.7.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S, Nagai T. The role of heme in gene expression. Int J Hematol. 1996;63:167–178. doi: 10.1016/0925-5710(96)00449-5. [DOI] [PubMed] [Google Scholar]
- Sharma R, Lopez-Juez E, Nagatani A, Furuya M. Identification of photo-inactive phytochrome A in etiolated seedlings and photo-active phytochrome B in green leaves of the aurea mutant of tomato. Plant J. 1993;4:1035–1042. doi: 10.1046/j.1365-313x.1993.04061035.x. [DOI] [PubMed] [Google Scholar]
- Sharrock RA, Parks BM, Koornneef M, Quail PH. Molecular analysis of the phytochrome deficiency in an aurea mutant of tomato. Mol Gen Genet. 1988;213:9–14. [Google Scholar]
- Sperling U, Franck F, van Cleve B, Frick G, Apel K, Armstrong GA. Etioplast differentiation in Arabidopsis: both PORA and PORB restore the prolamellar body and photoactive protochlorophyllide-F655 to the cop1 photomorphogenic mutant. Plant Cell. 1998;10:283–296. doi: 10.1105/tpc.10.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WC. Regulatory interactions between nuclear and plastid genomes. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:211–233. [Google Scholar]
- Terry MJ. Phytochrome chromophore-deficient mutants. Plant Cell Environ. 1997;20:740–745. [Google Scholar]
- Terry MJ, Kendrick RE. The aurea and yellow-green-2 mutants of tomato are deficient in phytochrome chromophore synthesis. J Biol Chem. 1996;271:21681–21686. doi: 10.1074/jbc.271.35.21681. [DOI] [PubMed] [Google Scholar]
- Terry MJ, McDowell MD, Lagarias JC. (3Z)- and (3E)-Phytochromobilin are intermediates in the biosynthesis of the phytochrome chromophore. J Biol Chem. 1995;270:11111–11119. doi: 10.1074/jbc.270.19.11111. [DOI] [PubMed] [Google Scholar]
- Terry MJ, Wahleithner JA, Lagarias JC. Biosynthesis of the plant photoreceptor phytochrome. Arch Biochem Biophys. 1993;306:1–15. doi: 10.1006/abbi.1993.1473. [DOI] [PubMed] [Google Scholar]
- van Tuinen A, Hanhart CJ, Kerckhoffs LHJ, Nagatani A, Boylan MT, Quail PH, Kendrick RE, Koornneef M. Analysis of phytochrome-deficient yellow-green-2 and aurea mutants of tomato. Plant J. 1996;9:173–182. [Google Scholar]
- van Tuinen A, Kerckhoffs LHJ, Nagatani A, Kendrick RE, Koornneef M. Far-red light-insensitive, phytochrome A-deficient mutants of tomato. Mol Gen Genet. 1995a;246:133–141. doi: 10.1007/BF00294675. [DOI] [PubMed] [Google Scholar]
- van Tuinen A, Kerckhoffs LHJ, Nagatani A, Kendrick RE, Koornneef M. A temporarily red light-insensitive mutant of tomato lacks a light-stable, B-like phytochrome. Plant Physiol. 1995b;108:939–947. doi: 10.1104/pp.108.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Arnim A, Deng X-W. Light control of seedling development. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:215–243. doi: 10.1146/annurev.arplant.47.1.215. [DOI] [PubMed] [Google Scholar]
- von Wettstein D, Gough S, Kannangara CG. Chlorophyll biosynthesis. Plant Cell. 1995;7:1039–1057. doi: 10.1105/tpc.7.7.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wettstein D, Henningsen KW, Boynton JE, Kannangara CG, Nielsen OF. The genic control of chloroplast development in barley. In: Boardman NK, Linnane AW, Smillie RM, editors. Autonomy and Biogenesis of Mitochondria and Chloroplasts. Amsterdam, The Netherlands: North Holland; 1971. pp. 205–223. [Google Scholar]
- Vothknecht UC, Kannangara CG, von Wettstein D. Expression of catalytically active barley glutamyl tRNAGlu reductase in Escherichia coli as a fusion protein with glutathione S-transferase. Proc Natl Acad Sci USA. 1996;93:9287–9291. doi: 10.1073/pnas.93.17.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JL, Terry MJ, Rameau C, Reid JB, Kendrick RE. The phytochrome-deficient pcd1 mutant of pea is unable to convert heme to biliverdin IXα. Plant Cell. 1996;8:55–67. doi: 10.1105/tpc.8.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JL, Terry MJ, Reid JB, Kendrick RE. The phytochrome-deficient pcd2 mutant of pea is unable to convert biliverdin IXα to 3(Z)-phytochromobilin. Plant J. 1997;11:1177–1186. doi: 10.1105/tpc.8.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam GC, Smith H. Retention of phytochrome-mediated shade avoidance responses in phytochrome-deficient mutants of Arabidopsis, cucumber and tomato. J Plant Physiol. 1991;139:119–125. [Google Scholar]