Abstract
Recent experimental work has demonstrated the existence of extremely rapid saccades toward faces in natural scenes that can be initiated only 100 ms after image onset (Crouzet et al., 2010). These ultra-rapid saccades constitute a major challenge to current models of processing in the visual system because they do not seem to leave enough time for even a single feed-forward pass through the ventral stream. Here we explore the possibility that the information required to trigger these very fast saccades could be extracted very early on in visual processing using relatively low-level amplitude spectrum (AS) information in the Fourier domain. Experiment 1 showed that AS normalization can significantly alter face-detection performance. However, a decrease of performance following AS normalization does not alone prove that AS-based information is used (Gaspar and Rousselet, 2009). In Experiment 2, following the Gaspar and Rousselet paper, we used a swapping procedure to clarify the role of AS information in fast object detection. Our experiment is composed of three conditions: (i) original images, (ii) category swapped, in which the face image has the AS of a vehicle, and the vehicle has the AS of a face, and (iii) identity swapped, where the face has the AS of another face image, and the vehicle has the AS of another vehicle image. The results showed very similar levels of performance in the original and identity swapped conditions, and a clear drop in the category swapped condition. This result demonstrates that, in the early temporal window offered by the saccadic choice task, the visual saccadic system does indeed rely on low-level AS information in order to rapidly detect faces. This sort of crude diagnostic information could potentially be derived very early on in the visual system, possibly as early as V1 and V2.
Keywords: natural scenes, fast saccades, Fourier transform, amplitude spectrum, face detection
Introduction
The time needed to detect the presence of an object in a complex natural scene can be remarkably short (Potter, 1975; Thorpe et al., 1996; Kirchner and Thorpe, 2006). The Kirchner and Thorpe study showed that if two images are presented to human subjects left and right of a fixation, they can selectively saccade on the image containing an animal as early as 120–130 ms after stimulus onset. Recently, it has been show that this processing time could be even shorter if targets are human faces, with reliable saccadic responses starting only 100 ms after image display (Crouzet et al., 2010). Additionally, this extremely fast processing is associated with a very strong bias toward faces, such that when subjects attempt to saccade toward another category of stimulus such as vehicles (faces in this case were used as distractors), performance is particularly poor, especially when the saccades are initiated at short latencies.
This kind of extremely rapid processing puts very severe constraints on underlying visual processing. Given that the earliest saccades toward faces can be initiated at 100 ms, it follows that the brain mechanisms that trigger the response must be even earlier. It is often assumed that the delays in the oculomotor circuit leading to activation of the eye muscles are of the order of 20 ms or so, implying that the “decision” to move is presumably made at a latency of only 80 ms. Even a pure feed-forward processing sweep from the retina to the human homolog of inferotemporal cortex (IT), where object selective neurons are found (Tanaka, 1996), might be too long as it is suggested by single-cell recordings in monkeys (see Lamme and Roelfsema, 2000 for a review; see also Tsao et al., 2006) or Local Field Potentials in humans (Liu et al., 2009). Instead, the visual system might base these rapid behavioral decisions on information that is only partially processed, leading to what might be termed a “quick and dirty” processing strategy.
A good candidate to be the basis of this “quick and dirty” processing could be amplitude spectrum (AS) information of the image in the Fourier domain. The analogy between the early stages of processing in the visual system and Fourier analysis is long standing (Marr, 1982; see Westheimer, 2001 for an historical review). Early visual processing has often been described as a form of filtering operation (Campbell and Robson, 1968; Marr, 1982; Field, 1999). Although image semantics does not depend on spatial-frequency amplitude but rather on phase information (Oppenheim and Lim, 1981; Piotrowski and Campbell, 1982), the Fourier spectral signatures of scenes have been used by computational models to infer scene categories (Oliva and Torralba, 2001; Torralba and Oliva, 2003). Indeed, it has been suggested that the human visual system can take advantage of these low-level natural image statistics to perform more complex tasks. For example, rapid image recognition can be biased by priming using information concerning the AS (for global scene properties: Guyader et al., 2004; or animal detection: Kaping et al., 2007). However, studies that have directly manipulated the target images for recognition suggest that it is not used for global scene categorization (Loschky et al., 2007; Loschky and Larson, 2008) but see (Joubert et al., 2009), animal detection (Wichmann et al., 2006; Gaspar and Rousselet, 2009), even using fast saccadic responses (Wichmann et al., 2010).
However, face detection could be a special case. Amplitude information has been claimed to be responsible for face pop-out in visual search (Vanrullen, 2006), but see also (Hershler and Hochstein, 2006). Recently, a study explicitly tested the role of amplitude information for fast saccades toward faces. Using a similar design to Wichmann et al. (2010) but replacing the manual response by a saccadic choice task, they showed that amplitude alone can bias fast saccadic responses toward faces (Honey et al., 2008). Furthermore, numerous studies have already showed that faces have specific characteristics in the frequency domain (Keil, 2008, 2009; Keil et al., 2008; Nestor et al., 2008; Dakin and Watt, 2009). As a demonstration, an amplitude-only based classification performs surprisingly well when separating the set of images used in Crouzet et al., 2010; 85% on faces; and 84% on vehicles, see Figure 1). This particularity could thus be used by the visual saccadic system.
Figure 1.
Performance of a linear classifier feed with amplitude-only information to classify face and vehicle images used in Crouzet et al. (2010). After having resized images to 256 × 256, they were passed trough a Hamming window function to remove boundary artifacts. The amplitude of the Fourier transform was then computed on each image. The resulting distribution of frequencies were divided into four bins of orientation (horizontal, vertical, and the two obliques), each one covering 45°. The distribution of frequencies for each orientation was then encoded by 20 points, resulting in 80 values representing the global features of each image to feed the classifier. A linear SVM was then trained on half of the images (50% faces, 50% vehicles) and tested on the other half. After 1000 cross-validations with random train and test subsets, we computed the mean performance of the classifier to correctly classify an image in its class. The error bars correspond to the SD. The computing of the global and unlocalized features was based on the MATLAB script AverageAndPowerSpectrum.m (Torralba and Oliva, 2003), and the classification was done using the LIBSVM 2.9-1 for MATLAB (http://www.csie.ntu.edu.tw/~cjlin/libsvm/).
In the present study, using the same set of images than in an earlier study, we investigated the role of AS information in ultra-rapid detection of faces. In Experiment 1, we compared the subject performance in a saccadic choice task where they had to discriminate between faces and vehicles, using either unmodified or amplitude normalized images. A performance drop in the normalized condition would mean that this type of information plays a significant role in the task. The observed decrease of performance when subjects had to saccade toward faces suggests that amplitude information is indeed important for fast face detection. However, this decrease might at least partially be caused by the edge noise added to images by the AS normalization rather than the absence of specific amplitude information (Gaspar and Rousselet, 2009). Experiment 2, designed to address this issue, showed that edge noise alone cannot explain the result, but rather suggested that amplitude information is effectively used to guide fast saccades, even if phase information is still the most important. Together, these results suggest that the visual system does indeed use incompletely processed information to help make the extremely rapid behavioral responses that are seen with face stimuli.
General Methods
Stimuli
Two hundred photographs selected from the Corel Photo library database and downloaded from the Internet were used to set up two object categories of 100 natural scenes: human faces and vehicles. The same set has already been used in a recent study by our group (Crouzet et al., 2010). All the images were converted to gray-scale and resized to 330 × 330 pixels. The global luminance (0.5) and contrast (RMS = 0.26) were set to be equal between images. All the image modifications were done using MATLAB.
Apparatus
Participants viewed the stimuli in a dimly lit room with their heads on a chin rest to maintain the viewing distance at 60 cm. Stimuli were presented on a IIYAMA Vision Master PRO 454 monitor with the screen resolution set to 800 × 600 pixels and a refresh rate of 100 Hz. The centers of the two images were always 8.6° from the fixation cross, resulting in a retinal size for each image of 14° by 14°. Stimuli presentation was done using MATLAB and the Psychophysics Toolbox 3 (Brainard, 1997; Pelli, 1997). The background color is the only apparatus difference between the two experiments. In Experiment 1, a black background was used. In Experiment 2, it has been changed to a mid gray background, which seems to slightly improve performance.
The saccadic choice task
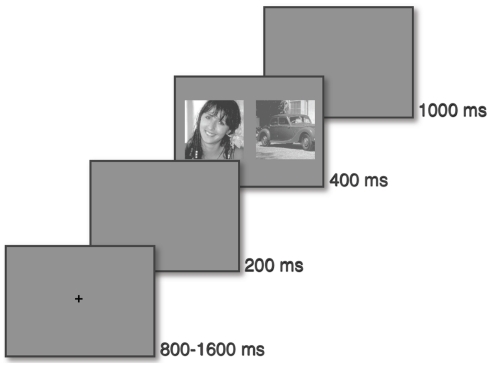
A trial takes place as follows: observers had to keep their eyes on a fixation cross which disappeared after a pseudo-random time interval (800–1600 ms). After a 200 ms-time gap, two natural scenes (one face and one vehicle) appeared on each side of the screen for 400 ms (see Figure 2). The task was to make a saccade as quickly and as accurately as possible to the side of the target.
Figure 2.
Protocol: the saccadic choice task. A fixation cross is displayed for a pseudo-random time (between 800 and 1600 ms), then, after a 200 ms gap, two images (a target and a distractor) are displayed on the left and right. After a 1000 ms period, a new trial can start.
Eye movement recording
Eye movements were monitored with an IView Hi-Speed eyetracker (SensoMotoric Instruments, Berlin, Germany). This infrared tracking system samples eye position at 240 Hz. Saccade detection was performed off-line using the saccade based algorithm of the SMI BeGaze Event Detection (Smeets and Hooge, 2003). Only the first saccade to enter one of the two images after display onset was analyzed. Only saccades with onsets longer than 80 ms and shorter than 500 ms were kept for the analysis in order to reduce the influence of outliers. Before each run, a 13-point calibration was performed.
Experiment 1
In order to test the influence of AS for the ultra-rapid detection of faces, we made this cue non-informative by averaging it between images. This corresponds to the normalized condition. In this case, the only information still available to discriminate between faces and vehicles is thus phase information. The performance of participants in this normalized condition was compared to their performance with original images.
Methods
Participants
Eight participants (six males, mean age = 29.5, two left-handed) including the two authors gave written informed consent before participating in the experiment.
Amplitude spectrum normalization
In order to normalize it, we computed the mean AS over all images of the two categories. This mean AS was then re-combined with the original phase of each image, resulting in a second equivalent set of images which differed only by their phase information (Figure 3). This method averages the spatial-frequency contents of all stimuli at each scale and orientation.
Figure 3.
Stimuli of Experiment 1. Examples of original images of faces and vehicles at the top. In the middle, the mean amplitude spectrum of faces and vehicles images is plotted using a representation where each section account for 60, 80, and 90% of the image energy. This mean amplitude spectrum was then applied to every image to create the normalized condition. Examples of images for this condition are at the bottom of the figure.
Design
Using a within-subject design, two tasks (saccading toward faces or toward vehicles) and two types of images (original, normalized) were tested here. The whole experiment was divided for each subject in two blocks (one for each task). In each block, runs were alternating between the two types of images. For example, a subject would start with eight runs of 50 trials with faces as the target – these runs alternating between original images and normalized images – and then the same with vehicles as target, blocks, and runs orders being counterbalanced between subjects. Indeed, each image was displayed two times in each condition (once on the left and once on the right hemifield), resulting in 200 trials per condition and per subject. Each block was preceded by a training session of 50 trials (25 with original images and then 25 with normalized images which were not used in the experiment).
Results
The results with original images (168 ms and 88.8% correct in the face task; 197 ms and 72.8% in the vehicle task) are very comparable to the values observed in a previous experiment by Crouzet et al., 2010; Figure 4). In the normalized condition, when participants can only rely on phase information, they were still able to do the task very quickly and accurately (180 ms and 75% correct in the face task; 201 ms and 63.8% in the vehicle task). Using a 2-way ANOVA with factors Task (face, vehicle) and Type (original, normalized), the results showed that even though participants are able to do the task above chance in the normalized condition, their performance is globally lower (Type global effect: F(1,7) = 52.8, p < 0.001 for mean RT; F(1,7) = 120.3, p < 0.001 for accuracy), a result which is consistent with previous studies using this type of normalization (Wichmann et al., 2006, 2010; Loschky et al., 2007; Loschky and Larson, 2008; Gaspar and Rousselet, 2009; Joubert et al., 2009). More specifically, and after correction for multiple comparisons, the normalization has no effect on mean RTs, and the decrease in accuracy following normalization is only significant in the face task (Tukey HSD post hoc test: p < 0.01 in the face task, p = 0.1 in the vehicle task). In other word, the AS information seemed to be used particularly when subjects had to target faces.
Figure 4.
Results of Experiment 1. On the top, results when participants had to saccade toward faces, on the bottom, results when participants had to saccade toward vehicles. (A) Mean RT and accuracy across subjects in the original (Orig) and normalized (Norm) conditions. Error bars represent bootstrapped 95% C. I. of the mean. (B) Distributions of RT in each condition and each task. Thick lines are for correct responses, thin lines for errors. Orange lines for saccades toward faces, blue lines for saccades toward vehicles. The transparent gray bar is placed according to the minimum RT (the first 10 ms bin of time where correct responses significantly outnumber incorrect ones using a χ2 test) when considering all subjects as one.
Furthermore, despite the normalization, the advantage for faces is still present although reduced in size, with an advantage for faces over vehicles of 21 ms and 11% accuracy in the normalized condition (accuracy: p < 0.05), 29 ms and 16% in the original one (accuracy: p < 0.01; Task global effect: F(1,7) = 18.4, p < 0.01 for mean RT; F(1,7) = 27.5, p < 0.01 for accuracy). A second conclusion is thus that the AS can explain a part of the bias toward faces, but is not sufficient.
As a summary of Experiment 1, and as expected from previous studies, the AS normalization significantly decreased subjects performance. However, this effect was larger in the face task than in the vehicle one. Even if phase information is largely sufficient to induce a bias toward faces, amplitude information could then play a significant role in driving fast saccades.
Experiment 2
A recent study demonstrated that a performance decrease caused by AS normalization is not sufficient to claim that it is effectively used by the visual system to perform the task. Indeed, Gaspar and Rousselet (2009) showed that the performance decrease in an animal detection task caused by AS normalization could be entirely explained by the edge noise added incidentally by the manipulation, rather than by the absence of amplitude information. To show that, they used an additional condition where every animal image phase spectrum was combined with the AS of another animal image (they did the same for non-animal images), resulting in images with phase and amplitude from the same category but from different individual images. The performance of their participants in this “identity swapped” condition was similar to the one in the “normalized” condition. This means that in a manual response animal detection task, what matters is not phase or amplitude information by themselves, but the interaction between the two.
However, it has been shown that the AS of faces alone could attract fast saccades in a saccadic choice task (Honey et al., 2008) since even when the phase information was completely scrambled, there was still a bias with saccades toward faces. In Experiment 2, we thus used two new image manipulations: (i) a category swapped condition (SWAcat), where the amplitude spectra of the face and vehicle images were exchanged and (ii) an identity swapped condition (SWAid) where the amplitude spectra applied to each image were taken from another image of the same category. Thus, in all conditions, amplitude differences were still informative, but in the case of SWAcat, they were inverted between categories. The only difference between SWAid and ORI being that amplitude and phase of each image was not consistent. This results in three different conditions: original (ORI), category swapped (SWAcat), and identity swapped (SWAid) that will be used to investigate further the role of AS in guiding fast saccades toward faces.
The logic here is as follows. If the amplitude is not used by subjects to perform the task, the performance in the SWAcat and SWAid conditions should be equal, and certainly lower than in ORI because of higher edge noise (Gaspar and Rousselet, 2009). On the contrary, if the AS is used, performance in the identity swapped condition should be significantly higher than in the category swapped. Our results clearly support the second alternative, arguing for the use of amplitude information for fast saccade guidance.
Methods
Participants
Twelve participants (seven males, mean age = 25.9, one left-handed) including the first author gave written informed consent before participating in the experiment.
Design
The design was essentially the same as in Experiment 1. The difference was the use of three experimental conditions: original (ORI), category swapped (SWAcat), and identity swapped (SWAid). Every image was seen two times for each of the three conditions by each participant, as a target and as distractor when the task is reversed (resulting in 100 images × 2 repetitions × 3 conditions × 2 tasks = 1200 trials per participant).
Image manipulation
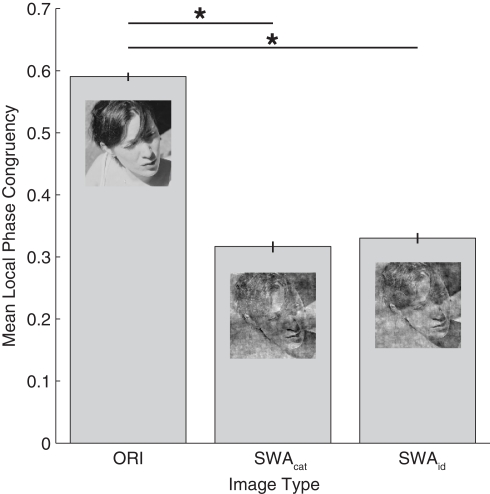
In the original condition, there was no manipulation of the images in the Fourier domain. In the category swapped condition, the amplitude spectra were switched between the two images presented on every trial. Thus, face images had the amplitude from vehicles, and vehicles from faces. In the identity swapped condition, on every trial, the amplitude of each image was replaced by an amplitude randomly selected among the 99 other exemplars of the category (each individual amplitude was used only once per run). For example, a face had the amplitude of another face, resulting in images with amplitude, and phase from the same category but not from the same image. Examples can be seen in Figure 5. A possible confound for this experiment would be different level of edge noise between the category and identity swapped conditions. To control this aspect, a measure of local phase congruency (LPC) based on the MATLAB function phasecong3.m written by Peter Kovesi was made on every image. The results of these measurements can be seen on Figure 6, and show that the level of edge noise between category and identity swapped conditions are effectively equal, and were both significantly higher than in the original condition.
Figure 5.
Images used in Experiment 2. (A) Principles of the different possible combinations between amplitude and phase spectrums with examples for an image. (B) Examples of images used in the three conditions, faces on top and vehicles on bottom.
Figure 6.
Averaged local phase congruency (LPC) over all images as a function of the three image modifications. The function phasecong3.m (Kovesi, 2000) was applied on every image of each set (ORI, SWAcat, and SWAid) to obtain an LPC value for each image. On an individual image, this value corresponds to the averaged value of the 10 most salient locations (as defined by the output map of the LPC on the original image, see Gaspar and Rousselet, 2009). The results show that the edge strengths are different between the three conditions (F(2,597) = 1392.6, p < 0.001) and significantly higher in the original than in the category and identity swapped sets (test post hoc Tukey HSD). The important result here is that there is no significant difference between the category and identity swapped sets.
Results
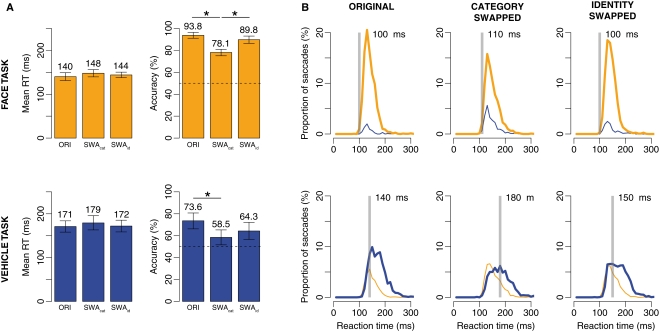
A global look at the results reveals that the performance in Experiment 2 for the original condition was somewhat better than in Experiment 1 despite the fact that the images used were exactly the same (see Figure 7). A large part of this difference is certainly caused by the change in background color (from black to gray), although inter-subject variability may also be involved. A first analysis of the global effects, using a two-way ANOVA with factors Task (face or vehicle) and Type (original, category, and identity swapped), showed an effect of both on mean RT (Type: F(2,22) = 13.56, p < 0.001; Task F(1,11) = 44.80, p < 0.001) and accuracy (Type: F(2,22) = 84.06, p < 0.001; Task: F(1,11) = 43.56, p < 0.001).
Figure 7.
Results of Experiment 2. Top: when participants had to saccade toward faces. Bottom: when participants had to saccade toward vehicles. (A) Mean RT and accuracy across subjects in the original (ORI), category swapped and identity swapped conditions. Error bars represent bootstrapped 95% C.I. of the mean (B) Distributions of RT in each condition and each task. Thick lines are for correct responses, thin lines for errors. Orange lines for saccades toward faces, blue lines for saccades toward vehicles. The transparent gray bar is placed according to the minimum RT (the first 10 ms bin of time where correct responses become significantly higher than incorrect ones).
However, the principal aim of this experiment was to test the difference between the original condition and the two conditions involving image manipulations: category and identity swapped. A post hoc analysis using correction for multiple comparisons (Tukey HSD) was used, and revealed no effect on mean RT, thus only analysis of accuracy will be developed. In the face task, the original (accuracy = 93.8%) and identity swapped (89.8%) conditions led to comparable levels of performance and were significantly better performed than the category swapped (78.1%). A closer look at RT distributions reveals that even though accuracy was lower in the category swapped condition, the first selective responses still occur very early. In the vehicle task, the effect is less clear, original (73.6%) and identity swapped (64.3%) conditions are again comparable, the only difference being between with the category swapped one (58.5%).
Each image category can be divided into two different object sizes: close-up and middle view. It could have been hypothesized that size would have an effect here, caused by the specific image manipulations. However, remarkably, a post hoc analysis showed absolutely no effect: there is no speed or accuracy differences between all the different conditions of size mixing. For example, we found no evidence that mixing the phase information from a close-up face with the amplitude information from a mid-distance view (or the inverse) specifically impaired performance in comparison to other conditions.
As a global conclusion of Experiment 2, the results argue in favor of a significant use of amplitude information by the visual system to guide saccades. Surprisingly, it seems that there is a tendency for amplitude information to also be used by subjects when the task was to target vehicles, even if it is less clear than for faces. Another surprise was that this effect is not limited to just the fastest saccades. So even if, again, phase information seems to be the most important information, AS definitely plays a role in saccade generation.
General Discussion
Our first goal was to investigate the role of amplitude information in the Fourier spectrum in the generation of fast saccades toward faces. The question raised by this study follows from two recent results which showed that (i) saccades can be selectively oriented toward a target extremely fast (100–110 ms) in the specific case of this target being a face (Crouzet et al., 2010), (ii) in the absence of phase information, amplitude alone can attract fast saccades by its own if it comes from a face image (Honey et al., 2008). Our first aim was thus to test the influence of AS information in the effect observed in Crouzet et al. (2010) study. Experiment 1 showed that the absence of amplitude information tends to slow down saccadic responses and decrease accuracy. However, Gaspar and Rousselet recently demonstrated that this performance decrease could be explained, not by the lack of amplitude information, but rather by the edge noise incidentally added. This lead to Experiment 2 which, using two new image manipulations, showed that subjects had remarkably good performance even if images were composed with phase and amplitude from the same object category but different images (and thus had a very high-level of edge noise).
To sum up all the results. First, it seems that the bias toward faces over vehicles cannot be eliminated by the different manipulations made on AS. In other words, subjects were always better at saccading toward faces than toward vehicles, even when the amplitude spectra were normalized or swapped. Thus, even though a face-like AS alone can attract fast saccades (Honey et al., 2008), a large part of the bias toward faces is caused by phase related information. Second, information from the AS is effectively used by the visual saccadic system to drive fast saccades. This result echoes a recent finding showing that the early face-selective component of the ERP (observed around 100 ms after image presentation) was closely linked to the amplitude content of images, while the later component (usually observed around 170 ms after image presentation) was related to the phase content (Rossion and Caharel, 2011).
Why amplitude spectrum was used here and not in most of previous studies?
Several previous studies have argued that pure AS information is of limited use for natural scene recognition by human subjects. Nevertheless, most of these studies have used manual responses (Wichmann et al., 2006; Loschky et al., 2007; Loschky and Larson, 2008; Gaspar and Rousselet, 2009) that have relatively long latencies. This raises the possibility that the effects observed here might be specific to very rapid saccadic responses. However, a recent study had the same conclusion for an animal detection using a saccadic choice task similar to the one used here (Wichmann et al., 2010). In the experiment 2 of this study, the authors first showed that animal detection was slightly impaired by AS normalization. Then, they used amplitude information alone to classify their images as animals or non-animals, and divided their set between images for which the classifier was the more confident (easy images) and those for which the classifier was the less confident (difficult images). Finally, with a post hoc analysis, they showed that subjects accuracy differences between easy and difficult images was not only clear in the original condition, but also in the normalized one. The straightforward interpretation was that amplitude information had no causal role on their results for animal detection by human subjects. However, as can be seen in their table of results, SRT were very slow compare to ours (most of their mean RT were above 270 ms, whereas most of our mean RTs were below 200 ms). A contrasted analysis of their results between fast and slow reaction time would thus be of great interest (Honey et al., 2008), and we would predict that the interpretation could be different according to this criterion. Thereby, the difference in reaction time could potentially explain the difference between the two studies, and suggest that amplitude information would play a role especially in an early time of processing.
Eccentricity and summary statistics
Time is not the only factor that has to be considered to explain the difference between manual and saccadic studies. Presentation of images is typically foveal in studies with manual responses (Wichmann et al., 2006; Gaspar and Rousselet, 2009), whereas by design the presentation is peripheral in tasks that involve a saccadic choice paradigm (Honey et al., 2008, as well as the present study). Could this difference accounts for the use of AS information in saccadic studies? It is well-known that contour processing as well as positional accuracy (Levi, 2008; Greenwood et al., 2009) is less and less precise with eccentricity. Indeed, peripheral vision is characterized by a high degree of spatial uncertainty (Pelli, 1985). A result of this could be that patterns in a constrained region of the periphery would be processed as textures, so that individual patterns are not available for discrimination (Orbach and Wilson, 1999). This “jumbled” effect on contours would thus disrupt phase more than unlocalized amplitude information. In this case, information contained in the “summary statistics” of the AS could be used to detect objects in the visual field taking account of the low capacity of the visual system in the periphery. According to this hypothesis, the more eccentric the presentation, the more subjects would tend to rely on information in the AS. The relatively modest eccentricity used in the saccadic choice task (8° from fixation to stimuli centers, images covering 14° × 14° each of visual angle) could explain why phase information still plays a significant role, but it could well be that at even more extreme eccentricities, only the information in the AS would be available. To conclude, the two factors (slow vs. fast response, central vs. peripheral presentations) had not been completely dissociated here, although the results of Wichmann et al. (2010) would argue in favor of the timing hypothesis. Further investigations will definitely be needed to disentangle the contribution of time and eccentricity in the observed effect.
Our conclusion is thus that, contrary to most studies that have used longer RT responses, AS is also used by the visual system to detect objects in the visual field and produce extremely fast behavioral responses. More than a global Fourier analysis of the entire visual scene (Torralba and Oliva, 2003), a process which is rather unlikely in the real world, because it would be absolutely non-informative about the localization of objects, a more plausible mechanism would be the fast extraction of AS information in a localized fashion. This could take a patch-wise and multi-scale form, as in a wavelet analysis or in the Spatial Envelope model (Oliva and Torralba, 2006). This amplitude-based but localized information seems well-suited to be the basis for ultra-rapid visual processing such as the one observed with a saccadic choice task. Further investigations will be needed to determine how exactly this type of information, present in the early visual system, is used to guide eye movement.
Quick and dirty processing for faces
A final point concerns the possibility that the very fast processing based on relatively crude low-level information in the AS may be a specific feature of face processing. Faces are clearly extremely important stimuli for humans, and it is probably vital for survival that we detect and localize any faces in the environment as quickly as possible. As a consequence, it would make good evolutionary sense to devote neural circuits early in visual processing to face detection (Johnson, 2005). In a sense, the feasibility of devising relatively simple algorithms for face detection is demonstrated by the availability of relatively efficient face-detection algorithms in many currently available cameras, often based on popular computer vision algorithms (Viola and Jones, 2004). Could equivalent strategies be implemented in neural circuits? Cortical areas such as V1 and V2 have direct connections to superficial areas of the superior colliculus from a relatively small number of specialized cortico-tectal projection neurons in layer V (Lock et al., 2003; Collins et al., 2005). These cells are known to be able to generate very strong short latency responses in the colliculus (Bereshpolova et al., 2006), and could provide a way to initiate very rapid eye movements. Could these cells have the sort of selectivity that would be needed to allow very rapid responses to faces? They certainly have some of the features that would be needed for this: they have extensive dendritic arborizations that extend through many cortical layers including superficial layers and could therefore sample much of the information that is available. If we suppose that some particular combination of orientations and spatial frequencies was in some way diagnostic for the presence of a face, it could be that this might be detected by a suitably configured cortico-tectal cell that could then directly trigger activity in the colliculus. Modeling studies have shown that relatively simple neural architectures can allow both face detection and face identification to be implemented with little more than a set of orientation tuned filters as an input, although it has generally been assumed that it be impractical to implement such strategies because of the excessively large number of neurons that would be required (VanRullen et al., 1998; Delorme and Thorpe, 2001). However, by using the unlocalized information that could be obtained from cortical complex cells, it may be possible to implement similar architectures with far fewer neurons. Other modeling studies have demonstrated that face-selective responses can be produced simply as the result of a repeated experience with face-like stimuli (Masquelier and Thorpe, 2007), something that may occur early during life because of the high probability of exposure to faces.
In conclusion, we would argue that the results presented here imply that the brain may well use some very specific strategies for detecting and localizing faces that could potentially be implemented remarkably early on in the visual processing sequence. Such strategies may not be sufficient for performing more demanding tasks such as determining facial expression or identity, but they could allow useful behavior to be generated at very short latencies.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Guillaume Rousselet for his useful comments on the original idea and design of Experiment 2. This work was supported by the Délégation Générale pour l’Armement (DGA), the Fondation pour la Recherche Médicale (FRM), and the Agence Nationale pour la Recherche (ANR).
References
- Bereshpolova Y., Stoelzel C. R., Gusev A. G., Bezdudnaya T., Swadlow H. A. (2006). The impact of a corticotectal impulse on the awake superior colliculus. J. Neurosci. 26, 2250–2259 10.1523/JNEUROSCI.4402-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard D. (1997). The psychophysics toolbox. Spat. Vis. 10, 433–436 10.1163/156856897X00357 [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Robson J. G. (1968). Application of Fourier analysis to the visibility of gratings. J. Physiol. (Lond.) 197, 551–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. E., Lyon D. C., Kaas J. H. (2005). Distribution across cortical areas of neurons projecting to the superior colliculus in new world monkeys. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 285, 619–627 [DOI] [PubMed] [Google Scholar]
- Crouzet S. M., Kirchner H., Thorpe S. J. (2010). Fast saccades toward faces: face detection in just 100 ms. J. Vis. 10, 1–17 10.1167/10.3.12 [DOI] [PubMed] [Google Scholar]
- Dakin S., Watt R. (2009). Biological “bar codes” in human faces. J. Vis. 9, 2. 10.1167/9.13.2 [DOI] [PubMed] [Google Scholar]
- Delorme A., Thorpe S. J. (2001). Face identification using one spike per neuron: resistance to image degradations. Neural. Netw. 14, 795–803 10.1016/S0893-6080(01)00049-1 [DOI] [PubMed] [Google Scholar]
- Field D. J. (1999). Wavelets, vision and the statistics of natural scenes. Philos. Trans. R. Soc. Lond. A 357, 2527–2542 10.1098/rsta.1999.0446 [DOI] [Google Scholar]
- Gaspar C. M., Rousselet G. A. (2009). How do amplitude spectra influence rapid animal detection? Vision Res. 49, 3001–3012 10.1016/j.visres.2009.09.021 [DOI] [PubMed] [Google Scholar]
- Greenwood J. A., Bex P. J., Dakin S. C. (2009). Positional averaging explains crowding with letter-like stimuli. Proc. Natl. Acad. Sci. U.S.A. 106, 13130–13135 10.1073/pnas.0901352106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyader N., Chauvin A., Peyrin C., Herault J., Marendaz C. (2004). Image phase or amplitude? Rapid scene categorization is an amplitude-based process. C. R. Biol. 327, 313–318 10.1016/j.crvi.2004.02.006 [DOI] [PubMed] [Google Scholar]
- Hershler O., Hochstein S. (2006). With a careful look: still no low-level confound to face pop-out. Vision Res. 46, 3028–3035 10.1016/j.visres.2006.03.023 [DOI] [PubMed] [Google Scholar]
- Honey C., Kirchner H., VanRullen R. (2008). Faces in the cloud: Fourier power spectrum biases ultrarapid face detection. J. Vis. 8, 1–13 10.1167/8.10.1 [DOI] [PubMed] [Google Scholar]
- Johnson M. H. (2005). Subcortical face processing. Nat. Rev. Neurosci. 6, 787–798 10.1038/nrn1766 [DOI] [PubMed] [Google Scholar]
- Joubert O. R., Rousselet G. A., Fabre-Thorpe M., Fize D. (2009). Rapid visual categorization of natural scene contexts with equalized amplitude spectrum and increasing phase noise. J. Vis. 9, 1–16 10.1167/9.6.1 [DOI] [PubMed] [Google Scholar]
- Kaping D., Tzvetanov T., Treue S. (2007). Adaptation to statistical properties of visual scenes biases rapid categorization. Vis. Cogn. 15, 12–19 10.1080/13506280600856660 [DOI] [Google Scholar]
- Keil M. S. (2008). Does face image statistics predict a preferred spatial frequency for human face processing? Proc. R. Soc. Lond. B Biol. Sci. 275, 2095–2100 10.1098/rspb.2008.0486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil M. S. (2009). “I look in your eyes, honey”: internal face features induce spatial frequency preference for human face processing. PLoS Comput. Biol. 5, e1000329. 10.1371/journal.pcbi.1000329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil M. S., Lapedriza A., Masip D., Vitria J. (2008). Preferred spatial frequencies for human face processing are associated with optimal class discrimination in the machine. PLoS ONE 3, e2590. 10.1371/journal.pone.0002590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Thorpe S. J. (2006). Ultra-rapid object detection with saccadic eye movements: visual processing speed revisited. Vision Res. 46, 1762–1776 10.1016/j.visres.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Kovesi P. D. (2000). MATLAB and Octave Functions for Computer Vision and Image Processing. Available at: http://www.csse.uwa.edu.au/(pk/research/matlabfns/
- Lamme V. A., Roelfsema P. R. (2000). The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 23, 571–579 10.1016/S0166-2236(00)01657-X [DOI] [PubMed] [Google Scholar]
- Levi D. M. (2008). Crowding – an essential bottleneck for object recognition: a mini-review. Vision Res. 48, 635–654 10.1016/j.visres.2007.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Agam Y., Madsen J. R., Kreiman G. (2009). Timing, timing, timing: fast decoding of object information from intracranial field potentials in human visual cortex. Neuron 62, 281–290 10.1016/j.neuron.2009.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock T. M., Baizer J. S., Bender D. B. (2003). Distribution of corticotectal cells in macaque. Exp. Brain Res. 151, 455–470 10.1007/s00221-003-1500-y [DOI] [PubMed] [Google Scholar]
- Loschky L. C., Larson A. M. (2008). Localized information is necessary for scene categorization, including the Natural/Man-made distinction. J. Vis. 8, 1–9 10.1167/8.10.1 [DOI] [PubMed] [Google Scholar]
- Loschky L. C., Sethi A., Simons D. J., Pydimarri T. N., Ochs D., Corbeille J. L. (2007). The importance of information localization in scene gist recognition. J. Exp. Psychol. Hum. Percept. Perform. 33, 1431–1450 10.1037/0096-1523.33.6.1431 [DOI] [PubMed] [Google Scholar]
- Marr D. (1982). Vision: A Computational Investigation into the Human Representation and Processing of Visual Information. New York, NY: Henry Holt and Co., Inc [Google Scholar]
- Masquelier T., Thorpe S. J. (2007). Unsupervised learning of visual features through spike timing dependent plasticity. PLoS Comput. Biol. 3, e31. 10.1371/journal.pcbi.0030031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor A., Vettel J. M., Tarr M. J. (2008). Task-specific codes for face recognition: how they shape the neural representation of features for detection and individuation. PLoS ONE 3, e3978. 10.1371/journal.pone.0003978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A., Torralba A. (2001). Modeling the shape of the scene: a holistic representation of the spatial envelope. Int. J. Comput. Vis. 42, 145–175 10.1023/A:1011139631724 [DOI] [Google Scholar]
- Oliva A., Torralba A. (2006). Building the gist of a scene: the role of global image features in recognition. Prog. Brain Res. 155, 23–36 10.1016/S0079-6123(06)55002-2 [DOI] [PubMed] [Google Scholar]
- Oppenheim A. V., Lim J. S. (1981). The Importance of Phase in Signals. Proc. IEEE 69, 529–541 10.1109/PROC.1981.12022 [DOI] [Google Scholar]
- Orbach H. S., Wilson H. R. (1999). Factors limiting peripheral pattern discrimination. Spat. Vis. 12, 83–106 10.1163/156856899X00058 [DOI] [PubMed] [Google Scholar]
- Pelli D. (1997). The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 10, 437–442 10.1163/156856897X00366 [DOI] [PubMed] [Google Scholar]
- Pelli D. G. (1985). Uncertainty explains many aspects of visual contrast detection and discrimination. J. Opt. Soc. Am. A 2, 1508–1532 10.1364/JOSAA.2.001508 [DOI] [PubMed] [Google Scholar]
- Piotrowski L. N., Campbell F. W. (1982). A demonstration of the visual importance and flexibility of spatial- frequency amplitude and phase. Perception 11, 337–346 10.1068/p110337 [DOI] [PubMed] [Google Scholar]
- Potter M. C. (1975). Meaning in visual search. Science 187, 965–966 10.1126/science.1145183 [DOI] [PubMed] [Google Scholar]
- Rossion B., Caharel S. (2011). ERP evidence for the speed of face categorization in the human brain: disentangling the contribution of low-level visual cues from face perception. Vision Res. 51, 1297–1311 10.1016/j.visres.2011.04.003 [DOI] [PubMed] [Google Scholar]
- Smeets J. B., Hooge I. T. (2003). Nature of variability in saccades. J. Neurophysiol. 90, 12–20 10.1152/jn.01075.2002 [DOI] [PubMed] [Google Scholar]
- Tanaka K. (1996). Inferotemporal cortex and object vision. Annu. Rev. Neurosci. 19, 109–139 10.1146/annurev.ne.19.030196.000545 [DOI] [PubMed] [Google Scholar]
- Thorpe S., Fize D., Marlot C. (1996). Speed of processing in the human visual system. Nature 381, 520–522 10.1038/381520a0 [DOI] [PubMed] [Google Scholar]
- Torralba A., Oliva A. (2003). Statistics of natural image categories. Network 14, 391–412 10.1088/0954-898X/14/3/302 [DOI] [PubMed] [Google Scholar]
- Tsao D. Y., Freiwald W. A., Tootell R. B. H., Livingstone M. S. (2006). A cortical region consisting entirely of face-selective cells. Science 311, 670–674 10.1126/science.1119983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanrullen R. (2006). On second glance: still no high-level pop-out effect for faces. Vision Res. 46, 3017–3027 10.1016/j.visres.2006.07.030 [DOI] [PubMed] [Google Scholar]
- VanRullen R., Gautrais J., Delorme A., Thorpe S. (1998). Face processing using one spike per neurone. Biosystems 48, 229–239 10.1016/S0303-2647(98)00070-7 [DOI] [PubMed] [Google Scholar]
- Viola P., Jones M. J. (2004). Robust real-time face detection. Int. J. Comput. Vis. 57, 137–154 10.1023/B:VISI.0000013087.49260.fb [DOI] [Google Scholar]
- Westheimer G. (2001). The Fourier theory of vision. Perception 30, 531–541 10.1068/p3193 [DOI] [PubMed] [Google Scholar]
- Wichmann F. A., Braun D. I., Gegenfurtner K. R. (2006). Phase noise and the classification of natural images. Vision Res. 46, 1520–1529 10.1016/j.visres.2005.11.008 [DOI] [PubMed] [Google Scholar]
- Wichmann F. A., Drewes J., Rosas P., Gegenfurtner K. R. (2010). Animal detection in natural scenes: critical features revisited. J. Vis. 10, 1–27 10.1167/10.3.12 [DOI] [PubMed] [Google Scholar]